Abstract
Blood coagulation plays a key role in the pathogenesis of infective endocarditis (IE). Conditions associated with thrombophilia could enhance IE vegetation formation and promote embolic complications.
In this study, we assessed prevalence, correlates, and clinical consequences of hyper-homocysteinemia (h-Hcy) in IE.
Homocysteine (Hcy) plasma levels were studied in 246 IE patients and 258 valvular heart disease (VHD) patients, as well as in 106 healthy controls.
IE patients showed Hcy levels comparable to VHD patients (14.9 [3–81] vs 16 [5–50] μmol/L, respectively; P = 0.08). H-Hcy was observed in 48.8% of IE patients and 55.8% of VHD (P = 0.13). Vegetation size and major embolic complications were not related to Hcy levels. IE patients with h-Hcy had a higher prevalence of chronic kidney disease and a higher 1-year mortality (19.6% vs 9.9% in those without h-Hcy; OR 2.21 [1.00–4.89], P = 0.05). However, at logistic regression analysis, h-Hcy was not an independent predictor of 1-year mortality (OR 1.87 [95% CI 0.8–4.2]; P = 0.13).
Our data suggest h-Hcy in IE is common, is related to a worse renal function, and may be a marker of cardiac dysfunction rather than infection. H-Hcy does not appear to favor IE vegetation formation or its symptomatic embolic complications.
Keywords: embolism, endocarditis, homeostasis, infective, mortality, thrombosis
1. Introduction
Infective endocarditis (IE) remains a challenging clinical condition showing increasing incidence and an unchanged, high mortality rate.[1] The endocardial vegetation is the disease-defining lesion of IE, generating the common embolic complications of the disease.[2]
IE vegetation originates at the site of endocardial lining damage or after an intracardiac device has been implanted as a consequence of hemostasis system activation and subsequent bacterial seeding on an initially sterile clot.[3,4] In light of the central role played by the hemostasis system in IE vegetation formation, we hypothesized that a prothrombotic state might increase the risk of IE onset or trigger the early IE vegetation development.[4] Moreover, a thrombophilic condition could also promote the embolic complications of the disease. Recently, we observed a significant association between the G20210A mutation of the prothrombin gene, a major inherited thrombophilic polymorphism, and prosthetic valve endocarditis.[5] Several other conditions affecting inflammation and hemostasis could play a role in IE pathogenesis.[4,6,7] Homocysteine (Hcy), a sulfur-containing amino acid, absent in natural diets, is a metabolic intermediary in transmethylation and transsulfuration reactions. Such reactions are essential to normal cellular growth, differentiation, and function. Excess homocysteine is associated with vascular disease and related disorders.[8] Hyperhomocysteinemia (h-Hcy) commonly results from inherited or acquired metabolic defects of Hcy metabolism.[9] It is associated with an increased risk of cardiovascular diseases mediated by endothelial damage and induction of both arterial and venous thrombosis.[10–13] H-Hcy due to the C677T transition in the methylene-tetra-hydro-folate reductase (MTHFR) gene is currently listed among the major genetically determined thrombophilias.[14] Thus far, no study has evaluated the potential role of this condition in the pathogenesis of IE.
Hence, we performed this study with the aim to assess the prevalence, correlates, and possible clinical significance of h-Hcy in patients with IE. Our hypothesis was that IE patients could have an inherently higher rate of h-Hcy.
2. Methods
2.1. Study design
In this case-control study, we included patients with a diagnosis of “definite” IE according to the modified Duke University criteria, admitted to our unit between 2004 and 2014. Additional eligibility criteria were age >18 years, Caucasian ethnicity, hospitalization in our center because of IE, and ability to provide the informed consent. As a result, the enrolled group did not include consecutive IE cases, as those in worse conditions or who went to cardiac surgery emergently were less likely to undergo additional blood sampling for research purposes. As controls, we recruited in our hospital valvular heart disease (VHD) patients with documented absence of IE, including uncomplicated native and prosthetic VHD patients, patients with heart valve disease undergoing cardiac surgical repair/replacement, and carriers of cardiac implantable electronic devices (CIED). Routine blood donors (BD) from our Hospital Transfusion Service were also studied as a further control group made of apparently healthy subjects. They were age- and sex-matched with IE patients in a 2:1 ratio and were selected between 2008 and 2009 among routine BD based on willingness to undergo additional blood sampling and a negative clinical history of heart disease and no prior thromboembolic event. Our hypothesis was that IE patients had an Hcy level that was 25% higher than healthy controls. Assuming a mean Hcy level of 9.2 μmol/L in healthy BD,[8] a power of 0.8, and an alpha error of 5%, the sample size needed was of about 55 IE patients.
A written informed consent to participation in this study and the collection and storage of DNA for genetic analyses was obtained from each participant. Our Department's Review Board approved the study protocol and procedures, which complied with the Declaration of Helsinki.
2.2. Type of and rationale for the analyses
Hcy plasma levels and the C677T polymorphism in the MTHFR gene were studied because of their association with a prothrombotic state as well as their measurable prevalence in the general population.[12,13] H-Hcy was defined as a plasma Hcy concentration >15 μmol/L.[13] 5,10-MTHFR is a key enzyme in the conversion pathway of Hcy into methionine.[15] The C677T transition in the MTHFR gene impairs its enzyme activity causing Hcy accumulation, that in turn exerts oxidant, atherogenic, and procoagulant effects.[8] The phenotypic effects of this polymorphism fully exploit when it occurs in the homozygous form. As a measure of C677T prevalence, allelic frequency was used, that is, the number of mutated alleles divided by twice the number of subjects studied.
To assess whether h-Hcy was possibly associated with the risk of developing IE, we compared IE patients (cases) with BDs (controls). In order to exclude that h-Hcy was associated to heart disease rather than IE, we compared IE patients with VHD patients. Subsequently, to verify the possible association between Hcy levels and a particular form of IE (NVE, PVE, CIEDE), subgroup analyses were carried out. Further, we evaluated whether Hcy levels affected IE vegetation size or the occurrence of embolic events. Finally, we looked at the possible effect of h-Hcy on the major outcomes of IE, including complications, need for surgery, and mortality.
2.3. Clinical procedures
Upon admission to our unit, patients signed their informed consent and underwent a detailed baseline clinical evaluation, including history, physical examination, chest x-ray and abdominal ultrasound scan. During the in-hospital stay, patients were carefully assessed for signs or symptoms suggestive of symptomatic embolic complications, such as sudden neurologic or visual dysfunction, abdominal pain, hematuria, chest discomfort, abrupt dyspnea, or limb pain.[16] No active search for silent embolic events was systematically done. Contrast-enhanced computed tomography scanning was performed to confirm the clinical suspicion of major or symptomatic cerebral, pulmonary, or intra-abdominal embolism.[17]
Also, 2 to 4 sets of blood cultures were obtained from each patient on the day of admission. Molecular microbiology on whole blood (Septifast) was also performed in cases with recent prior antimicrobial therapy, and valve/device cultures were always obtained in surgical patients. No routine serology for Coxiella and Bartonella or molecular biology on valve samples was performed.
All patients underwent trans-thoracic and, whenever needed and feasible, trans-esophageal echocardiography, measuring number, length (greatest diameter), and location of endocardial vegetations.
A standard panel of laboratory investigations was performed in both patients and controls by our Hospital Central Laboratory, including full blood count and measurement of prothrombin time, expressed as International Normalized Ratio (INR), activated partial thromboplastin time, plasma fibrinogen, and serum creatinine. In IE patients, C-reactive protein (CRP) serum levels were also assessed.
Patients were followed up until discharge from hospital or death. Patients discharged alive were invited to come back within 4 weeks at our outpatient IE clinic. All subjects, irrespective of whether or not returning to visit, were called by phone in order to obtain 1 year after discharge status.
2.4. Analytical procedures
Whole blood, serum, and plasma samples were obtained on the first day of admission from all patients included in the study and from VHD and BD controls. Samples were rapidly processed (centrifuged or refrigerated at 4 °C) and eventually stored in our laboratory at –80 °C. After centrifugation at 3500 rpm for 10 minutes, plasma was separated from EDTA blood and stored. Subsequently, plasma Hcy levels were measured on the single sample obtained on admission by means of a semi-automatic chemiluminescent microparticle immunoassay run on an Architect i2000SR platform (Abbott Diagnostics, Rome, Italy). By means of a rapid spin-column method (QIAquick PCR purification, Qiagen, Milan, Italy), whole blood was used to extract genomic DNA, which in turn was stored at –80 °C. DNA samples were then subjected to polymerase chain reaction assays (Hot Master Mix Taq, 5 Prime, Milan, Italy) with primers (Biofab Research, Milan, Italy) specific for the genetic regions of interest, as previously described.[17] To detect the C677T SNP in the MTHFR gene, PCR products were digested with restriction endonucleases (Biofab Research, Milan, Italy) and subsequently run on 2% agarose gel electrophoresis (Carlo Erba Reagenti, Milan, Italy).
2.5. Data analysis
Multiple IE patient data, including demographics, predisposing conditions and pre-existing illnesses, causative pathogens, medications taken, presenting signs/symptoms, echocardiographic findings, treatment strategies, disease complications and outcome, were recorded by means of a data collection form and entered in a computerized datasheet for subsequent analysis.
For the purpose of this study, the following variables were considered: age, sex; causative microorganism; site of infection; creatinine and coagulation parameters; vegetation size. One goal of the study was to evaluate whether h-Hcy was associated with major IE outcomes, including intracardiac complications of IE, outcome of hospitalization, and 1-year follow-up status after discharge.
Differences between groups in numerical variables were assessed through Mann–Whitney or Kruskal–Wallis tests for 2 or multiple independent samples, respectively. Categorical variables were compared with the Fisher's exact test. Odds ratios for the differences with 95% confidence intervals were calculated. Correlation between numerical variables was assessed by Spearman's coefficient. Logistic regression analysis of independent predictors of 1-year mortality was performed by block entering in the model all variables associated with this outcome on the univariate analysis (P < 0.1).
Genotype data were analyzed for deviation from the Hardy–Weinberg equilibrium by calculation of Pearson's chi-square.
Numerical data are presented as mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical data are presented as the number and the percent. Statistical analyses were carried out with the aid of the SPSS 20.0 software. The significance level was set at 5% and all tests were 2-tailed.
3. Results
Included in the analysis were 246 IE patients, showing either left heart or right heart infection (149 and 97 patients, respectively), and including 116 patients with native valve IE (NVE), 55 with prosthetic valve IE (PVE), and 75 with cardiac implantable electronic device (CIED)-related IE. As controls, we studied 2 groups: (a) 106 healthy blood donors (BD); (b) 258 VHD patients without IE, including 154 uncomplicated native and prosthetic VHD patients, 30 patients with heart valve disease undergoing cardiac surgical repair/replacement, and 74 carriers of a cardiac implantable electronic device (CIED). BD were significantly younger than IE patients; therefore, only a subgroup of the latter, comprising the youngest IE patients, was compared with BDs.
3.1. Homocystein levels in Infective Endocarditis and controls
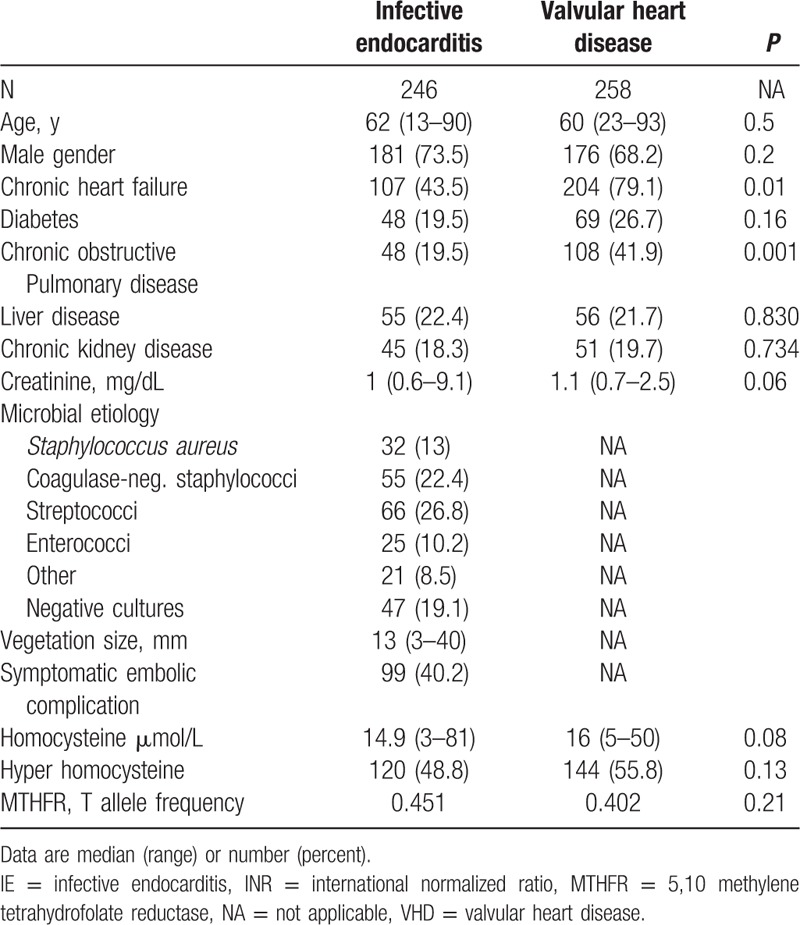
As shown in Table 1, IE patients showed higher levels of plasma Hcy compared to an age- and sex-matched group of healthy BDs. A condition of hyper-Hcy was more common among IE patients than their healthy counterparts (Table 1). Only a small subgroup of IE patients was compared to BD as the overall IE group had a much higher age (see below and Table 2). To assess whether h-Hcy was associated with heart disease rather than IE itself, we compared IE patients with a cohort of VHD patients. Characteristics of these 2 groups are comparatively shown in Table 2. Hcy levels were not significantly different between IE and VHD patients.
Table 1.
Hcy levels in IE patients compared to an age and sex-matched group of healthy BDs.
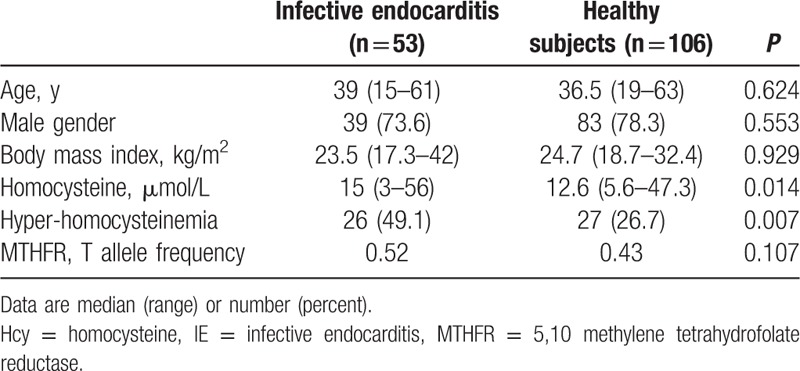
Table 2.
Clinical and biochemical features of IE and VHD patients.
3.2. Correlates of hyperhomocysteinemia in IE
In each of the 3 groups studied, TT homozygotes at position 677 of the MTHFR gene showed significantly higher Hcy levels (data not shown). The allelic frequency of this polymorphism was not significantly different when we compared IE patients and BD (Table 1). Similarly, there were no differences in allele frequency between IE and VHD patients (Table 2). Despite of this, h-Hcy was observed significantly more often in IE and VHD patients, suggesting the effect of factors other than the MTHFR genotype. We, therefore, studied clinical variables associated with h-Hcy. As shown in Table 3, IE patients with h-Hcy were older and had a higher prevalence of chronic kidney disease. In IE patients, Hcy levels were directly correlated to those of creatinine (r = 0.337; P = 0.0001) and age (r = 0.177; P = 0.006). Hcy did not correlate with C-reactive protein (r = –0.053; P = 0.443).
Table 3.
Clinical features of IE patients with or without hyperhomocysteinemia.
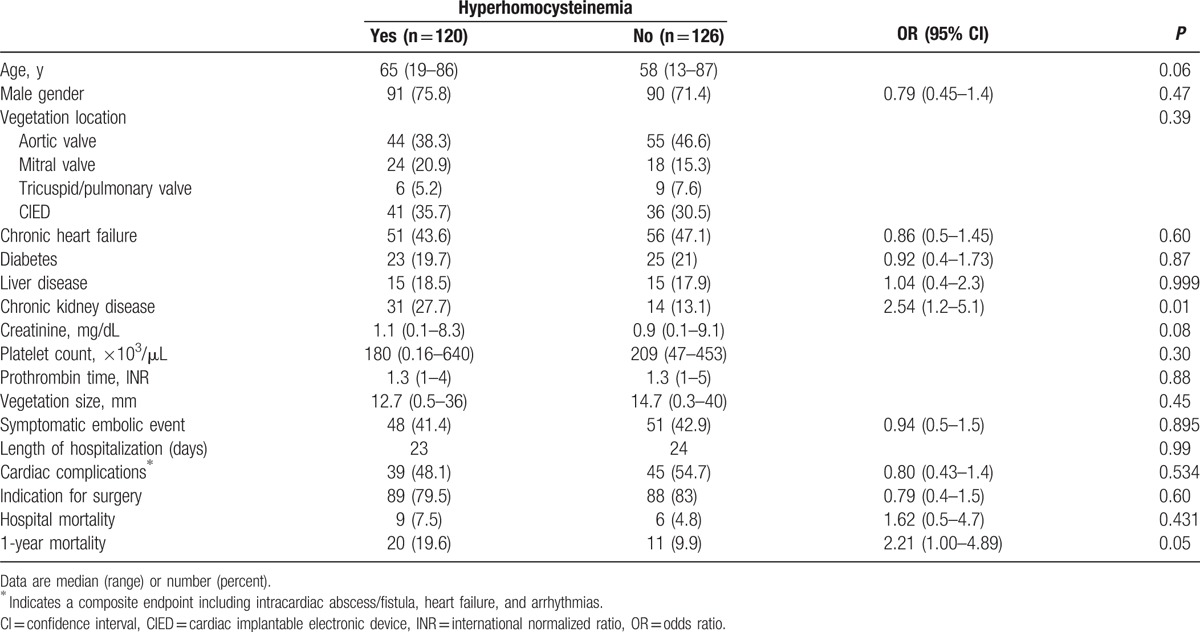
A trans-esophageal echocardiography was performed in 162 (66%) patients, including 58% of NVE, 78% of PVE, and 69% of CIED-IE cases. IE patients with h-Hcy had neither larger vegetations nor a higher rate of symptomatic embolic complications (Table 3). In particular, there were no differences in median Hcy values according to vegetation size (15.5 μmol/L for vegetation ≤10 mm and 16.0 μmol/L for vegetation >10 mm; P = 0.71). Likewise, there was no difference in the prevalence of patients with h-Hcy between small and large vegetation subgroups (53% vs 52.8%, respectively). Similarly, IE patients suffering an embolic complication did not have a higher prevalence of h-Hcy compared to those who did not (48.5% vs 50.0%; P = 0.89). In particular, median Hcy levels were 14.3 μmol/L in patients with and 15.2 μmol/L in those without symptomatic embolism (P = 0.75).
Hcy levels were not significantly higher in left-sided IE cases (14.7 vs 15.9 μmol/L in right-sided IE; P = 0.67). Likewise, no differences in Hcy levels were observed between patients with NVE, PVE, or CIED-related IE (data not shown). IE patients who were deemed clinically to have heart failure as a consequence of IE did not show higher Hcy levels (14.1 vs 15.3 μmol/L in those without de novo heart failure; P = 0.93).
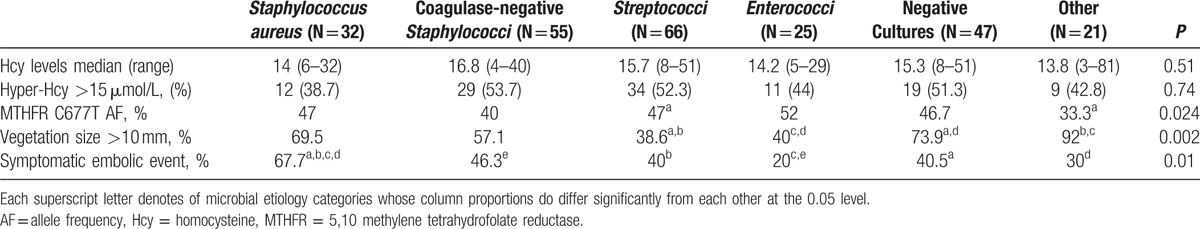
Table 4 presents data on Hcy levels, MTHFR polymorphism, vegetation size, and symptomatic embolic complications according to IE microbial etiology. No association was found between the causative pathogen and Hcy levels. In particular, there were no differences between cases due to Staphylococcus aureus and other etiologies. Significantly larger vegetations were observed among S aureus IE cases (Table 4). Embolic complications were also significantly associated with the microbial etiology of IE, with higher rates in cases due to S aureus and lower rates in enterococcal IE (Table 4).
Table 4.
Hcy levels, vegetation size, and rate of embolic complications in infective endocarditis patients grouped according to microbial etiology.
3.3. Association of hyper-homocysteinemia with major IE outcomes
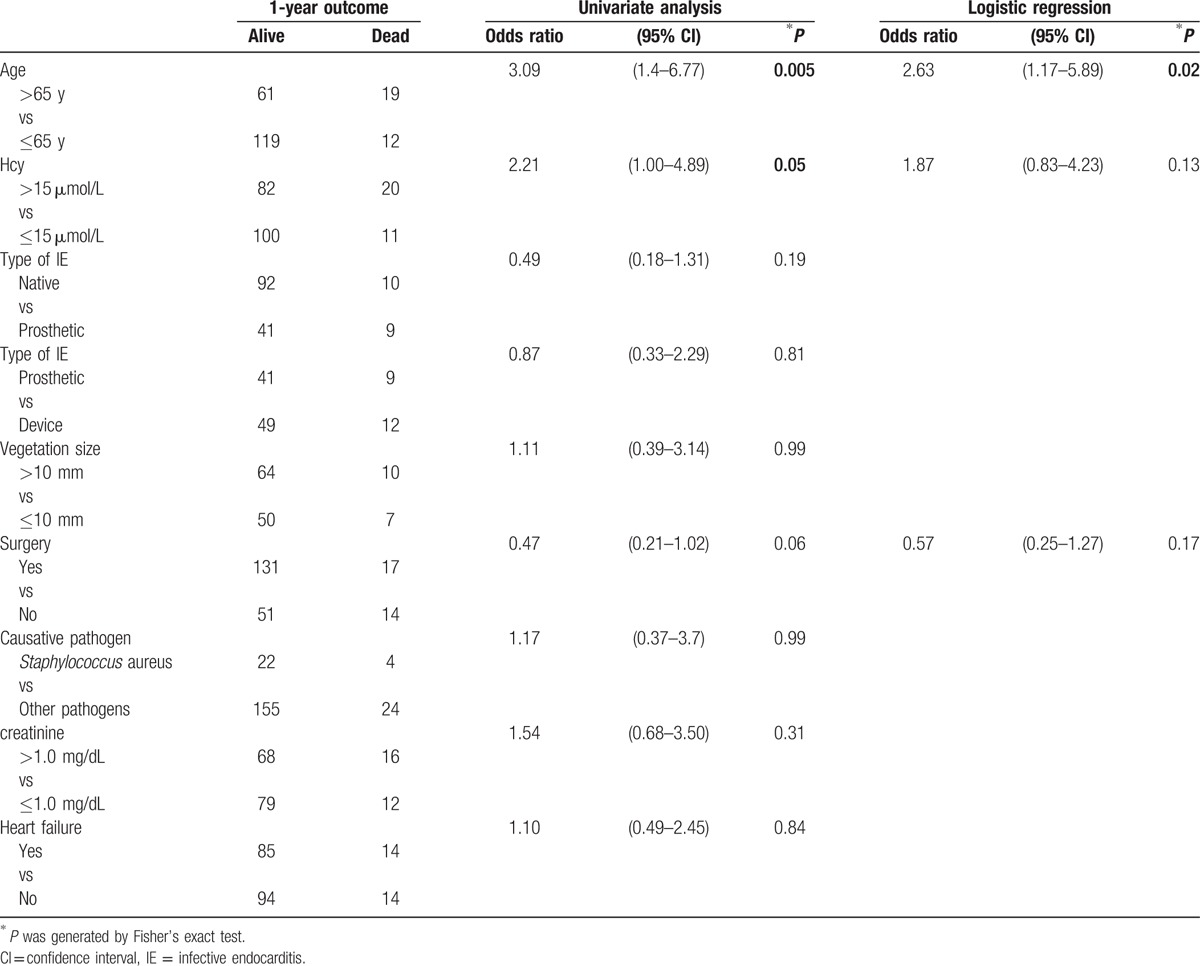
The major outcomes of IE according to the presence of h-Hcy are presented in Table 3. There were no significant differences in hospitalization length, rate of major complications, and need for cardiac surgery between patients grouped according to the presence of h-Hcy. Hospital mortality was not associated with h-Hcy (Table 3). In contrast, 1-year mortality was higher in IE patients with h-Hcy (19.6% vs 9.9%; OR 2.21 [95% CI 1.0–4.9]; P = 0.05). Median Hcy was higher in subjects not surviving at 1 year (17.3 [5–81] vs 14 [3–56] in those surviving; P = 0.09). In order to further assess the relationship between h-Hcy and 1-year mortality, we analyzed factors possibly associated with this outcome. As shown in Table 5, a significant association was found between 1-year mortality and patient age (23.7% in patients >65 years vs 9.1% in those ≤65 years; OR 3.1 [95% CI 1.4–6.77]; P = 0.005) as well as h-Hcy. At logistic regression analysis, age, but not h-Hcy, was an independent predictor of 1-year mortality (Table 5).
Table 5.
Multivariate analysis of 1-year mortality predictors in the 246 IE patients.
4. Discussion
The IE vegetation is made of bacteria embedded in a platelet-fibrin mesh.[3,18,19] We hypothesized that h-Hcy, by inducing a prothrombotic state, could favor IE vegetation formation, influencing incidence and clinical course of the disease. In this study, Hcy levels and the prevalence of h-Hcy were higher in IE compared with healthy BD. This finding pointed to a possible role of h-Hcy in the pathogenesis of IE. Previous clinical observations had indeed led us to hypothesize a potential role for h-Hcy with MTHFR C677T homozygous mutation in nonbacterial thrombotic endocarditis[20] and in pace-maker lead IE.[21] However, the equally higher levels observed in VHD as well as the correlation of Hcy with creatinine levels suggest h-Hcy could be a marker of cardiac dysfunction related to IE rather than a condition predisposing to IE. This interpretation is also consistent with the absence of differences in MTHFR C677T genotype across the 3 study groups. However, we lack data showing increased Hcy levels in IE with symptomatic heart failure.
H-hcy could not be a mere marker of cardiac dysfunction in IE. Interestingly, we observed a significant association of h-Hcy with 1-year mortality. Hcy levels also tended to be higher in IE patients dying in hospital. Further studies are needed to ascertain whether h-Hcy is solely a marker of risk or whether it contributes to a worse prognosis due to its potential atherogenic, proinflammatory, and prothrombotic effects.
At variance with what we expected, Hcy levels did not affect vegetation size or major embolic complications. This finding further suggests that embolic events in IE are the result of a complex interaction between host and microbial factors. An investigation of the risk factors for embolism in IE was, however, beyond the aims of the present study.
This work has some important limitations. We compared with age-matched healthy BD only a selected subgroup IE patients made of the youngest subjects, and we lacked a control group made of patients with bacteremia without IE. Only symptomatic embolic events were diagnosed and analyzed. The overall mortality which we observed was low, likely as we were less successful to obtain samples of blood for Hcy and genetics from the most severely ill patients or those undergoing emergency cardiac surgery. Despite of these lower rates of mortality, differences were observed in patient outcome at 1 year.
In conclusion, we screened a cohort of 246 IE patients for h-Hcy and its major genetic ground. Based on our current data, we are unable to support a role for this condition in the susceptibility to IE. However, IE patients have a high rate of h-Hcy and this is associated with late mortality, but not vegetation size or symptomatic embolic events. Further studies are surely warranted to better understand the role of h-Hcy and other inherited or acquired homeostasis defects in the pathogenesis and clinical course of IE.
Acknowledgments
For collaboration in the enrolment of control subjects, the authors are indebted to Dr. Bruno Zuccarelli, Head of the Transfusion Medicine Service, Monaldi Hospital.
For clinical assistance the authors thank Drs. Enrico Ragone, Rosina Albisinni, Giovanni Dialetto, Franco E. Covino, and Sabrina Manduca. For technical assistance, we thank Fabiana D’Amico, Francesco Crispi, Giovanni Fusco.
Footnotes
Abbreviations: BD = blood donors, CI = confidence interval, CIED = cardiac implantable electronic device, CIEDE = cardiac implantable electronic device endocarditis, CRP = C-reactive protein, Hcy = homocysteine, h-Hcy = hyper-homocysteinemia, IE = infective endocarditis, INR = international normalized ratio, IQR = interquartile range, MTHFR = 5,10 methylene tetrahydrofolate reductase, NVE = native valve IE, OR = odds ratio, PVE = prosthetic valve IE, SD = mean ± standard deviation, VHD = valvular heart disease.
Funding: This work was supported by the Department of Cardiothoracic Sciences of the University of Naples S.U.N., Napoli, Italy (grant Ricerca di Ateneo 2012–2013 to RU and EDM).
The study sponsor had no role in study design, collection, analysis, and interpretation of data, writing of the report or decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
No writing assistance was utilized in the production of this manuscript and there are no guest authors.
References
- 1.Hoen B, Duval X. Infective endocarditis. N Engl J Med 2013; 369:785. [DOI] [PubMed] [Google Scholar]
- 2.Werdan K, Dietz S, Löffler B, et al. Mechanisms of infective endocarditis: pathogen–host interaction and risk states. Nat Rev Cardiol 2014; 11:35–50. [DOI] [PubMed] [Google Scholar]
- 3.Que YA, Moreillon P. Infective endocarditis. Nat Rev Cardiol 2011; 8:322–336. [DOI] [PubMed] [Google Scholar]
- 4.Durante-Mangoni E, Molaro R, Iossa D. The role of hemostasis in infective endocarditis. Curr Infect Dis Rep 2014; 16:435–443. [DOI] [PubMed] [Google Scholar]
- 5.Durante-Mangoni E, Iossa D, Molaro R, et al. Prevalence and significance of two major inherited thrombophilias in infective endocarditis. Inter Emerg Med 2015; 10:587–594. [DOI] [PubMed] [Google Scholar]
- 6.Schouten M, Wiersinga WJ, Levi M, et al. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol 2008; 83:536–545. [DOI] [PubMed] [Google Scholar]
- 7.Verhamme P, Hoylaerts MF. Hemostasis and inflammation: two of a kind? Thromb J 2009; 7:15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldibany MM, Caprini JA. Hyperhomocysteine and thrombosis: an overview. Arch Pathol Lab Med 2007; 131:872–884. [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo M. Hyperhomocysteinemia, atherosclerosis and thrombosis. Thromb Haemost 1999; 81:165–176. [PubMed] [Google Scholar]
- 10.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA 2002; 288:2015–2022. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg RD, Aird WC. Vascular-bed-specific hemostasis and hypercoagulable states. N Engl J Med 1999; 340:1555–1564. [DOI] [PubMed] [Google Scholar]
- 12.Antoniades C, Antonopoulos AS, Tousoulis D, et al. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J 2009; 30:6–15. [DOI] [PubMed] [Google Scholar]
- 13.Malinow MR, Bostom AG, Krauss RM. Homocyst(e)ine, diet, and cardiovascular diseases: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation 1999; 99:178–182. [DOI] [PubMed] [Google Scholar]
- 14.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetra hydrofolate reductase. Nat Genet 1995; 10:111–113. [DOI] [PubMed] [Google Scholar]
- 15.Refsum H, Smith AD, Ueland PM, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 2004; 50:3–32. [DOI] [PubMed] [Google Scholar]
- 16.Durante Mangoni E, Adinolfi LE, Tripodi MF, et al. Risk factors for ‘major’ embolic events in hospitalized patients with infective endocarditis. Am Heart J 2003; 146:55–61. [DOI] [PubMed] [Google Scholar]
- 17.Inanir A, Yigit S, Tural S, et al. MTHFR gene C677T mutation and ACE gene I/D polymorphism in Turkish patients with osteoarthritis. Dis Markers 2013; 34:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreillon P, Que YA, Bayer AS. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect Dis Clin North Am 2002; 16:297–318. [DOI] [PubMed] [Google Scholar]
- 19.Widmer E, Que YA, Entenza JM, et al. New concepts in the pathophysiology of infective endocarditis. Curr Infect Dis Rep 2006; 8:271–279. [DOI] [PubMed] [Google Scholar]
- 20.Durante-Mangoni E, Iossa D, Nappi F, et al. Inherited hyper-homocysteinemia as a cause of nonbacterial thrombotic endocarditis. J Heart Valve Dis 2011; 20:232–233. [PubMed] [Google Scholar]
- 21.Durante-Mangoni E, Nappi G. Giant endocarditis vegetation on a pace-maker lead. Intern Emerg Med 2010; 5:251–252. [DOI] [PubMed] [Google Scholar]


