Abstract
The purpose of this study was to preoperatively evaluate the value of aortic arch lesions by multidetector computed tomography (MDCT) angiography in type A aortic dissection (AD).
From January 2013 to December 2015, we enrolled 42 patients with type A AD who underwent MDCT angiography in our hospital. The institutional database of patients was retrospectively reviewed to identify MDCT angiography examinations for type A AD. Surgical corrections were conducted in all patients to confirm diagnostic accuracy.
In this study, the diagnostic accuracy of MDCT angiography was 100% in all 42 patients. The intimal tear site locations that were identified in patients included the ascending aorta (n = 25), aortic arch (n = 12), and all other sites (n = 5). Compared with the control group, there were significant differences in the aortic arch anatomy among the cases. Regarding the distance between the left common carotid and left subclavian arteries, compared with the control group, most cases with type A AD had a significant variation.
MDCT angiography plays an important role in detecting aortic arch lesions of type A AD, especially in determining the location of the intimal entry site and change of branch blood vessels. Surgeons can formulate an appropriate operating plan, according to the preoperative MDCT diagnosis information.
Keywords: aortic arch lesions, diagnosis, multidetector computed tomography angiography, type A aortic dissection
1. Introduction
Acute type A aortic dissection (AD) is a life-threatening disorder that occurs in approximately 1 in 10,000 emergency department patients.[1,2] According to reports in the literature, the mortality of AD is 40% on presentation, with a 1-year mortality of 90%,[3] and aortic rupture was identified as the common cause of death in approximately one-third of patients admitted to the hospital.[4] In recent years, the incidence of AD has been rising yearly, and the overall outcome is determined by the type and extent of dissection, the presence of associated complications, and the timing of medical intervention. Thus, early and accurate diagnosis with a noninvasive modality is of great importance for the optimal treatment and prognosis of patients with type A AD. The purpose of this study was to preoperatively evaluate the value of aortic arch lesions by multidetector computed tomography (MDCT) angiography in type A AD in a Chinese cardiac center.
2. Materials and methods
Approval was obtained from the Institutional Review Board of the University of Fujian Medical University, China for a retrospective review of patients with type A AD who received MDCT angiography. In addition, informed written consent was obtained from the patient or their relatives.
We reviewed the charts of 42 consecutive patients with type A AD who were admitted to our hospital between January 2013 and December 2015 and were subjected to MDCT angiography. All patients suffered from a long history of hypertension, and none received regular antihypertensive medication. During admission, patients presented with a classic history of acute-onset tearing chest pain radiating to the back. Some complained of a variety of other symptoms, which included syncope, hypotension, pericardium and pleural effusion, cardiac tamponade, oliguria and anuria, abdominal pain, and so on. Patients’ standard demographic information was also collected (Table 1). Among the 42 patients, there were 11 females and 31 males included in this study. The patients were aged 45 to 68 years (mean ± standard deviation, 52.1 ± 5.8 years). Their weights ranged from 48 to 85 kg (62.5 ± 6.8 kg). None of the patients included in the study group had a diagnosis of Marfan syndrome or other connective tissue diseases. Routine examinations were performed, which included standard lead electrocardiogram, transthoracic echocardiography (TTE), and routine blood and biochemical tests. Out of all the patients, 40 had mild–moderate aortic valve regurgitation and 2 patients had severe aortic valve regurgitation. The initial diagnosis was obtained with MDCT angiography, and the diagnosis of AD type was made using the Stanford (types A and B) classification. All patients were managed conservatively with antihypertensive and analgesic therapy, according to the medical standards of care.
Table 1.
Clinical data of patients with type A AD undergoing MDCT angiography.
For all patients in this study, the baseline MDCT angiography study was available for review. MDCT angiography examinations were performed with a DSCT scanner (Somatom Definition; Siemens, Germany). Patients were examined while supine, and images were taken extending from the base of the neck to the thigh. We used retrospective Electrocardiogram (ECG)-gated cardiac computed tomogarphy (CT) scan. A low-dose protocol was used to reduce the radiation dose as follows: slice thickness 1 mm; pitch 1.75, depending on heart rate; tube voltage 120 kVp; and tube current 350 mA. In all patients, a dose of 90 mL iodinated contrast medium and iopromide 350 mgI/mL (Schering Ultravist, Iopromide, Berlin, Germany) was injected. The rate of injection was 4.5 mL/s, followed by 30 mL of saline at the same rate. The scanning delay was determined with a bolus-tracking technique. All images were transferred to an external workstation (Leonardo; Siemens Medical Solutions, Forchheim, Germany) using the volume rendering technique. Established radiologists with 10 years of radiology experience evaluated the data sets for each of the cases.
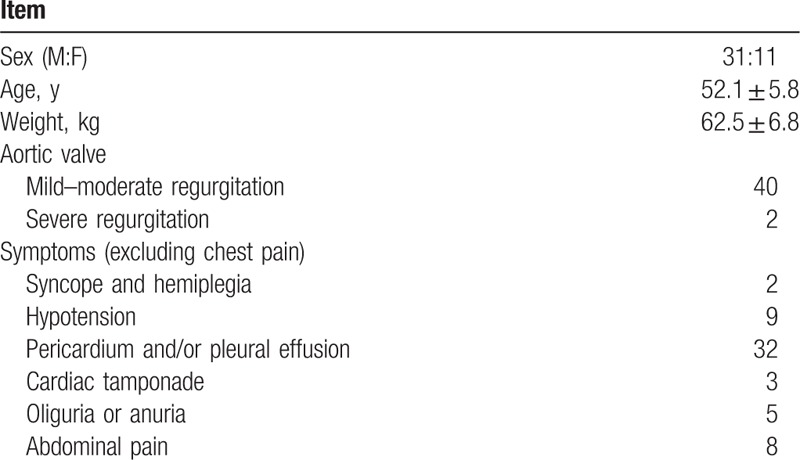
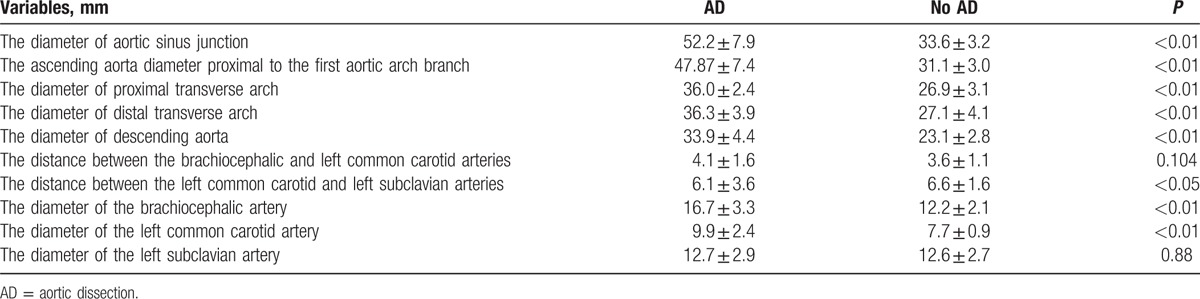
The location of the intimal entry site and the change of the branch blood vessels were detected by MDCT angiography. Some of the parameters were measured, which included the diameter of the aortic sinus junction, ascending aorta diameter proximal to the first aortic arch branch, proximal transverse arch diameter (between the brachiocephalic and left common carotid arteries), distal transverse arch diameter (between the left common carotid and the left subclavian arteries), diameter of descending aorta, distance between the brachiocephalic and left common carotid arteries, distance between the left common carotid and left subclavian arteries, diameter of the brachiocephalic artery, diameter of the left common carotid artery, and diameter of the left subclavian artery. Measurements were recorded according to published guidelines[5,6] (Figs. 1–5).
Figure 1.
Multidetector computed tomography angiography showed the parameters that were measured, which included the diameter of aortic sinus junction, ascending aorta diameter proximal to the first aortic arch branch, proximal transverse arch diameter (between the brachiocephalic and left common carotid arteries), distal transverse arch diameter (between the left common carotid and the left subclavian arteries), the diameter of descending aorta, the distance between the brachiocephalic and left common carotid arteries, the distance between the left common carotid and left subclavian arteries, the diameter of the brachiocephalic artery, the diameter of the left common carotid artery, and the diameter of the left subclavian artery.
Figure 5.
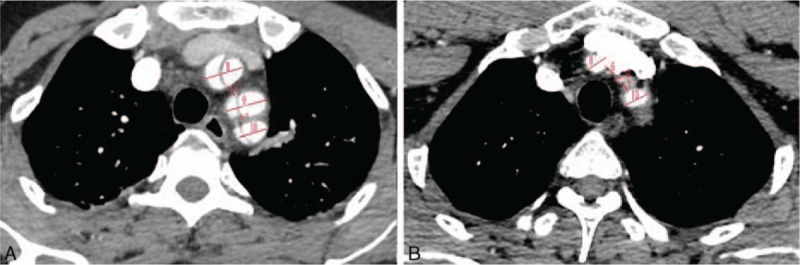
Multidetector computed tomography angiography showed the diameter of descending aorta, the distance between the brachiocephalic and left common carotid arteries, the distance between the left common carotid and left subclavian arteries, the diameter of the brachiocephalic artery, the diameter of the left common carotid artery, and the diameter of the left subclavian artery. (A) The type A aortic dissection group and (B) the control group.
Figure 2.
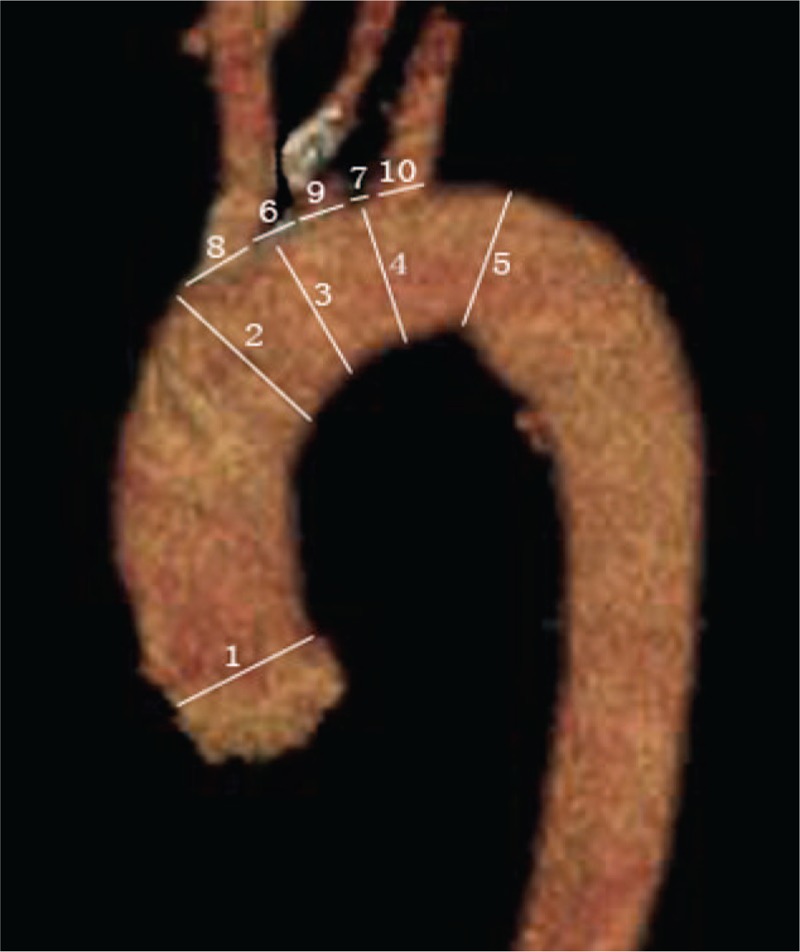
Multidetector computed tomography angiography showed the diameter of aortic sinus junction. (A) The type A aortic dissection group and (B) the control group.
Figure 3.
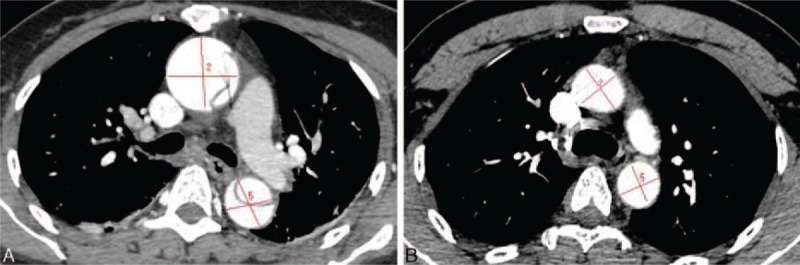
Multidetector computed tomography angiography showed the ascending aorta diameter proximal to the first aortic arch branch and the diameter of descending aorta. (A) The type A aortic dissection group and (B) the control group.
Figure 4.
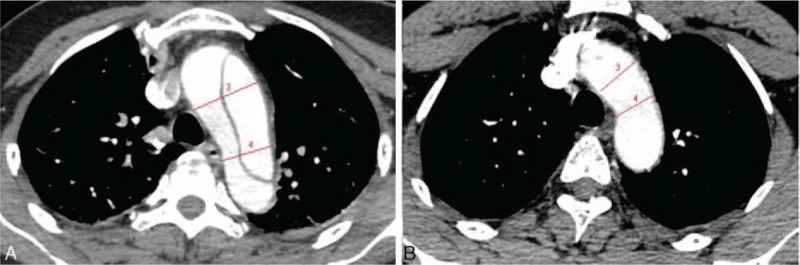
Multidetector computed tomography angiography showed the proximal transverse arch diameter and the distal transverse arch diameter. (A) The type A aortic dissection group and (B) the control group.
2.1. Statistical analysis
Continuous data are presented as the mean ± standard deviation and range. Clinical parameters were compared with the independent samples t test. Nominal variables were compared using Fisher exact test. A P value <0.05 was defined as significant.
3. Results
In this study, 42 patients were confirmed with the diagnosis of type A AD via the surgical findings, and all patients received aortic valvuloplasty (1 case for valve replacement), ascending aorta replacement, and open placement of a triple-branched stent graft for aortic arch reconstruction. The clinical results were satisfactory.
The diagnostic accuracy of MDCT angiography was 100%. In the intraoperative findings, the intimal tear site locations that were identified in all 42 patients included the ascending aorta (n = 25) and the aortic arch (n = 12); the rest (n = 5) were retrograde dissections from the tear of the descending aorta.
A comparison between the type A AD group and control group (patients with hypertension) is presented in Table 2. There were no significant differences in demographic characteristics. Compared with the control group, the diameter of the aortic sinus junction, ascending aorta, proximal transverse arch, distal transverse arch, descending aorta, brachiocephalic artery, and left common carotid artery were significantly longer in the type A AD group (P < 0.05). Regarding the distance between the left common carotid and left subclavian arteries, compared to the control group, most cases with type A AD had a significant variation.
Table 2.
Comparison of clinical data of the aortic arch between those patients with and without AD.
None of the patients presented with vascular complications or drug side effects when MDCT angiography was performed. The average time taken to finish MDCT angiography was 3.5 to 7.5 minutes (4.5 ± 1.4 minutes).
4. Discussion
Type A AD is the most common acute emergency condition of the aorta and often results in catastrophic results for patients, which depends on the extent of dissection and presence of associated complications. The most common risk factor for the development of an AD is hypertension, which occurs in 60% to 90% of cases.[7,8] Type A AD involves the ascending thoracic aorta and may extend into the descending aorta, and the intimal tear site is usually located in the ascending aorta and aortic arch. Some reports identified aortic rupture as the cause of death in approximately one-third of patients admitted to the hospital.[9–11] Complications of type A AD include aortic valve regurgitation, aortic rupture, tamponade and compromise of the arch branches or coronary arteries leading to myocardial infarction, cerebral sequelae, aortic branch involvement, and peripheral circulation involvement. Thus, type A AD typically requires urgent surgical treatment, and early and accurate diagnosis with a noninvasive modality is required for optimal management and improvement in the prognosis of these patients.[12]
There are a variety of inspection methods for the diagnosis of type A AD, including TTE, CT angiography, and magnetic resonance (MR) angiography. TTE is an available, noninvasive, relatively low-cost, and easy processing examinational modality that can be repeated as frequently as necessary and has been used in the preliminary diagnostic approach to type A AD. It has a reported sensitivity of 59% to 83% and a specificity of 63% to 93% for the diagnosis of AD.[13,14] The main purpose of TTE is to determine aortic valves and root lesions, which helps surgeons to develop an aortic valve medical plan. In our study, according to the TTE results that were confirmed during the operation, most of the patients with aortic valve regurgitation required aortic valvuloplasty, and few patients required valve replacement. MR angiography is accurate and noninvasive, has excellent spatial and contrast resolution, and allows multivascular imaging phases with fast postprocessing.[15–17] Although MR angiography has several advantages, including a lack of radiation and greater vessel coverage at high resolution, it may not be suitable for unstable patients due to the longer acquisition time and monitoring difficulties in an emergency situation, as well as in those patients with implanted electronic devices. Recently, MDCT angiography was shown to be less invasive and time consuming and is more popularly performed in clinical practice for the diagnosis of type A AD. Its contrast-enhanced scanning can rapidly and accurately localize the intimal entry site and help assess branch vessel lesions as well as the relationship of the branch vessels, which may aid surgeons in deciding on a treatment plan with either root or aortic valve replacement, as well as intravascular stent placement.
There are several advantages in using MDCT angiography for the diagnosis of type A AD, and this modality can show a high accuracy rate in assessing the severity of dissection using 3-dimensional images, which can be processed and analyzed by using several postreconstruction techniques. The highest imaging resolution is an important reason that MDCT angiography may be helpful in the preoperative imaging study of type A AD. Some information can be easily identified, including the sites of intimal tears, intimomedial flap, false and true lumen morphology, coronary and aortic arch branches, and scope of the dissection. AMDCT scan can be performed in a few seconds to complete inspection, which can increase the security of the inspection process. Meanwhile, MDCT angiography yielded higher sensitivity and specificity and was superior to the other 2 modalities in ruling out type A AD.[18,19] Some reports indicated that MDCT angiography has a sensitivity and specificity of close to 100% for diagnosis of type A AD[20–22]; our results also confirmed these views.
Preoperative identification of the locations and characteristics of intimal tears is one of the most important steps for surgeons to help procedural planning. To our knowledge, many studies have focused on CT angiography to determine the location and characteristics of intimal tears.[19,23,24] Kim et al reported their study on the validation of the accuracy of CT in predicting intimal tear locations. They concluded that sensitivity was higher for ascending aorta tears and that specificity was higher for arch tears.[25] In our study, most of the intimal tears were located in the ascending aorta and aortic arch. The ascending aorta replacement is the optimal surgical strategy. The anastomotic position of the artificial vessel depended on the location of the intimal tears and the scope of the true and false lumen.[26,27] For patients with intimal tears located in the aortic arch, although the treatment is still controversial, we still recommend that total arch replacement can result in better long-term prognosis.[28,29]
Total aortic arch reconstruction for type A AD with anastomosis of the brachiocephalic vessel to a Dacron tube graft or 4-branched prosthetic graft replacement of the aortic arch are popular surgical procedures.[30,31] These methods remain controversial because they are very complex and highly invasive, which makes the risk of these procedure very high.[32] In recent years, Chen et al[33] developed an open triple-branched stent graft placement technique for type A AD, in which total arch repair could be simply completed by inserting a triple-branched stent graft into the proximal descending aorta, arch, and 3 arch vessels through aortic incision. Their results showed that their new technique could reduce the risk and technical difficulty of total arch repair. In addition, they found that this new technique could not be applied in most patients because the diameters of the native arch vessels and distances between the 2 neighboring arch vessels did not always match the available sizes of the triple-branched stent grafts. They also modified the stent graft to several new generations for a good match with different diameters of the arch and branch vessels and achieved good clinical results[34–37] (Fig. 6). Given the many advantages, their technology may have good prospects. From their new report,[37] they obtained encouraging short-term results (a nearly 97.6% operation success rate, relatively short operation time, and very few complications). However, it is important to preoperatively evaluate the anatomic lesions of aortic arch and branch vessels, which was the other main purpose of our study.
Figure 6.
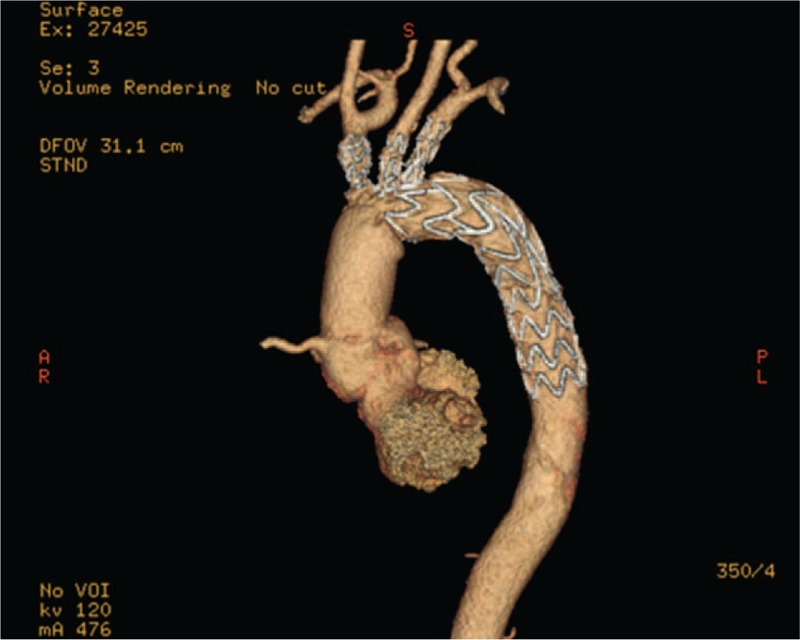
Postoperative computed tomographic scans showed that the triple-branched vascular stent graft without kinking, the stents were well stretched in the aortic arch, and the branching artery, with satisfactory adherence to the vessel wall.
From our study, we found that mutations in the size and position of the ascending aorta, aortic arch, and branch vessels during AD had occurred. In our treatment strategy, the pathological ascending aorta was replaced by a Dacron artificial tube graft, and the true lumen of the descending aorta, aortic arch, and brachiocephalic vessels were replaced with a triple-branched stent graft. The diameters of the arch and descending aorta were measured by preoperative MDCT angiography, which was then used to select the stent models. From our data, there were significant differences in the position change of the arch branch vessels, which was a challenge, intraoperatively, using the integration stent. Compared with normal data, there were significant differences in the distance of the 3 branch vessels in patients with type A AD. It was important for intraoperative selection and the installation of stents to preoperatively accurately measure these data. The use of the modified triple-branched stent graft by Dr Chen could provide a good match with the different diameters of the native arch vessels and the various distances between the 2 neighboring arch vessels in type A AD status.
Like any retrospective study, ours included bias associated with data collection and incomplete data for some patients. Although the small number of patients in the study precluded reaching significance for all of the study end-points, important differences were demonstrated. Much larger numbers of patients must be evaluated to establish the results of the preoperative evaluation value of aortic arch lesions by MDCT angiography. This study was limited to 1 institution, and other institutions may obtain different results.
In conclusion, MDCT angiography plays an important role in the preoperative evaluation value of aortic arch lesions and guides the development of reasonable surgical plans in the clinical setting. It is necessary to preoperatively evaluate the structures of the aortic arch and branching vessels, especially for patients who are ready for an intraoperative triple-branched stent graft.
Acknowledgments
We wish to extend our gratitude to Xin-ming Huang and Jian-hua Chen. They helped us to deal with a large number of images.
Footnotes
Abbreviations: AD = aortic dissection, MDCT = multidetector computed tomography.
Author contributions: FH, QC, and Q-qL designed the study, collected the clinical data, performed the statistical analysis, participated in the operation, and drafted the manuscript. W-hH, HW, W-cL, and Q-qL participated in the operation and revised the article. All authors read and approved the final manuscript.
FH and QC contributed equally to the article and share first authorship.
The authors report no conflicts of interest.
References
- 1.Strayer RJ, Shearer PL, Hermann LK. Screening, evaluation, and early management of acute aortic dissection in the ED. Curr Cardiol Rev 2012; 8:152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua M, Ibrahim I, Neo X, et al. Acute aortic dissection in the ED: risk factors and predictors for missed diagnosis. Am J Emerg Med 2012; 30:1622–1626. [DOI] [PubMed] [Google Scholar]
- 3.Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol 2007; 99:852–856. [DOI] [PubMed] [Google Scholar]
- 4.Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type a aortic dissection. Circulation 2002; 105:200–206. [DOI] [PubMed] [Google Scholar]
- 5.Al Akhfash AA, Almesned AA, Al Harbi BF, et al. Two-dimensional echocardiographic predictors of coarctation of the aorta. Cardiol Young 2015; 25:87–94. [DOI] [PubMed] [Google Scholar]
- 6.Lai WW, Geva T, Shirali GS, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 2006; 19:1413–1430. [DOI] [PubMed] [Google Scholar]
- 7.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000; 283:897–903. [DOI] [PubMed] [Google Scholar]
- 8.Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J Am Coll Cardiol 2015; 66:350–358. [DOI] [PubMed] [Google Scholar]
- 9.Emmett M. Predicting death in patients with acute type a aortic dissection. Circulation 2002; 106:e224. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type a aortic dissection. Circulation 2002; 105:200–206. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Jiang Y, Gao C, Wang A. Risk factors for hospital death in patients with acute aortic dissection. Heart Lung Circ 2015; 24:348–353. [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto S, Taniguchi N, Nakajima S, et al. Diagnostic value of nonenhanced multidetector computed tomography for ruling out acute aortic dissection inpatients presenting with chest or back pain. Int J Cardiol 2013; 168:734–738. [DOI] [PubMed] [Google Scholar]
- 13.Meredith EL, Masani ND. Echocardiography in the emergency assessment of acute aortic syndromes. Eur J Echocardiogr 2009; 10:i31–i39. [DOI] [PubMed] [Google Scholar]
- 14.Jánosi RA, Buck T, Erbel R, et al. Role of echocardiography in the diagnosis of acute aortic syndrome. Minerva Cardioangiol 2010; 58:409–420. [PubMed] [Google Scholar]
- 15.Willmann JK, Wildermuth S, Pfammatter T, et al. Aortoiliac and renal arteries: prospective intraindividual comparison of contrast-enhanced three-dimensional MR angiography and multi-detector row CT angiography. Radiology 2003; 226:798–811. [DOI] [PubMed] [Google Scholar]
- 16.García A, Ferreirós J, Santamaría M, et al. MR angiographic evaluation of complications in surgically treated type A aortic dissection. Radiographics 2006; 26:981–992. [DOI] [PubMed] [Google Scholar]
- 17.Halefoğlu AM. Emergency diagnosis of acute aortic dissection using magnetic resonance imaging. Ulus Travma Acil Cerrahi Derg 2007; 13:106–114. [PubMed] [Google Scholar]
- 18.McMahon MA, Squirrell CA. Multidetector CT of aortic dissection: a pictorial review. Radiographics 2010; 30:445–460. [DOI] [PubMed] [Google Scholar]
- 19.Weiss G, Wolner I, Folkmann S, et al. The location of the primary entry tear in acute type B aortic dissection affects early outcome. Eur J Cardiothorac Surg 2012; 42:571–576. [DOI] [PubMed] [Google Scholar]
- 20.Manghat NE, Morgan-Hughes GJ, Roobottom CA. Multi-detector row computed tomography: imaging in acute aortic syndrome. Clin Radiol 2005; 60:1256–1267. [DOI] [PubMed] [Google Scholar]
- 21.Salvolini L, Renda P, Fiore D, et al. Acute aortic syndromes: role of multi-detector row CT. Eur J Radiol 2008; 65:350–358. [DOI] [PubMed] [Google Scholar]
- 22.Yoo SM, Lee HY, White CS. MDCT evaluation of acute aortic syndrome. Radiol Clin North Am 2010; 48:67–83. [DOI] [PubMed] [Google Scholar]
- 23.Quint LE, Platt JF, Sonnad SS, et al. Aortic intimal tears: detection with spiral computed tomography. J Endovasc Ther 2003; 10:505–510. [DOI] [PubMed] [Google Scholar]
- 24.Menke J. Aortic dissection with sheared-off intimal flap. Eur J Cardiothorac Surg 2011; 40:1544. [DOI] [PubMed] [Google Scholar]
- 25.Kim JS, Park KH, Lim C, et al. Prediction of intimal tear site by computed tomography in acute aortic dissection type A. Korean Circ J 2016; 46:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park KH, Sung K, Kim K, et al. Ascending aorta replacement and local repair of tear site in type A aortic dissection with arch tear. Ann Thorac Surg 2003; 75:1785–1790. [DOI] [PubMed] [Google Scholar]
- 27.Monsefi N, Miskovic A, Moritz A, et al. Long-term results of the David Procedure in patients with acute type A aortic dissection. Int J Surg 2015; 22:99–104. [DOI] [PubMed] [Google Scholar]
- 28.Unosawa S, Hata M, Niino T, et al. Prognosis of patients undergoing emergency surgery for type A acute aortic dissection without exclusion of the intimal tear. J Thorac Cardiovasc Surg 2013; 146:67–71. [DOI] [PubMed] [Google Scholar]
- 29.Mori Y, Hirose H, Takagi H, et al. Aortic arch repair for Stanford type A aortic dissection with distal anastomosis to the proximal level of the distal aortic arch. J Thorac Cardiovasc Surg 2003; 126:415–419. [DOI] [PubMed] [Google Scholar]
- 30.Rylski B, Milewski RK, Bavaria JE, et al. Long-term results of aggressive hemiarch replacement in 534 patients with type A aortic dissection. J Thorac Cardiovasc Surg 2014; 148:2981–2985. [DOI] [PubMed] [Google Scholar]
- 31.Sun LZ, Qi RD, Chang Q, et al. Surgery for acute type A dissection using total arch replacement combined with stented elephant trunk implantation: experience with 107 patients. J Thorac Cardiovasc Surg 2009; 138:1358–1362. [DOI] [PubMed] [Google Scholar]
- 32.Dougenis D. Repair of acute type A aortic dissection: moving towards a more aggressive approach but keeping the old gold standards. Eur J Cardiothorac Surg 2016; 49:131–133. [DOI] [PubMed] [Google Scholar]
- 33.Chen LW, Dai XF, Lu L, et al. Extensive primary repair of the thoracic aorta in acute type A aortic dissection by means of ascending aorta replacement combined with open placement of triple-branched stent graft: early results. Circulation 2010; 122:1373–1378. [DOI] [PubMed] [Google Scholar]
- 34.Chen LW, Wu XJ, Lu L, et al. Total arch repair for acute type A aortic dissection with 2 modified techniques: open single-branched stent graft placement and reinforcement of the dissected arch vessel stump with stent graft. Circulation 2011; 123:2536–2541. [DOI] [PubMed] [Google Scholar]
- 35.Chen LW, Wu XJ, Dai XF, et al. Total arch repair for acute type A aortic dissection with open placement of a modified triple-branched stent graft and the arch open technique. J Cardiothorac Surg 2014; 9:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen LW, Wu XJ, Dai XF, et al. A self-adaptive triple-branched stent graft for arch repair during open type A dissection surgery. J Thorac Cardiovasc Surg 2015; 149:1278–1283. [DOI] [PubMed] [Google Scholar]
- 37.Chen LW, Wu XJ, Dai XF, et al. Repair of acute type A aortic dissection with ascending aorta replacement combined with open fenestrated stent graft placement. Ann Thorac Surg 2016; 101:644–649. [DOI] [PubMed] [Google Scholar]


