Abstract
In our series of patients with primary aldosteronism, we compared diagnostic concordance and clinical outcomes after adrenalectomy between adrenal venous sampling (AVS) and computed tomography (CT) imaging.
Our retrospective analysis included 886 patients with primary aldosteronism diagnosed in our hospital between 2005 and 2014. Of them, 269 patients with CT unilateral adrenal disease were included in the analysis on the diagnostic concordance and 126 patients with follow-up data in the analysis on clinical outcomes after adrenalectomy. Hypertension was considered cured if systolic/diastolic blood pressure (BP) was controlled (<140/90 mm Hg) without medication and improved if BP was controlled with a reduced number of antihypertensive drugs.
In 269 patients with CT unilateral adrenal disease, the overall concordance rate between AVS and CT was 50.5% for lateralization on the same side. The concordance rate decreased with increasing age, with highest rate of 61% in patients aged <30 years (n = 16). In 126 patients with follow-up data after adrenalectomy, the AVS- (n = 96) and CT-guided patients (n = 30) had similar characteristics before adrenalectomy. After andrenalectomy, the AVS-guided patients had a significantly higher serum potassium concentration (4.3 ± 0.3 vs 4.0 ± 0.5 mmol/L, P = 0.04) and rate of cured and improved hypertension (98% vs 87%, P = 0.03). The AVS-guided patients (n = 50) had slightly higher cured rate than the CT-guided patients (n = 11) in those older than 50 years (26.0% vs 18.2%, P = 0.72). The age below which the cured rate in the CT-guided patients was 100% was 30 years.
AVS guidance had moderate concordance with CT and slightly improved clinical outcomes after adrenalectomy. The age below which CT unilateralization achieved 100% cured rate was approximately 30 years.
Keywords: adrenal venous sampling, adrenalectomy, age, blood pressure, computed tomography, primary aldosteronism
1. Introduction
With the increasing use of aldosterone-to-renin ratio and computed tomography (CT) imaging for screening, the detection of primary aldosteronism has been substantially improved. According to recent reports, the prevalence of primary aldosteronism was as high as 11.2% and 17% to 23% in patients with newly diagnosed[1] or resistant hypertension,[2–8] respectively. In patients with primary aldosteronism, 50% to 70% do not show evidence of a unilateral adenoma or lateralized aldosterone hypersecretion and classified as bilateral adrenal hyperplasia and receive medical treatment. However, 30% to 50% of patients do have unilateral adrenal disease, including aldosterone-producing adenoma and unilateral adrenal hyperplasia and require adrenalectomy.[9] After surgical removal of the gland or the tumor, hypertension might be cured without medication in 29.5%[10] to 31.5%[11] of patients, and blood pressure (BP) control might be improved in an additional 42.3%[11] to 53.0%[10] of patients.
CT imaging is sensitive in the differentiation of adrenal gland lesions, but inaccurate in the determination of functional disorders.[12,13] Current guidance documents therefore recommend adrenal venous sampling (AVS) for the diagnosis of unilateral adrenal disease for surgery except those patients aged less than 40 years, with a typical profile of primary aldosteronism and a clear unilateral adenoma and a normal contralateral adrenal gland on CT imaging.[14] Based on complete concordance (100%) between AVS and adrenal imaging, Lim et al[15] recently proposed that adrenal imaging performed well, and therefore AVS was not needed in patients younger than 35 years. This age-related discrimination on the necessity for AVS was mainly based on the fact that in younger subjects the prevalence of nonfunctional incidentaloma was low[16] and the concordance between CT imaging and AVS was high.[17] There is some but not much evidence from prospective outcome studies on adrenalectomy.[1,18] We therefore investigated our series of patients with primary aldosteronism and compared the diagnostic concordance and clinical outcomes after adrenalectomy between adrenal CT imaging alone and the combination of adrenal CT imaging with AVS.
2. Methods
2.1. Study designs and population
This retrospective analysis was performed in our series of patients who were diagnosed with primary aldosteronism between May 2005 and September 2014 at the Department of Hypertension, Ruijin Hospital, Shanghai, China. The ethics committee of Ruijin Hospital approved the study protocol. All patients gave written informed consent.
The diagnosis of primary aldosteronism was based on an aldosterone/renin ratio of >240 (ng/dL)/(ng/mL/h) with 2 independent samples and confirmed by either a fludrocortisone test or a saline infusion test. Before and during the work-up, patients were advised to be on a diet of regular salt intake and withdraw mineralocorticoid receptor antagonists for at least 6 weeks; nonpotassium-sparing diuretics for 4 weeks; and β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II type 1 receptor blockers for 2 weeks. Nondihydropyridine calcium blockers and/or α1-blockers were prescribed for BP control, as necessary. Hypokalemia was corrected by oral potassium chloride supplementation.
2.2. AVS and CT procedures
All patients first underwent adrenal CT scan (1.25–3.75 mm/slice) for subtyping. The case was considered unilateral if a unilateral radiolucent nodule (<10 Hounsfield units on noncontrast) was detected with a diameter of at least 5 mm and with the remaining ipsilateral and contralateral glands being normal. Patients underwent AVS if they were considered candidates for adrenalectomy according to the clinical manifestations of primary aldosteronism with no contraindication for surgery and were willing to receive the operation. AVS was carried out without cosyntropine stimulation and with subsequent catheterization. A selectivity index (defined as the ratio of adrenal venous plasma cortisol concentration to peripheral plasma cortisol concentration) of ≥3 was considered to indicate correct catheterization. A lateralization index (defined as the ratio of cortisol-corrected aldosterone from dominant side to nondominant side) of ≥2 was considered to indicate lateralization.
2.3. Follow-up after adrenalectomy
Adrenalectomy was carried out laparoscopically. Operated patients were followed up either through clinic visits or by telephone interview. The data of the last visit were used for analysis. Hypertension was considered cured if systolic/diastolic BP was <140/90 mm Hg without medication, and improved if BP was <140/90 mm Hg with a reduced number of antihypertensive drugs. Treatment was considered failed if BP was ≥140/90 mm Hg with an increase or no change in the number of preoperative antihypertensive drugs.
2.4. Statistical analysis
Statistical analysis was performed using SPSS Statistics 19.0 (Chicago, IL). Differences were considered statistically significant if the P value was <0.05. Data were expressed as mean ± standard deviation or median (25th–75th percentile), depending on distribution. Skewed variables were transformed logarithmically or with square transformation. Continuous variables were analyzed using the Student t test. Categorical variables were compared using the chi-squared test.
3. Results
3.1. Flow of patients
Our series included 886 patients with primary aldosteronism (Fig. 1). Adrenal CT imaging showed unilateral disease in 610 (68.8%) patients and nonunilateral disease in 276 (31.2%) patients. AVS was performed in 288 (47.2%) and 135 patients (48.9%), respectively, and succeeded in 269 (93.4%) and 125 (92.6%) patients, respectively. Adrenalectomy was performed in 125 and 55 patients as guided by AVS lateralization and CT imaging, respectively. Of these patients, 96 (76.8%) and 30 (54.5%), respectively, were followed up for clinical outcomes.
Figure 1.
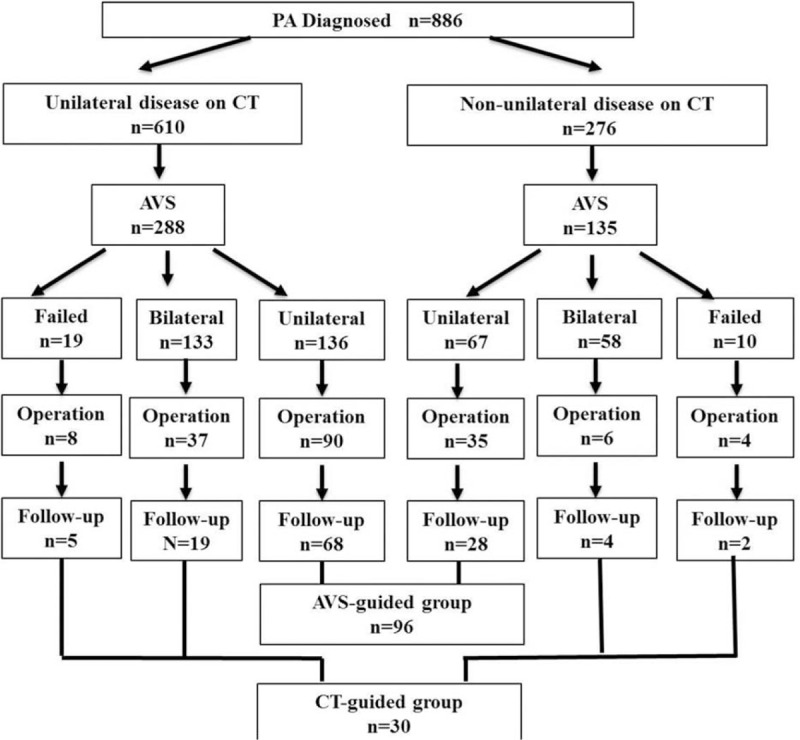
Flow of patients. AVS = adrenal venous sampling, CT = computed tomography.
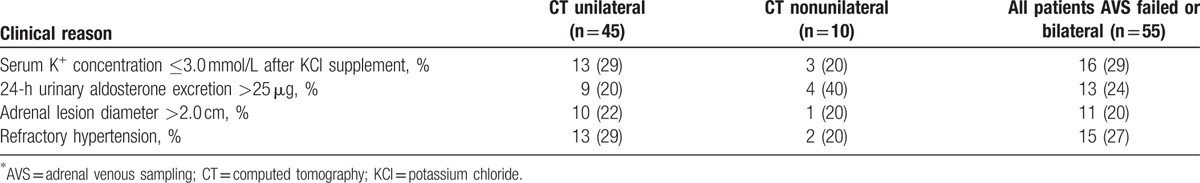
Of the 55 patients who had adrenalectomy as guided by CT imaging, 45 and 10 patients had unilateral and nonunilateral diseases, respectively (Fig. 1 and Table 1). For the 45 patients with unilateral disease on CT imaging, the reasons for adrenalectomy included persistent hypokalemia after oral supplement of potassium chloride (n = 13), 24-hour urinary aldosterone excretion ≥25 μg (n = 9), adrenal lesion diameter ≥2.0 cm (n = 10) and refractory hypertension (n = 13), and the choice of the adrenal gland to remove was based on the unilateral adenoma presentation. For the 10 patients with nonunilateral disease on CT imaging, the reasons for adrenalectomy included persistant hypokalemia after oral supplement of potassium chloride (n = 3), 24-hour urinary aldosterone excretion ≥25 μg (n = 4), adrenal lesion diameter ≥2.0 cm (n = 1) and refractory hypertension (n = 2), and the choice of the adrenal gland to remove was based on typical adenoma presentation on 1 side (diameter 8–20 mm) and nodular presentation on the opposite side on CT imaging, with a higher adrenal aldosterone/cortisol ratio on the adenoma side.
Table 1.
Classification of clinical reasons for adrenalectomy in patients whose adrenal venous sampling failed or showed bilateral disease.
3.2. Diagnostic concordance between AVS and adrenal CT imaging
We first studied the diagnostic concordance between adrenal CT imaging and AVS in patients with CT unilateral disease and with successful AVS (n = 269, Fig. 2). Overall, the concordance rate was 50.5%; it decreased with age increasing, with highest rate of 61% in patients younger than 30 years (n = 16). None of the patients younger than 36 years had CT and AVS showing opposite lateralization.
Figure 2.
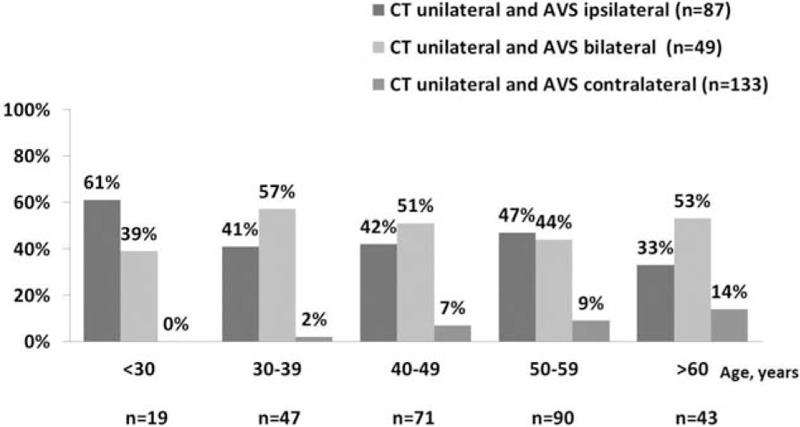
Concordance between adrenal venous sampling (AVS) and computed tomography (CT) according to age in patients with CT unilateral adrenal disease (n = 269). The complete disconcordance (CT unilateral and AVS contralateral) rate was higher with age increasing (P = 0.001).
3.3. Clinical outcomes after adrenalectomy
We then investigated clinical outcomes of adrenalectomy in 96 patients guided by AVS lateralization and 30 patients guided by CT imaging (AVS bilateral n = 23 and AVS failure n = 7, Fig. 1). Table 2 shows the characteristics of patients before adrenalectomy. Before adrenalectomy, patients guided by CT and AVS were similar, except that the AVS-guided patients had lower plasma renin activity (P = 0.045) and higher 24-hour urinary aldosterone excretion (P = 0.043). After adrenalectomy, the AVS-guided patients had a shorter duration of follow-up than the CT-guided patients (P ≤ 0.001). They had similar BP control with a similar number of antihypertensive drugs (Table 3). Fewer patients required the use of spironolactone in the AVS- than CT-guided group (1 vs 3 patients; P = 0.04), because of surgical treatment failure. However, the AVS-guided patients had a significantly higher serum potassium concentration (4.3 ± 0.3 vs 4.0 ± 0.5 mmol/L, P = 0.04) and lower prevalence of hypokalemia (1.0% vs 10.0%, P = 0.04). The total rate of cured and improved hypertension in the AVS group was significantly higher than in the CT group (98% vs 87%; P = 0.03, Fig. 3). The AVS-guided patients (n = 50) had slightly higher cured rate than the CT-guided patients (n = 11) in those older than 50 years (26.0% vs 18.2%, P = 0.72). Among the CT-guided patients, the 6 patients with nonunilateral disease on CT imaging and with failed (n = 2) or bilateral AVS (n = 4) had similar BP control (3 cured and 3 improved) as the rest of the 24 patients (14 cured, 6 improved, and 4 failed).
Table 2.
Clinical characteristics before adrenalectomy in patients with follow-up data.
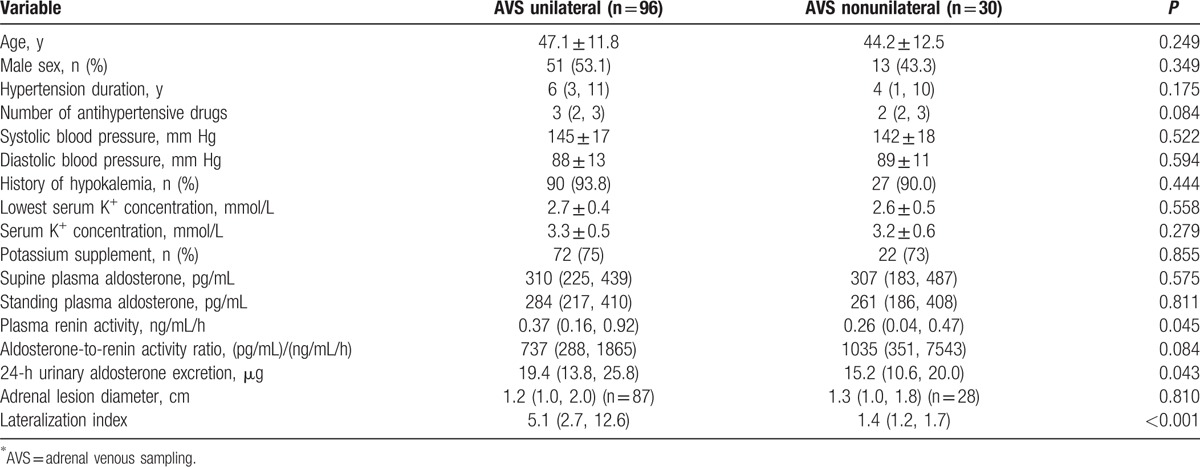
Table 3.
Clinical outcomes after adrenalectomy in patients with follow-up data.

Figure 3.
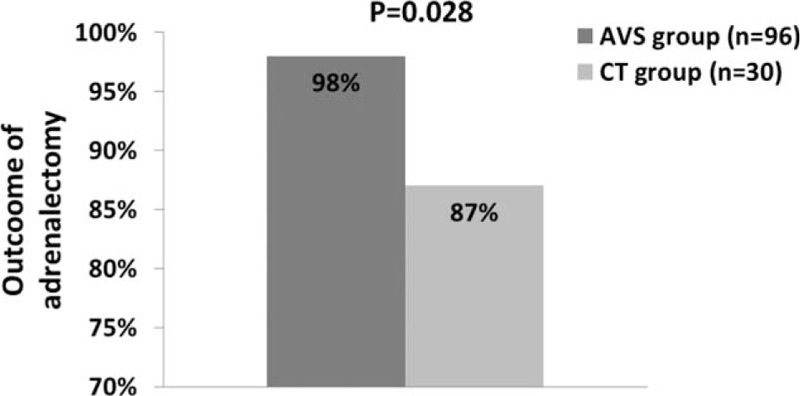
Rate of cured and improved hypertension after adrenalectomy as guided by adrenal venous sampling (AVS) or computed tomography (CT) imaging. The P value for the comparison between AVS and CT imaging is given. For the definitions of “cured” and “improved” hypertension, see Section 2.
In further analysis in patients younger than 40 years and the AVS-guided patients as comparator, the age below which the cured rate in the CT-guided patients was 100% was 30 years. The corresponding cured rate in the AVS-guided patients was 64%.
4. Discussion
Although AVS and adrenal CT imaging had only moderate agreement in patients with CT unilateral adrenal disease, adrenalectomy guided by both techniques improved control of hypertension, with a total cured and improved rate of 87% to 98%. AVS further improved clinical outcomes after adrenalectomy especially in subjects older than 50 years. The age below which the cured rate in the CT-guided patients was 100% was 30 years.
Our finding is in line with the results of a recent study in 263 patients who underwent unilateral adrenalectomy for the treatment of primary aldosteronism at the Mayo Clinic (Rochester, MN).[15] In 143 patients with long-term follow-up data, hypertension was cured in 53 (41.7%) and improved in 59 (46.5%) patients. In 133 patients with long-term follow-up and known cure status of primary aldosteronism, the rate of concordance with the surgically documented side was much higher for AVS (97.1%) than for adrenal imaging with CT or magnetic resonance (58.6%). Nonetheless, adrenal imaging and AVS were 100% concordant in 6 patients younger than 35.1 years. The authors of the study therefore concluded that adrenal imaging performed poor in general but sufficiently well in those younger than 35 years.
The results of several earlier studies also support that AVS may be mandatory for the diagnosis of primary aldosteronism.[12,19–21] In a study of 62 patients with primary aldosteronism, 38 had adrenal CT imaging and successful bilateral AVS. In patients with AVS proven adrenal producing adenoma (n = 15) and bilateral adrenal hyperplasia (n = 21), respectively, only 8 and 4 had concordant findings with CT imaging. The investigators therefore concluded that adrenal CT imaging was not a reliable method, and AVS was essential for the diagnosis of primary aldosteronism.[19] Similar results were observed in a study in 203 patients with primary aldosteronism (age range, 17–80 years; 163 men). AVS was successful in 194 patients (95.6%) and showed unilateral aldosterone hypersecretion in 110 patients (56.7%), including 24 (41.4%) of 58 patients with normal adrenal CT findings, 24 (51.1%) of 47 patients with unilateral micronodule (≤10 mm) apparent on CT (7 had unilateral aldosterone hypersecretion from the contralateral adrenal), 21 (65.6%) of 32 patients with unilateral macronodule (>10 mm) apparent on CT (1 had unilateral aldosterone hypersecretion from the contralateral adrenal), 16 (48.5%) of 33 patients with bilateral micronodules, and 2 (33%) of 6 patients with bilateral macronodules. Overall, if the diagnosis was based on CT findings alone, 42 patients (21.7%) would have missed the opportunity for adrenalectomy and 48 patients (24.7%) might have had unnecessary or inappropriate adrenalectomy.[12] In a retrospective study, CT showed unilateral and bilateral abnormalities, respectively, in 57 and 52 patients with a biochemical diagnosis of primary aldosteronism. Of them, AVS demonstrated concordant results in 46 (80.7%) and 9 patients (17.3%), respectively.[20] Similarly, in a retrospective analysis of 41 patients with primary aldosteronism, unilateral (n = 26) but not nonunilateral lesions on CT (n = 12) had a high positive predictive value of AVS-proven adrenal lesions (85% vs 50%).[21]
Our study was not randomized and hence not the best for the comparison of the efficacy between AVS and CT imaging in guiding adrenalectomy. Nevertheless, our study showed that AVS was associated with a slightly but significantly improved clinical outcomes, that is, 99% correction of hypokalemia and 98% improved BP control. However, AVS in general did not increase the cured rate of hypertension in comparison with the CT imaging method. Our finding is in keeping with the results of several previously published studies.[15,22] In the aforementioned study reported by Lim et al,[15] the total rate of cured and improved hypertension was 88.2%. In a study of 13 patients with doubtful lateralization on adrenal imaging, AVS was performed in 8 patients. The AVS-guided adrenalectomy cured the disease biochemically in 100% and cured and improved hypertension in 85% of the patients.[22]
Our study provided new evidence on the age limit for the decision of AVS in younger subjects. Our finding suggests that 30 years might be the proper age for not having AVS, as appropriate. Our finding is slightly different from the results of several recent studies[15,21] and the current recommendations.[14] In the aforementioned study from Lim et al,[15] the authors proposed 35 years as the age limit for exemption of AVS according to 100% of concordance rate between AVS and adrenal CT imaging in 6 patients of 35.1 years or younger. In a recent study of 41 patients with primary aldosteronism, 16 were 40 years or younger.[21] Eleven had adrenal CT unilateral lesions. One patient declined surgery. Ten patients had histological confirmation of adenoma. These authors concluded that all patients aged under 40 years with unilateral CT lesions may be directed to surgery without AVS. More and larger sample studies are apparently required to address this important question.
Our study had several limitations. First, although our study included follow-up data after adrenalectomy, it was retrospective. To some extent, because of this retrospective nature, our follow-up was incomplete in terms of the number of patients and the clinical and biochemical data. Second, because of the high exclusion rate, the sample size of our present analysis was relatively small, especially for the age-stratified analysis. Third, in our follow-up study, BP was only measured once in the clinic. Ambulatory or home BP monitoring would be preferred for the evaluation of BP control. Finally and importantly, selection bias was apparent, especially for the CT-guided patients. The CT-guided group included patients whose AVS failed or did not show unilateral aldosterone hypersecretion. Although patients’ wishes played an important part in the decision of surgery, these patients were surgically treated for clinical reasons and after careful lateralization according to the best available imaging and biochemical evidence. The positive outcome results of adrenalectomy suggest that future research should address the issue of unilateral adrenalectomy in patients whose AVS fails (it may fail) and who have bilateral aldosterone hypersecretion, as also indicated by a recent study by Sukor et al[23] on unilateral adrenalectomy in bilateral aldosteronism.
In conclusion, AVS guidance had moderate agreement with CT imaging guidance and was associated with improved clinical outcomes after adrenalectomy. The age below which CT unilateralization achieved 100% of cured rate was 30 years. An implication of our study is that AVS is mandatory in patients with primary aldosteronism for surgery except those very young patients with CT unilateralization.
Footnotes
Abbreviations: AVS = adrenal venous sampling, BP = blood pressure, CT = computed tomography.
LZ and YZ have contributed equally to the article.
This study was supported by grant 20134109 from the Shanghai Municipal Health Bureau, Shanghai, China and grant 81500324 from National Natural Science Foundation of China (Beijing).
The authors have no conflicts of interest to disclose.
References
- 1.Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 2006; 48:2293–2300. [DOI] [PubMed] [Google Scholar]
- 2.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2016; 2:jc20154061. [DOI] [PubMed] [Google Scholar]
- 3.Gallay BJ, Ahmad S, Xu L, et al. Screening for primary aldosteronism without discontinuing hypertensive medications: plasma aldosterone-renin ratio. Am J Kidney Dis 2001; 37:699–705. [DOI] [PubMed] [Google Scholar]
- 4.Benchetrit S, Bernheim J, Podjarny E. Normokalemic hyperaldosteronism in patients with resistant hypertension. Isr Med Assoc J 2002; 4:17–20. [PubMed] [Google Scholar]
- 5.Calhoun DA, Nishizaka MK, Zaman MA, et al. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896. [DOI] [PubMed] [Google Scholar]
- 6.Eide IK, Torjesen PA, Drolsum A, et al. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens 2004; 22:2217–2226. [DOI] [PubMed] [Google Scholar]
- 7.Calhoun DA, Nishizaka MK, Zaman MA, et al. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest 2004; 125:112–117. [DOI] [PubMed] [Google Scholar]
- 8.Umpierrez GE, Cantey P, Smiley D, et al. Primary aldosteronism in diabetic subjects with resistant hypertension. Diabetes Care 2007; 30:1699–1703. [DOI] [PubMed] [Google Scholar]
- 9.Mulatero P, Monticone S, Rainey WE, et al. Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat Rev Endocrinol 2013; 9:104–112. [DOI] [PubMed] [Google Scholar]
- 10.Rossi GP, Bolognesi M, Rizzoni D, et al. Vascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patients. Hypertension 2008; 51:1366–1371. [DOI] [PubMed] [Google Scholar]
- 11.Letavernier E, Peyrard S, Amar L, et al. Blood pressure outcome of adrenalectomy in patients with primary hyperaldosteronism with or without unilateral adenoma. J Hypertens 2008; 26:1816–1823. [DOI] [PubMed] [Google Scholar]
- 12.Young WF, Stanson AW, Thompson GB, et al. Role for adrenal venous sampling in primary aldosteronism. Surgery 2004; 136:1227–1235. [DOI] [PubMed] [Google Scholar]
- 13.Nwariaku FE, Miller BS, Auchus R, et al. Primary hyperaldosteronism: effect of adrenal vein sampling on surgical outcome. Arch Surg 2006; 141:497–502.discussion 502–503. [DOI] [PubMed] [Google Scholar]
- 14.Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension 2014; 63:151–160. [DOI] [PubMed] [Google Scholar]
- 15.Lim V, Guo Q, Grant CS, et al. Accuracy of adrenal imaging and adrenal venous sampling in predicting surgical cure of primary aldosteronism. J Clin Endocrinol Metab 2014; 99:2712–2719. [DOI] [PubMed] [Google Scholar]
- 16.Kloos RT, Gross MD, Francis IR, et al. Incidentally discovered adrenal masses. Endocr Rev 1995; 16:460–484. [DOI] [PubMed] [Google Scholar]
- 17.Kupers EM, Amar L, Raynaud A, et al. A clinical prediction score to diagnose unilateral primary aldosteronism. J Clin Endocrinol Metab 2012; 97:3530–3537. [DOI] [PubMed] [Google Scholar]
- 18.Sigurjonsdottir HA, Gronowitz M, Andersson O, et al. Unilateral adrenal hyperplasia is a usual cause of primary hyperaldosteronism. Results from a Swedish screening study. BMC Endocr Disord 2012; 12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magill SB, Raff H, Shaker JL, et al. Comparison of adrenal vein sampling and computed tomography in the differentiation of primary aldosteronism. J Clin Endocrinol Metab 2001; 86:1066–1071. [DOI] [PubMed] [Google Scholar]
- 20.Mathur A, Kemp CD, Dutta U, et al. Consequences of adrenal venous sampling in primary hyperaldosteronism and predictors of unilateral adrenal disease. J Am Coll Surg 2010; 211:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salem V, Hopkins TG, El-Gayar H, et al. Adrenal venous sampling as a diagnostic procedure for primary hyperaldosteronism: experience from a tertiary referral centre. Hormones (Athens, Greece) 2012; 11:151–159. [DOI] [PubMed] [Google Scholar]
- 22.Tan YY, Ogilvie JB, Triponez F, et al. Selective use of adrenal venous sampling in the lateralization of aldosterone-producing adenomas. World J Surg 2006; 30:879–885.discussion 886–887. [DOI] [PubMed] [Google Scholar]
- 23.Sukor N, Gordon RD, Ku YK, et al. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. J Clin Endocrinol Metab 2009; 94:2437–2445. [DOI] [PubMed] [Google Scholar]


