Abstract
We investigated recurrence pattern and oncologic outcomes after treatment of metachronous isolated liver metastases from colorectal cancer according to treatment modality.
We retrospectively analyzed 123 patients treated with hepatic resection and 82 patients treated with radiofrequency ablation (RFA) for metachronous isolated hepatic metastasis from colorectal cancer (HMCRC). We compared clinicopathological data, recurrence pattern, and recurrence-free survival (RFS) rates after the treatment of hepatic metastasis between patients treated with RFA and resection.
The patients in the 2 groups were similar in gender, location of primary tumor, disease-free interval to hepatic metastasis, pathologic stage of primary tumor, and number of hepatic metastasis. The age was older in RFA group but it was not statistically different. The mean diameter of the largest hepatic mass was greater in the resection group than in the RFA group (3.1 vs 1.9 cm, P < 0.001). Chemotherapy after the treatment of hepatic metastasis was more commonly given in hepatic resection group (76.4% vs 62.2%, P = 0.04). Recurrence after the treatment of hepatic metastasis was not significantly different between the 2 groups (54.5% vs 65.9% in the resection and RFA groups). However, intrahepatic recurrence without extra-hepatic metastases was more common in the RFA group than in the resection group (47.5% vs 12.1%, P < 0.001). The RFS rate after the treatment of hepatic metastasis was significantly higher in resection group (48.6% vs 33.7%, P = 0.015). The size and number of hepatic metastasis, primary tumor stage, disease-free interval to hepatic metastasis, and the modality of treatment (RFA vs resection) for hepatic metastasis were confirmed as associated factors with re-recurrence after the treatment of hepatic metastasis. Among patients with solitary hepatic metastases of ≤3 cm, marginal recurrence was higher in the RFA group (3% vs 17.2%) and re-RFA was performed to achieve comparable recurrence rate (3% vs 5.2%, P = 0.662), the RFS rate was not different between the resection and RFA group (52.4% vs 53.4%, P = 0.491).
Surgical resection for HMCRC showed higher RFS. However, the RFS rate in patients with a solitary hepatic metastasis of ≤3 cm was similar between the resection and RFA groups.
Keywords: colorectal cancer, hepatic metastasis, resection, RFA
1. Introduction
Liver is one of the most frequently encountered sites of recurrence in colorectal cancer, and nearly 50% of patients with colorectal cancer develop hepatic metastases during the course of their disease.[1–3] Surgical resection has been the universally accepted standard of treatment for resectable hepatic metastases from colorectal cancer. However, only 10% to 25% of patients with hepatic metastases are qualified for hepatic resection; others are not suitable for resection due to anatomically ill-located lesions, functional insufficiency of hepatic reserve, medical comorbidities, and extra-hepatic metastases.[4]
Radiofrequency ablation (RFA) is considered to be the alternative treatment in certain indications for patients not suitable to undergo surgical resection.[5] RFA has been increasingly utilized as a treatment modality equivalent to hepatic resection in patients with isolated hepatic metastases, leading to 5-year survival rates of 14% to 55% for some patients.[6] Thus, RFA is being considered to replace hepatic resection in certain indications; however, inferior local control remains one of the greatest challenges of RFA for the treatment of hepatic metastases.
There are many conflicting reports that have compared the efficacy of RFA and hepatic resection for hepatic metastasis from colorectal cancer. Some authors reported comparable oncologic outcomes between RFA and hepatic resection,[7] while other reports indicated that RFA is inferior to hepatic resection in terms of local control and survival.[6,8]
We frequently encounter patients who develop hepatic metastasis after curative resection of colorectal cancer and question which treatment modality would result in the most effective outcome in terms of oncologic outcome and morbidity. Although previous studies reported outcomes of hepatic metastasis from colorectal cancer treated with RFA and resection, the results could not be directly translated for clinical application due to the heterogeneous characteristics of the study subjects. Therefore, we narrowed down the study subjects with a relatively homogeneous condition, and evaluated the oncologic outcomes of patients with metachronous isolated hepatic metastasis from colorectal cancer (HMCRC). We compared the patterns of re-recurrence after the treatment of hepatic metastasis, and the oncologic outcomes after the treatment of hepatic metastasis with RFA and surgical resection and investigated the prognostic factors associated with the recurrence-free survival (RFS) after the treatment of HMCRC.
2. Methods
2.1. Patients
Patients who were diagnosed HMCRC after curative treatment for colorectal cancer between January 2000 and December 2010 were included. We further defined the study subjects to those whose hepatic metastases were the 1st metastatic site found without any extrahepatic metastasis, based on computed tomography, magnetic resonance imaging, ultrasonography, positron emission tomography, and biopsy findings. Hepatic metastasis was determined by serial changes of a suspicious lesion by a certain imaging modality or by a combination of other diagnostic methods. Metachronous metastasis was defined as a metastatic lesion diagnosed at least 6 months after the diagnosis of primary colorectal cancer. Patients who received a combination of RFA and resection and those who received intraoperative RFA or other treatment modalities for hepatic metastases were excluded. Thus, 205 patients fulfilling these criteria were identified. Of these 205 patients, 123 were treated by hepatic resection and 82 were treated by RFA. RFS which was defined as RFS after the treatment of HMCRC was compared between 2 groups.
Treatment for hepatic resection was decided according to patient general condition, location, size of hepatic metastasis, and physician's favor to treatment. Resection was indicated for patients with resectable hepatic disease which was determined by surgeons specialized in hepatic surgery, under the condition that the remaining liver volume was adequate, the general condition of patient was acceptable for surgery and general anesthesia. RFA was considered for patients who were reluctant to undergo surgery, or if the remnant hepatic function was expected to be inadequate due to tumor location requiring major surgery for relatively small size of metastasis. It was also considered with curative intent when the probe could be optimally positioned to achieve complete destruction of the tumor with at least a 0.5 cm safety margin of normal liver parenchyma.
The present study protocol was approved by the institutional review committee of Asan Medical Center.
2.2. Radiofrequency ablation
All RFA procedures were performed percutaneously under imaging guidance with real-time ultrasonography by 1 of 3 radiologists with more than 8 years of experience in RFA. All patients received local anesthesia at the puncture site and were under conscious sedation during the procedure. We used internally cooled electrode system, either a single-type with a 2 or 3-cm active tip (Cool-tip RF System, Covidien, Mansfield, MA) or a cluster-type (Cluster, RF Medical Co. Ltd, Seoul, Korea), depending on tumor size and location. A multistep incremental power expansion method was used as an algorithm for energy deposition. We aimed to obtain an ablation margin of at least 0.5 cm in the hepatic parenchyma surrounding the index tumor. In cases where the ablation margin did not appear to be sufficient during the procedure, a multiple overlapping technique was applied as an intraprocedural modification. Before retracting electrodes, the electrode path was cauterized to avoid bleeding and tumor seeding which could possibly occur during electrode retraction.
Immediately after the completion of procedure, all patients underwent contrast-enhanced CT scans in order to confirm the technical success of the RFA session and to detect potential postprocedural complications.
We followed the reporting standards of the Society of Interventional Radiology to define success, outcomes, and complications.[9] Technical success was determined when the ablative zone completely encompassed the index tumor. Local tumor progression was determined by the appearance of tumor foci at the edge of the ablative zone on follow-up imaging studies.
2.3. Statistical analysis
Student t test, Mann–Whitney test, and χ2 tests were used for the statistical analyses of the data. Patient survival rates were calculated by the Kaplan–Meier method, and statistically significant differences in survival rates were identified by the log rank test. Multivariate analysis was performed by the Cox proportional hazards model. All statistical analyses were conducted using SPSS 21.0 (IBM, Chicago, IL). P-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Clinicopathological characteristics of patients
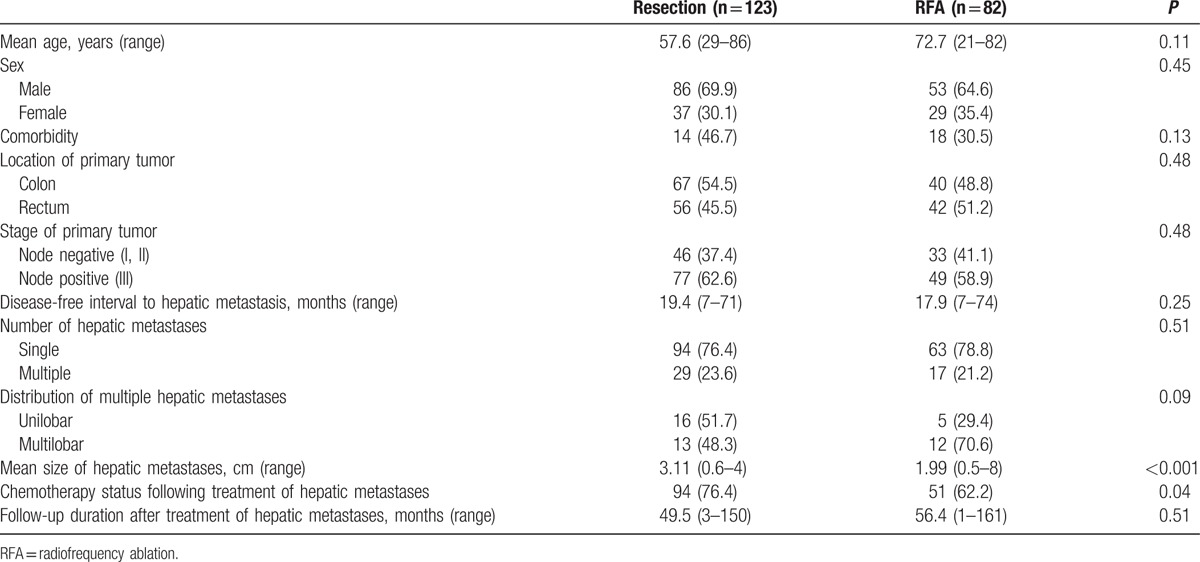
The clinicopathological characteristics of patients included in the final analyses are shown in Table 1. Patients in the resection and RFA groups were not different in terms of gender, primary tumor stage and location, and comorbidities. Patients treated with RFA were older but it was not statistically significant. Chemotherapy after the treatment of HMCRC was more frequently given in resection group. Disease-free interval to hepatic metastasis was similar between the 2 groups; 19 and 18 months in the resection and RFA groups, respectively. The number of hepatic metastases was also similar between the 2 groups; however, the mean diameter of the largest hepatic mass was greater in the resection group than in RFA group (3.11 vs 1.99 cm, respectively; P < 0.001). Among those with multiple hepatic metastases, unilobar metastases were observed in 16 of 29 patients (51.7%) in the resection group and in 5 of 17 patients (29.4%) in the RFA group (Table 1).
Table 1.
Clinicopathological characteristics of patients and primary tumors.
3.2. Recurrence after the treatment of hepatic metastasis
A total of 121 patients had recurrence after the treatment of hepatic metastasis. Recurrence at a single organ occurred in 99 patients (77.7%), and 22 (22.3%) at multiple organs as the 1st recurrence after the treatment of hepatic metastases. The site of recurrence and the number of intrahepatic recurrences were different according to the treatment groups. In patients with a single site recurrence after the treatment of solitary hepatic metastases, lung was the most common site in the resection group, while liver was the predominant site in the RFA group. In patients with multiple organ metastases after the treatment of solitary hepatic metastases, re-recurrence occurred most frequently in the liver both in the resection and the RFA group (Fig. 1).
Figure 1.

Recurrence pattern after treatment of metachronous isolated hepatic metastasis from colorectal cancer according to type of treatment.
There were 47 patients who had intrahepatic recurrence in the RFA group, and marginal recurrence developed in 14 patients (17%). In the resection group, there were 29 patients who had intrahepatic recurrence and 5 (4.1%) developed marginal recurrence (P = 0.003). Intrahepatic recurrence without extrahepatic metastasis occurred in 15 of 123 (12.1%) patients in the resection group and in 39 of 82 (47.5%) patients in the RFA group (P < 0.001). (Table 2)
Table 2.
Factors associated with re-recurrence after treatment of liver metastasis.

Treatment for intrahepatic recurrence without extra-hepatic metastasis was different between the 2 groups. Resection was the most commonly adopted treatment for intrahepatic recurrence in the resection group. In contrast, RFA was the predominant treatment in the RFA group. In the resection group, resection and chemotherapy, chemotherapy alone, RFA, and stereotactic radiosurgery were performed in 5 (23.8%), 4 (19%), 3 (14.4%), and 1 (4.8%) patients for intrahepatic re-recurrence. In the RFA group, RFA and/or chemotherapy, resection and chemotherapy, chemotherapy alone, and stereotactic radiosurgery were performed in 12 (30.7%), 5 (12.8%), 7 (17.9%), and 1 (2.5%) patients for intrahepatic re-recurrence.
In patients with solitary hepatic metastases ≤3 cm in diameter, intrahepatic recurrence developed in 13 patients (19.4%) in the resection group and 16 (27.6) in the RFA group (P = 0.280). The marginal recurrence occurred in 2 (3%) and 10 (17.2%) in the resection and RFA groups, respectively. Among 10 patients who developed marginal recurrence in the RFA group, 6 were treated with re-RFA successfully and 1 was treated with radiotherapy. Therefore, local recurrence rate in the liver at last follow-up was not different between resection group and RFA group (3% vs 5.2%, P = 0.662).
3.3. Factors associated with the re-recurrence-free survival rate after the treatment of hepatic metastasis
The RFS rate after the treatment of hepatic metastases was significantly higher in the resection group than in the RFA group among all patients (48.6% vs 33.7%, respectively; P = 0.015, Fig. 2). By multivariate analysis, the type of treatment for hepatic metastasis was associated with the RFS rate. Specifically, RFA was related with significantly higher risk of re-recurrence after the treatment of hepatic metastasis. In addition, the number of hepatic metastases was associated with the RFS rate. The presence of more than 2 hepatic metastases was associated with a 59% increase in the risk of re-recurrence. Furthermore, primary tumor stage categorized by the nodal status of the primary tumor was significantly associated with the RFS rate. Finally, a disease-free interval of more than 12 months to hepatic metastasis was associated with a decreased risk of re-recurrence. However, adjuvant chemotherapy after the treatment of hepatic metastasis was not associated with an improved RFS (Table 2).
Figure 2.

RFS rate between RFA and resection group. Resection group showed higher re-RFS rate in overall group. RFA = radiofrequency ablation, RFS = recurrence-free survival.
3.4. Oncologic outcomes in subgroups according to the characteristics of hepatic metastasis
Of 153 patients whose hepatic metastases was ≤3 cm in the largest diameter, the re-RFS rate was not significantly different between the 2 types of treatment for hepatic metastases (Fig. 3A, P = 0.142). A similar oncologic outcome was also observed in 125 patients with solitary hepatic metastases ≤3 cm in diameter (Fig. 3B, P = 0.491). Of 28 patients with multiple hepatic metastases with the largest diameter ≤3 cm, RFS rate was significantly lower in the RFA group than in the resection group (Fig. 3C, P = 0.039). Among those with longer than 12 months of disease-free interval to hepatic metastases, the RFS rate was similar between the resection and RFA groups. In contrast, among patients with shorter than 12 months of disease-free interval to hepatic metastases, those who underwent resection showed a significantly higher RFS rate (P = 0.053).
Figure 3.

RFS rate. (A) In patients with lesion ≤3 cm. (B) In patients with lesion ≤3 cm and solitary metastasis. (C) In patients with lesion ≤3 cm and multiple metastasis. RFA and resection group showed similar re-RFS for ≤3 cm solitary hepatic metastasis. RFA = radiofrequency ablation, RFS = recurrence-free survival.
4. Discussion
Our study showed that, in patients with HMCRC, the RFS rate of the resection group was higher than the RFA group, and the intrahepatic recurrence without extrahepatic metastases was more common in the RFA group than in the resection group. In patients with solitary hepatic metastasis ≤3 cm, however, the RFS rate was similar between the 2 groups. The type of treatment (RFA), the number of metastatic lesions (more than 2), the nodal status of the primary tumor (node positive), and the disease-free interval to hepatic metastasis (less than 12 months) were found to be the factors associated with higher risk of recurrence after the treatment of hepatic metastasis in multivariate analysis.
Hepatic resection, the current treatment of choice for resectable hepatic metastasis, is not possible or appropriate for patients with insufficient hepatic reserve, severe comorbidities, and multiple and/or bulky lesions.[10] RFA has proven its efficacy in certain indications for patients who are not suitable to undergo surgical resection.[10–12] The comparison of the 2 treatment modalities from previous studies has not provided sufficient evidence for clinical application due to their different indications and heterogeneous patient populations. Our study also showed no statistical difference in terms of age and comorbidities between the 2 groups. However, severity of comorbidities of individual patients could be different depending on the surgeon's point of view. There were some cases that hepatobiliary surgeons decided not to perform surgery due to adhesion of previous surgery and difficult area of surgery. Meanwhile, several studies have shown that RFA is an effective viable alternative to hepatic resection for small and solitary hepatic metastatic lesions.[7,8,13,14] Furthermore, the clinical use of RFA has been increasing due to several factors including reduced morbidity rates and cost, possible repeated procedure for recurrent metastases, reduce the chances of hepatic resection, and controlling the extent of resection when surgery is needed.[15,16]
Most studies comparing treatment outcome between resection and RFA of hepatic metastases from colorectal cancer have included both synchronous and metachronous hepatic metastases. In patients with synchronous hepatic metastasis, we usually have a wider range of treatment options available since they are scheduled to undergo surgery for the primary colorectal tumor, compared to the more limited treatment options possible for metachronous hepatic metastases. Thus, the outcome of a given treatment has to be differentially evaluated for synchronous and metachronous hepatic metastases. The central focus of this study was on HMCRC after curative surgery.
This study demonstrated that surgical resection for HMCRC was advantageous over RFA due to the higher RFS rate and lower local recurrence rate in the liver. RFA was previously reported to be inferior to hepatic resection due to the higher local recurrence rate and lower 5-year overall survival and 5-year disease-free survival rate; these results have also been observed in small lesions (diameter ≤3 cm);[6–8,10–12,17–23] however, several studies support RFA as an alternative treatment to hepatic resection for metastatic hepatic tumor.[13,14,18,19] Our results are in line with those studies demonstrating RFA as inferior to surgical resection; the RFS rate after the treatment of hepatic metastasis was 48.6% in resection group and 33.7% in RFA group. Considering other factors such as number, nodal stage of the primary tumor, and disease-free interval to hepatic metastasis, our results showed that the treatment modality was an associated factor of RFS. RFA might not be equivalent to hepatic resection; rather, it might be considered as an adjunctive therapy to surgery because the RFS rate with RFA in colorectal hepatic metastases was worse than that with surgical resection in all hepatic metastasis patients, according to previous and our results.[10–12,22,23]
RFA was suggested to equivalent to surgical resection for the treatment of solitary and small hepatic metastatic lesion, the oncologic outcomes of which remain controversial.[6–8,17,20,21,24–26] Recent studies have reported that RFA is similar to hepatic resection for solitary hepatic metastases ≤3 cm in diameter.[6–8,26] The results of our study also demonstrated that RFA could be considered as an alternative to hepatic resection for solitary hepatic metastases ≤3 cm with oncologic outcomes similar to those achieved by surgical resection. We found that the RFS rate was similar between hepatic resection and RFA in these patients, which is contrary to the results of a previous study conducted at our center; in that study, the RFA group with solitary hepatic metastasis ≤3 cm had a higher local recurrence rate, shorter time to recurrence, and lower overall survival rate than the resection group.[20] This inconsistency might be due to the small number of patients (n = 60), shorter follow-up duration, and different statistical methods used in the previous study.
Independent of the RFS rate, the local recurrence patterns in the liver was quite different between the resection and RFA groups. Previous studies have reported a wide range of local recurrence rates in the liver, ranging from 6.6% to 66.7%,[27–31] and many studies have demonstrated that hepatic resection was superior to RFA based on local recurrence rates, even in resectable hepatic metastases.[10,11,17,20,32] Our study showed that the rate of intrahepatic metastases was 12.1% in hepatic resection group and 47.5% in RFA group, and 16.2% and 34.1% for solitary lesion, respectively. The marginal recurrence rate was 4.1% and 17.1% in the resection and RFA groups, respectively. Intrahepatic and marginal recurrence rates were predominant in the RFA group, although the procedure was successfully done in 97.5% of all cases in the present study. In patients with solitary hepatic metastases ≤ 3 cm in diameter, the intrahepatic recurrence was not statistically different. However, the marginal recurrence rates were significantly higher in the RFA group and re-RFA was performed. After then, 60% of marginal recurrences were completely eradicated with re-RFA (3% vs 5.2%, P = 0.662). Consequently, local recurrence rate at last follow-up for patients with solitary hepatic metastases ≤3 cm did not show difference. Local disease control with RFA was not comparable to that with surgical resection among all patients; however, RFA might be an alternative in patients with solitary, small metastases with satisfactory local control.
Multivariate analysis showed that treatment modality such as hepatic resection or RFA was the most associated factor. Several studies including our previous study also indicated that treatment modality was the most influential factor for survival outcome.[8,10,12,17,20] We found that adjuvant chemotherapy after the treatment of hepatic metastases did not influence RFS. However, it was performed more frequently in the resection group (76.4%) than the RFA group (62.2%). This might be because that patients of the resection group were more active in treatment and their general condition was good enough to be given chemotherapy. There was no case of neoadjuvant chemotherapy before liver resection because our center has a policy of not performing neoadjuvant chemotherapy for curative hepatic resection. Adjuvant chemotherapy after colorectal surgery could influence RFS; however, the rate of receiving chemotherapy between the resection group and the RFA group did not differ significantly (91.1% vs 89.0%, respectively) and adjuvant chemotherapy did not influence RFS in multivariate analysis. Given these results, chemotherapy did not influence RFS in this study; however, chemotherapy regimen is heterogeneous and further study will be needed to evaluate the application of chemotherapy.
The present study has several limitations. This was a retrospective study of a single center and might have a potential bias in patient selection, which is clinically unavoidable. Although the 2 groups had comparable patients and tumor characteristics, we tried to mitigate influence of selection bias by subgroup analysis. Our study has a significant advantage over previous studies as the confounding effects of other factors were limited by including only metachronous isolated hepatic metastases cases.
In conclusion, the findings presented here provide evidence that hepatic resection should be preferred for the treatment HMCRC, whereas RFA might be considered as a reasonable alternative for solitary hepatic metastases ≤3 cm in diameter. The results of this study should be considered for the selection of treatment modality for HMCRC. In addition, effective surveillance methods and schedules need to be further studied for the early recognition of recurrence considering its patterns and timing.
Footnotes
Abbreviations: HMCRC = metachronous isolated hepatic metastasis from colorectal cancer, RFA = radiofrequency ablation, RFS = recurrence-free survival.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Ballantyne GH, Quin J. Surgical treatment of liver metastases in patients with colorectal cancer. Cancer 1993; 71:4252–4266. [DOI] [PubMed] [Google Scholar]
- 2.Millikan KW, Staren ED, Doolas A. Invasive therapy of metastatic colorectal cancer to the liver. Surg Clin North Am 1997; 77:27–48. [DOI] [PubMed] [Google Scholar]
- 3.South and West Cancer Intelligence Unit. Wessex Colorectal Cancer Audit: Final report, 5 year outcomes. Wessex: South and West Cancer Intelligence Unit, 2000. [Google Scholar]
- 4.Feliberti EC, Wagman LD. Radiofrequency ablation of liver metastases from colorectal carcinoma. Cancer Control 2006; 13:48–51. [DOI] [PubMed] [Google Scholar]
- 5.Shibata T, Niinobu T, Ogata N, et al. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer 2000; 89:276–284. [PubMed] [Google Scholar]
- 6.Mulier S, Ni Y, Jamart J, et al. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? Ann Surg Oncol 2008; 15:144–157. [DOI] [PubMed] [Google Scholar]
- 7.Kim KH, Yoon YS, Yu CS, et al. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc 2011; 81:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hur H, Ko YT, Min BS, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 2009; 197:728–736. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria: a 10 year update. Radiology 2014; 273:241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004; 239:818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WS, Yun SH, Chun HK, et al. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol 2008; 42:945–949. [DOI] [PubMed] [Google Scholar]
- 12.Lee KH, Kim HO, Yoo CH, et al. Comparison of radiofrequency ablation and resection for hepatic metastasis from colorectal cancer. Korean J Gastroenterol 2012; 59:218–223. [DOI] [PubMed] [Google Scholar]
- 13.Oshowo A, Gillams A, Harrison E, et al. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg 2003; 90:1240–1243. [DOI] [PubMed] [Google Scholar]
- 14.Elias D, Baton O, Sideris L, et al. Local recurrences after intraoperative radiofrequency ablation of liver metastases: a comparative study with anatomic and wedge resections. Ann Surg Oncol 2004; 11:500–505. [DOI] [PubMed] [Google Scholar]
- 15.Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology 1997; 205:367–373. [DOI] [PubMed] [Google Scholar]
- 16.Meloni MF, Andreano A, Laeseke PF, et al. Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation: intermediate and long-term survival rates. Radiology 2009; 253:861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006; 141:460–467. [DOI] [PubMed] [Google Scholar]
- 18.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006; 243:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siperstein AE, Berber E, Ballem N, et al. Survival after radiofrequency ablation of colorectal liver metastases: 10 year experience. Ann Surg 2007; 246:559–567. [DOI] [PubMed] [Google Scholar]
- 20.Park IJ, Kim HC, Yu CS, et al. Radiofrequency ablation for metachronous liver metastasis from colorectal cancer after curative surgery. Ann Surg Oncol 2008; 15:227–232. [DOI] [PubMed] [Google Scholar]
- 21.Ko S, Jo H, Yun S, et al. Comparative analysis of radiofrequency ablation and resection for resectable colorectal liver metastases. World J Gastroenterol 2014; 20:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ungureanu BS, Sandulescu L, Surlin V, et al. Surgical hepatic resection vs. ultrasonographic guided radiofrequency ablation in colorectal liver metastases: what should we choose? Med Ultrason 2014; 16:145–151. [DOI] [PubMed] [Google Scholar]
- 23.Bai H, Huangz X, Jing L, et al. The effect of radiofrequency ablation vs. liver resection on survival outcome of colorectal liver metastases (CRLM): a meta-analysis. Hepatogastroenterology 2015; 62:373–377. [PubMed] [Google Scholar]
- 24.Gravante G, Overton J, Sorge R, et al. Radiofrequency ablation versus resection for liver tumours: an evidence-based approach to retrospective comparative studies. J Gastrointest Surg 2011; 15:378–387. [DOI] [PubMed] [Google Scholar]
- 25.Weng M, Zhang Y, Zhou D, et al. Radiofrequency ablation versus resection for colorectal cancer liver metastases: a meta-analysis. PLoS One 2012; 7:e45493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishiwada S, Ko S, Mukogawa T, et al. Comparison between percutaneous radiofrequency ablation and surgical hepatectomy focusing on local disease control rate for colorectal liver metastases. Hepatogastroenterology 2014; 61:436–441. [PubMed] [Google Scholar]
- 27.Abitabile P, Hartl U, Lange J, et al. Radiofrequency ablation permits an effective treatment for colorectal liver metastasis. Eur J Surg Oncol 2007; 33:67–71. [DOI] [PubMed] [Google Scholar]
- 28.Berber E, Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Ann Surg Oncol 2008; 15:2757–2764. [DOI] [PubMed] [Google Scholar]
- 29.Kosari K, Gomes M, Hunter D, et al. Local, intrahepatic, and systemic recurrence patterns after radiofrequency ablation of hepatic malignancies. J Gastrointest Surg 2002; 6:255–263. [DOI] [PubMed] [Google Scholar]
- 30.Machi J, Uchida S, Sumida K, et al. Ultrasound-guided radiofrequency thermal ablation of liver tumors: percutaneous, laparoscopic, and open surgical approaches. J Gastrointest Surg 2001; 5:477–489. [DOI] [PubMed] [Google Scholar]
- 31.Veltri A, Sacchetto P, Tosetti I, et al. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol 2008; 31:948–956. [DOI] [PubMed] [Google Scholar]
- 32.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005; 241:715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]


