Abstract
Atopic dermatitis (AD) is one of the common allergic diseases in children. The presence of allergic diseases was found to have association with the risk of developing attention-deficit hyperactivity disorder (ADHD) or autistic spectrum disorder (ASD) in children, but it is still inconclusive. This study was to investigate the longitudinal relationship between AD developed during toddlerhood and subsequent development of ADHD or ASD in later childhood. Toddlers born between 1998 and 2008 and diagnosed with AD at the age younger than 3 years and older than 1 month were retrieved from Taiwan's National Health Insurance Research Database. Age- and gender-matched toddlers with no lifetime AD were enrolled as the control group. All enrolled toddlers were followed until 2011 to identify the development of ADHD or ASD. Multivariate Cox regression analysis was performed to analyze the hazard ratios (HRs). The risks associated with allergic comorbidities were analyzed. A total of 18,473 toddlers were enrolled into the AD group. The presence of AD significantly increased the risk of developing ADHD (HR = 2.92, 95% confidence interval [CI] = 2.48–3.45) or ASD (HR = 8.90, 95% CI = 4.98–15.92) when aged 3 years or older. Children from the AD group with 3 comorbidities together, namely, allergic rhinitis, allergic conjunctivitis, and asthma, had the greatest risk of developing ADHD and ASD (ADHD: HR = 4.67, 95% CI = 3.81–5.43; ASD: HR = 16.65, 95% CI = 8.63–30.60). In conclusion, toddlers who suffer from AD at the age younger than 3 years are at a higher risk of developing ADHD and ASD during later childhood. Pediatricians taking care of toddlers with AD should have knowledge of this increased risk of developing ADHD and ASD later in life, especially when children have certain comorbidities such as allergic rhinitis, allergic conjunctivitis, and asthma.
Keywords: atopic dermatitis, attention-deficit hyperactivity disorder, autistic spectrum disorder, children, toddler
1. Introduction
Allergic diseases, including atopic dermatitis (AD), allergic rhinitis, allergic conjunctivitis, and bronchial asthma, are common disorders among children. The prevalence of childhood AD has been noted to be increasing recently and the frequencies have been found to range from 10% to 20%, especially in developed countries.[1–4] Among chronic childhood inflammatory skin diseases, AD is considered to be the most common.[5] Since severe AD in children creates a burden to themselves, to their families and to society, it has become a serious public health issue across the world.[2,6,7] Importantly, AD has been reported to show a high degree of association with other nonallergic disease, including psychiatric and behavior disorders.[2,8,9]
Attention-deficit hyperactivity disorder (ADHD) and autistic spectrum disorder (ASD) are developmental disabilities affecting children that have also shown an increasing prevalence over recent years.[10] Both ADHD and ASD have a range of impacts on the patients themselves, on their families, and on health services.[11–13] Although a definite etiology for these diseases is still unknown,[14–16] neurobiological and genetic factors together with environmental interactions are thought to contribute to disease development.[14,16,17] Further investigations into the possible comorbidities of and the potential risk factors related to ADHD and ASD are 2 of the ways that may allow the elucidation of their possible pathophysiology.
The relationship between atopic disease and ADHD/ASD has been investigated for more than 10 years and the findings remain controversial.[1,9,15,18–21] Nevertheless, the increase in prevalence of and the burden created by atopic diseases over the past decades seems to have paralleled the recent increase in ADHD diagnoses.[1] It is well known that patients with AD have a higher risk of developing other allergic diseases.[7,22–24] The presence of a correlation between ADHD/ASD and AD is a strong possibility,[25] but not all investigators agree with this conclusion (Table 1).[1,9,15,18–21,25–33] Reviewing the latest reports, most of the studies find in favor of a positive association between AD and ADHD/ASD in children, but investigations targeting longitudinal associations are still not being carried out in sufficient numbers. Therefore, the temporal relationship between having AD and the subsequent development of ADHD and ASD during later childhood requires further investigation.
Table 1.
Recently published reports exploring the association between AD and ADHD or ASD.

Taiwan's National Health Insurance (NHI) program started in 1995, and its population coverage has been higher than 99% over the last 10 years.[34,35] The database is a reliable representation of the patterns and longitudinal relationships associated with children's diseases in Taiwan.[20,27,36–40] Although previous reports using this dataset have demonstrated an association between different allergic diseases and ADHD/ASD,[20,27,28,41] a thorough investigation into the role of early AD during toddlerhood and the subsequent development of ADHD or ASD during later childhood is still lacking.
We hypothesized that AD, when observed in early toddlerhood, will increase the risk of developing ADHD or ASD during later childhood and that the presence of other allergic comorbidities might further increase the risk. Therefore, the main purpose of this study was to analyze the temporal relationship between an early diagnosis of AD in toddlers who are aged 0 to 2 years old and the subsequent development of ADHD or ASD when the individual is aged 3 years and older. In addition, we also investigated the possible exacerbation of this risk by the presence of other allergic comorbidities.
2. Methods
2.1. Data sources
The National Health Institute Research Database (NHIRD) is provided by Taiwan's NHI and consists of comprehensive patient information, including demographic data, disease diagnoses, medication details, visit dates, and physician's specialisms.[34,35,37,40,42] The patient information is encrypted and deidentified in order to prevent researchers identifying individual data.[42] Many epidemiological studies and longitudinal surveys have been published based on Taiwan's NHIRD.[28,39,41,43]
The claims data from the Longitudinal Health Insurance Database (LHID) 2010 of Taiwan's NHIRD were used for this study. The LHID 2010 contains the claims data of 1 million randomly sampled beneficiaries from the 2010 registry of beneficiaries.[34,42] According to an announcement of the Bureau of the NHI in Taiwan, there is no significant difference in age distribution, gender distribution, or average insured payroll-related amount between the patients in the LHID 2010 and the original NHIRD.[42]
No consent is needed for the use of this data because the data are analyzed anonymously and no personal information can be connected via the database used in this study. This study was approved by the Institutional Review Board of Taipei Veterans General Hospital, Taipei, Taiwan (VGHIRB No.:2013–06–011BC).
2.2. Study design
This study was designed as a population-based case–control study.
The diagnoses of each individual's claim data are based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). To minimize misclassifications and ensure diagnostic validity, we defined that a given diagnosis should be documented at least twice by the corresponding physician or psychiatrist.
Children younger than 3 years old and older than 1 month old with a diagnosis of AD (ICD-9-CM: 691 or 691.8) given by board-certified dermatologist, pediatrician or rheumatologist and in the absence of any psychiatric disorder (ICD-9-CM code: 290–319) between January 1, 1998 and December 31, 2008, were enrolled into our study as the study group (AD group). The infants of age 0 to 1 month were not included because some of their medical claims might be reported as their parents’ identification number, and the diagnosis of AD at this age is questionable. Age- and gender-matched children (one case for every patient in AD group) were randomly identified as the control group after eliminating the study case group. Subjects who had been given a diagnosis of AD at any time and those with any psychiatric disorder before enrollment were excluded from the control group. All enrolled subjects were followed up to the end of 2011 or death to allow identification of a diagnosis of either ASD (ICD-9-CM code: 299) or ADHD (ICD-9-CM code: 314), which had to be given by board-certified psychiatrist. Other atopic diseases, including asthma (ICD-9-CM codes: 493, 493.0, 493.1, or 493.9), allergic rhinitis (ICD-9-CM code: 477), and allergic conjunctivitis (ICD-9-CM codes: 372.05, 372.10, and 372.14) were identified as comorbidities and/or possible confounding factors. The case selection process is demonstrated in Fig. 1.
Figure 1.
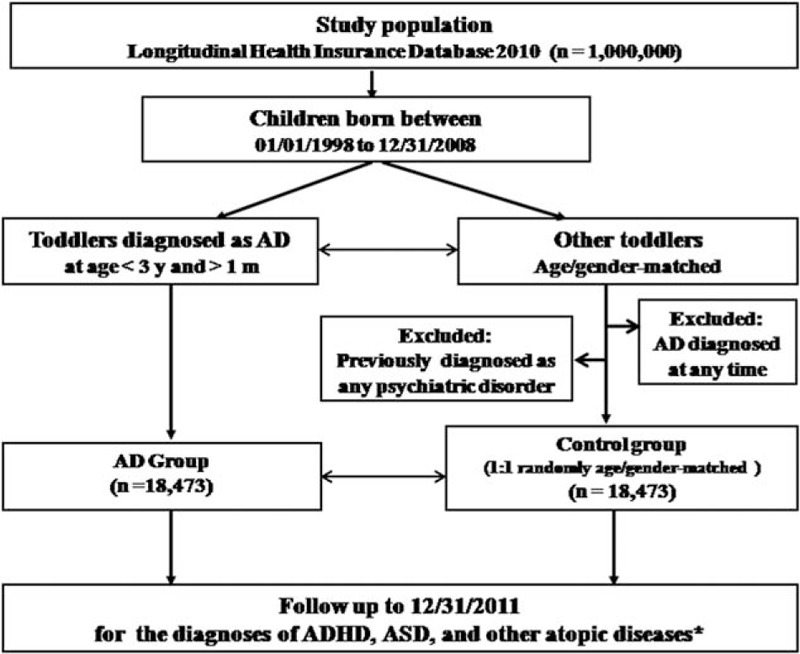
Flow chart for the subject selection process. ∗Other atopic diseases include asthma, allergic rhinitis, and allergic conjunctivitis.
2.3. Statistical analysis
Statistical Package for Social Science (SPSS, version 21, SPSS Inc., Chicago, IL) and Statistical Analysis Software (SAS, version 9.2, SAS Institute, Cary, NC) were used for data processing and statistical analysis. For the between-group comparisons, independent t tests were used for continuous variables and chi-squared tests were used for nominal variables as appropriate. Multivariate Cox regression analyses were performed to investigate the hazard ratios (HRs) with 95% confidence intervals (CIs) of ASD and ADHD after adjusting for the demographic data and the atopic comorbidities. Furthermore, we investigated asthma, allergic rhinitis, and allergic conjunctivitis as atopic comorbidities with AD and explored their effect on the risk of subsequent ASD and ADHD during later life. The aim was to clarify whether there was an additive effect of atopic diseases associated with the development of ASD and ADHD. A 2-sided P value of less than 0.05 was considered statistically significant.
3. Results
A total of 18,473 toddlers born between 1998 and 2008 and diagnosed as AD at age younger than 3 years and older than 1 month old were enrolled into our AD group, and another 18,473 age- and gender-matched toddlers were enrolled into the control group. The overall mean age at enrollment was 0.78 years old (9.4 months old). The follow-up duration for these individuals ranged from 3 to 13 years. The children with AD showed a higher incidence of developing ADHD (4.94% vs 1.46%, P < 0.001) and ASD (0.99% vs 0.09%, P < 0.001) than the control group during the follow-up period. The mean age of being diagnosed with ADHD was 6 to 7 years old, while that for ASD was 4 years old. The age of diagnosis of ADHD was significantly younger in AD group (P < 0.001) and the time between enrollment and the diagnosis of ADHD was significantly shorter for the AD group than the control group (P < 0.001), but there was no significant difference for a diagnosis of ASD (P = 0.668) (Table 2).
Table 2.
Demographic data and incidence of ADHD and ASD among children with AD and the control group.
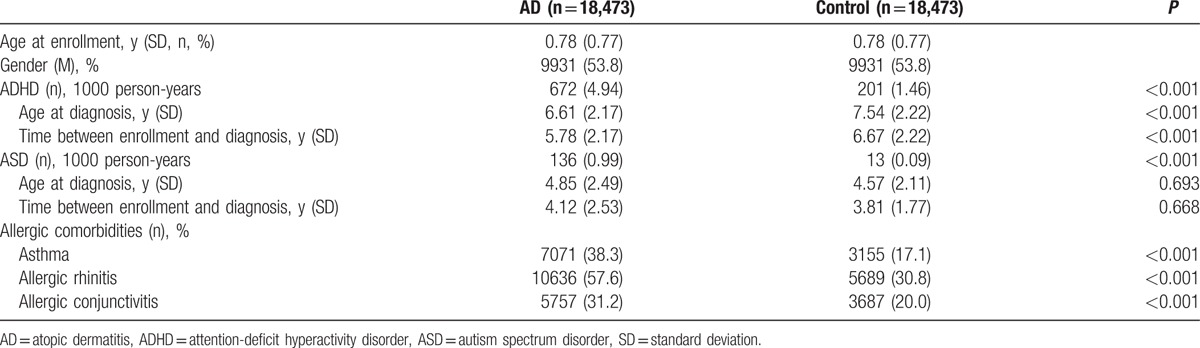
Additionally, significantly higher rates of a combined diagnosis with another allergic disease, namely asthma at 38.3% vs 17.1% (P < 0.001), allergic rhinitis at 57.6% versus 30.8% (P < 0.001) and allergic conjunctivitis at 31.2% versus 20.0% (P < 0.001), were found for the AD group than the control group (Table 2).
The event-free ratios (Kaplan–Meier survival curve) of the enrolled children are shown in Fig. 2 for ADHD and Fig. 3 for ASD. It can be seen that the event-free ratios for both ADHD and ASD dropped markedly when the children with AD were compared to the control group. Furthermore, the event-free ratios dropped most deeply when children of AD had all 3 allergic disease comorbidities, namely asthma, allergic rhinitis, and allergic conjunctivitis (Figs. 2 and 3).
Figure 2.
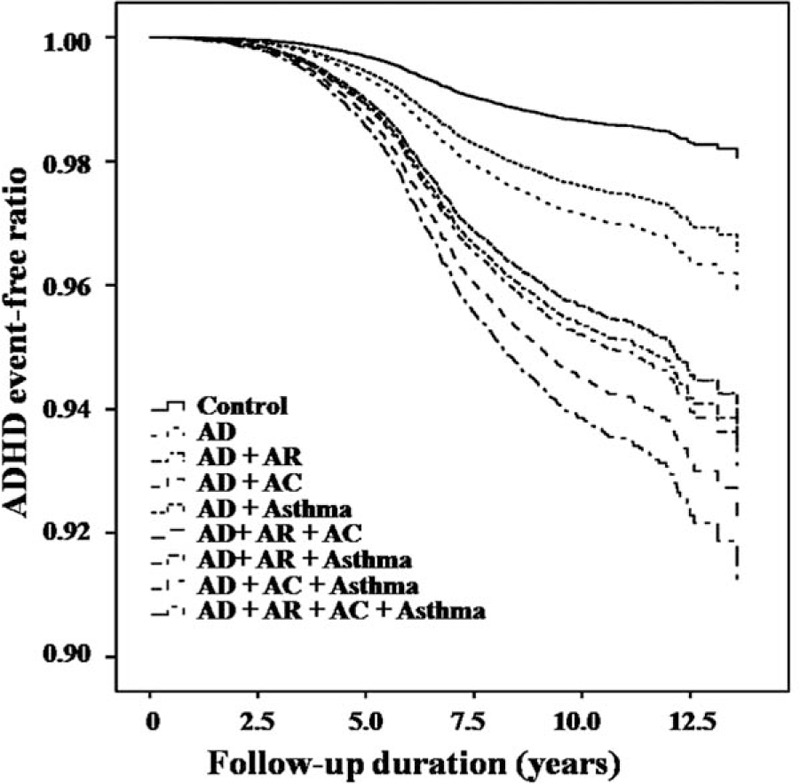
Event-free ratios for developing attention-deficit hyperactivity disorder among children with atopic dermatitis and other comorbid allergic diseases and among the control group (P < 0.001). AC = allergic conjunctivitis, AD = atopic dermatitis, ADHD = attention-deficit hyperactivity disorder, AR = allergic rhinitis.
Figure 3.
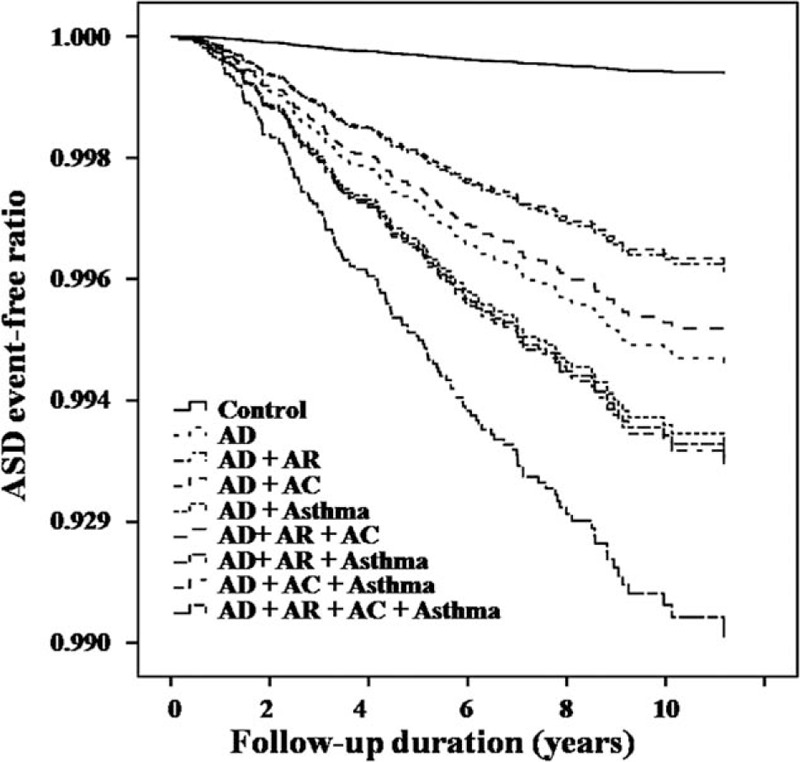
Event-free ratios for developing autistic spectrum disorder among children with atopic dermatitis and other comorbid allergic diseases, and among the control group (P < 0.001). AC = allergic conjunctivitis, AD = atopic dermatitis, AR = allergic rhinitis, ASD = autism spectrum disorder.
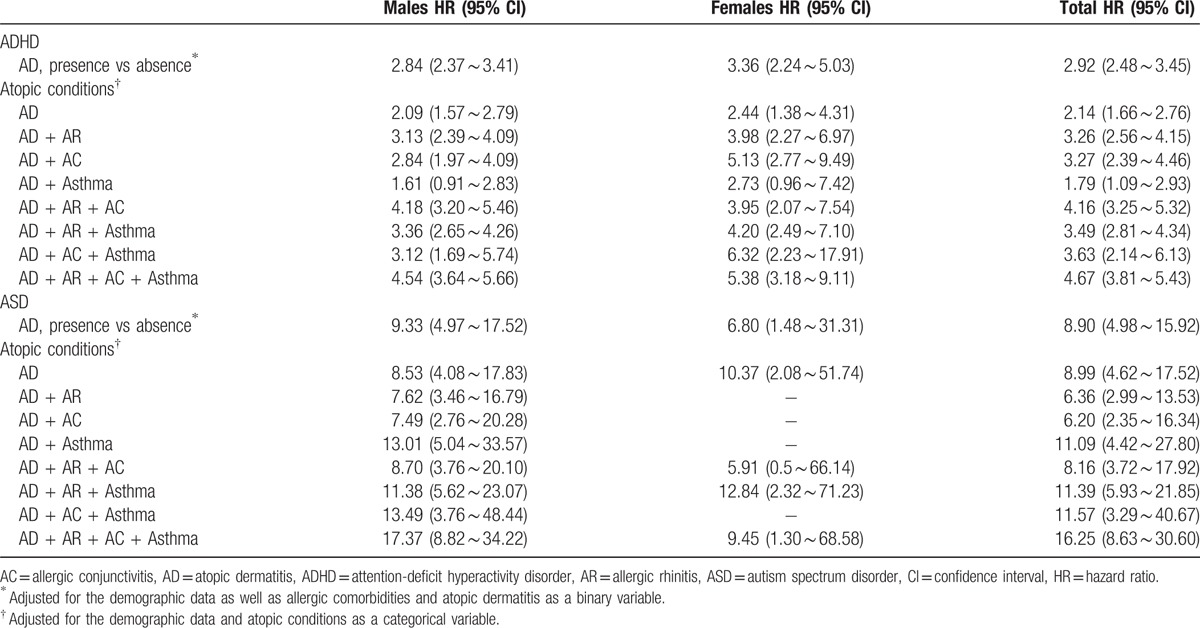
The multivariate Cox regression analyses revealed that the children who formed the AD cohort had a significantly elevated risk of developing ADHD (HR: 2.92, 95% CI: 2.48–3.45) or ASD (HR: 8.90, 95% CI: 4.98–15.92) compared with the control cohort after adjustment for the demographic data and the allergic comorbidities. Furthermore, the HR of each disease combination for ASD was higher than that of ADHD. Children who had all 4 allergic diseases had the greatest risk of having ADHD (HR: 4.67, 95% CI: 3.81–5.43) or ASD (HR: 16.25, 95% CI: 8.63–30.60) (Table 3).
Table 3.
Risk of developing ADHD and ASD among children with AD and the control group.
4. Discussion
Our results support the study hypotheses that AD occurring during early toddlerhood increases the risk of developing ADHD or ASD in later childhood. AD is an independent risk factor. The presence of other allergic comorbidities further increases the risk of ADHD or ASD.
The relationship between AD and ADHD had been studied over the past decades. Although the results have been inconsistent, most studies have pointed to a positive association between AD and ADHD.[1,9,18,25,26,31] In an early study published in 1993, McGee et al[18] reported no significant association between ADHD behavior and a history of allergic disorders and that a diagnosis of ADHD was also not related to a positive skin test or serum IgE levels. In addition, Shyru et al[20] showed that bronchial asthma and allergic rhinitis, but not AD, were the risk factors for ADHD. However, the meta-analyses by Schmitt et al[1,26,33] in 2010 and 2013 demonstrated that most investigations indicate a positive association between AD and ADHD. The comment by Gee et al[26] outlined that AD and asthma but not allergic rhinitis are positively associated with ADHD or ADHD symptoms among children and adults. Furthermore, published reports in 2013 and later all have pointed towards a positive association between AD and ADHD (Table 1). In Taiwan, previous reports based on nationwide population-based studies have also supported the hypothesis that the risk of ADHD is increased in patients with allergic diseases,[20,27] but none of them have focused on the role of AD. The present study reports our investigation of AD diagnosed during the early life of children (< 3 years old) and follows up these individuals for 3 to 16 years. It identifies a significant longitudinal relationship between early toddlerhood AD and later childhood ADHD. Therefore, our study is in agreement with most other reports.
Previous reports on the relationship between AD and ASD have also been inconsistent, although most of them are in favor of a positive association (Table 1).[9,15,21,30] Bakkaloglu et al[15] reported that allergic features were not frequent in young autistic children. Yaghmaie et al[9] reported that patients with AD had significantly increased odds of having ASD and the severity of AD altered the strength of the association. The meta-analysis by Billeci et al,[30] who reviewed 18 studies, found that the odds ratios for individuals who had AD of developing ASD ranged from 1.52 to 7.17. Our previous study targeting all atopic diseases including AD also found an elevated risk of developing ASD. In this study, we focused on AD and demonstrated that AD is an independent risk factor for the later development of ASD.
The underlying mechanism behind the development of ADHD or ASD in patients with AD remains unclear. In both ADHD and ASD, an association between inflammatory processes and neuropathology is becoming more evident. In ADHD, some specific gene polymorphisms have been reported to have significant connections with the pathogenesis of ADHD.[44] Different proinflammatory cytokines, including interleukin (IL)-2, IL-6, interferon (INF)-gamma, IL-16, IL-10, and IL-13, have been found to be higher in ADHD patients compared to controls.[45] With ASD, investigators have noticed that AD is able to induce mast cell activation, which releases proinflammatory cytokines.[46] Significantly increased levels of IL-6 have also been found in ASD patients and such an elevation in the brains of mice has been shown to mediate autistic-like behaviors.[47,48] Croonenberqhs et al[49] have also reported that there is an increased production of proinflammatory cytokines in patients with autism. Additionally, increased levels of circulating cytokine levels have been found to be associated with damage to the blood-brain barrier and such damage may play an important role in the pathogenesis of neuropsychiatric disorders.[50] All of these investigations and findings imply that early childhood inflammatory processes may play an important role in the later development of ADHD or ASD. Although our study has demonstrated the temporal relationship between early AD and the later development of ADHD/ASD, further studies are required to elucidate a definitive causal–effect relationship.
The presence of allergic comorbidities in addition to AD itself seems to further increase the risk of ADHD or ASD. In the report of Shyru et al,[20] it was found that although AD was not an independent risk factor for the development of ADHD, a combination of different allergic diseases was able to significantly increase the risk of ADHD. Our previous study demonstrated that there is a dose–dependent relationship between suffering from more atopic comorbidities and an increased risk of developing later ADHD or ASD compared to children who have never been diagnosed with any type of allergic disease. Furthermore, the HRs of children with 4 allergic diseases (asthma, allergic rhinitis, allergic conjunctivitis, and AD) were 2.25 (2.09–3.07) for ADHD and 4.29 (2.25–8.19) for ASD.[30] The present study used children who had never been diagnosed as AD as the control group and we found that a combination of 4 allergic diseases had the highest HRs (4.67 for ADHD, and 16.25 for ASD, P < 0.001). Further investigations to clarify the pathogenic role of AD in ADHD and, specifically, ASD, are necessary.
There are some limitations in the present study. Firstly, the diagnoses of AD and other allergic diseases may be overestimated. However, the fact that we had set the identification criteria for AD cases as having at least 2 diagnoses ought to minimize this problem. Secondly, the incidence of ADHD or ASD may be underestimated because only those who have sought medical help are able to be identified as part of our study. Thirdly, the severity of AD was not taken into account during the present analysis because such information is not included in the claims data of Taiwan's NHIRD. Similarly, the role of family history, personal lifestyle, and/or environmental factors cannot be obtained from a claims data. Therefore, this study has focused on analyzing the longitudinal relationship between AD and ADHD/ASD based on diagnosis by a physician and compared the AD group to a control group who had a similar situation in terms of possibility of diagnosis.
The strength of the present study is that it is a nationwide study, so great number of patients was enrolled, the difference of local districts could be minimized, and the statistical power was strong. Furthermore, the longitudinal design of this study clarified the temporal relationship between early toddlerhood AD and later childhood ADHD or ASD. Therefore, the findings of this study possess important clinical implication.
5. Conclusion
Toddlers who suffer from AD at the age younger than 3 years are at a higher risk of developing ADHD or ASD during later childhood. Pediatricians taking care of toddlers with AD should have knowledge of this increased risk of developing ADHD or ASD later in life, especially when children have allergic rhinitis, allergic conjunctivitis, and/or asthma as comorbidities.
Acknowledgments
The authors acknowledge the statistical assistance of Tzeng-Ji Chen. This study was supported by the grants from Taipei Veterans General Hospital, Taipei, Taiwan, R.O.C. (research grant V102D-001–3 and V102D-001–1). The funder has no role on the study design, data collection and analysis, or the manuscript's preparation. Additionally, this work was based on the datasets of the National Health Insurance Research Database provided by the Bureau of National Health Insurance (BNHI), Department of Health, Executive Yuan, Taiwan. The data interpretation and conclusions do not represent those of the institutions or agencies.
Footnotes
Abbreviations: AD = atopic dermatitis, ADHD = attention-deficit hyperactivity disorder, ASD = autistic spectrum disorder, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification, IL = interleukin, INF = interferon, LHID = Longitudinal Health Insurance database, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, OR = odds ratio.
C-YL and M-HC contributed equally to this article.
The authors have no conflicts of interest to declare.
References
- 1.Schmitt J, Buske-Kirschbaum A, Roessner V. Is atopic disease a risk factor for attention-deficit/hyperactivity disorder? A systematic review. Allergy 2010; 65:1506–1524. [DOI] [PubMed] [Google Scholar]
- 2.Totri CR, Diaz L, Eichenfield LF. 2014 update on atopic dermatitis in children. Curr Opin Pediatr 2014; 26:466–471. [DOI] [PubMed] [Google Scholar]
- 3.Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. J Invest Dermatol 2011; 131:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamburro J. Dermatology for the pediatrician: advances in diagnosis and treatment of common and not-so-common skin conditions. Cleve Clin J Med 2015; 82 (11 suppl 1):S19–S23. [DOI] [PubMed] [Google Scholar]
- 5.Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics 2007; 120:e527–e534. [DOI] [PubMed] [Google Scholar]
- 6.Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol 2005; 22:192–199. [DOI] [PubMed] [Google Scholar]
- 7.Suh DC, Sung J, Gause D, et al. Economic burden of atopic manifestations in patients with atopic dermatitis—analysis of administrative claims. J Manag Care Pharm 2007; 13:778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deckert S, Schmitt J. Nonallergic comorbidities of atopic dermatitis. Hautarzt 2015; 66:103–107. [DOI] [PubMed] [Google Scholar]
- 9.Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol 2013; 131:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle CA, Boulet S, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 2011; 127:1034–1042. [DOI] [PubMed] [Google Scholar]
- 11.Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51:879–888. [DOI] [PubMed] [Google Scholar]
- 12.Knapp M, Romeo R, Beecham J. Economic cost of autism in the UK. Autism 2009; 13:317–336. [DOI] [PubMed] [Google Scholar]
- 13.Klassen AF, Miller A, Fine S. Health-related quality of life in children and adolescents who have a diagnosis of attention-deficit/hyperactivity disorder. Pediatrics 2004; 114:e541–e547. [DOI] [PubMed] [Google Scholar]
- 14.Bhat S, Acharya UR, Adeli H, et al. Autism: cause factors, early diagnosis and therapies. Rev Neurosci 2014; 25:841–850. [DOI] [PubMed] [Google Scholar]
- 15.Bakkaloglu B, Anlar B, Anlar FY, et al. Atopic features in early childhood autism. Eur J Paediatr Neurol 2008; 12:476–479. [DOI] [PubMed] [Google Scholar]
- 16.Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry 2005; 57:1215–1220. [DOI] [PubMed] [Google Scholar]
- 17.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet 2005; 366:237–248. [DOI] [PubMed] [Google Scholar]
- 18.McGee R, Stanton WR, Sears MR. Allergic disorders and attention deficit disorder in children. J Abnorm Child Psychol 1993; 21:79–88. [DOI] [PubMed] [Google Scholar]
- 19.Romanos M, Gerlach M, Warnke A, et al. Association of attention-deficit/hyperactivity disorder and atopic eczema modified by sleep disturbance in a large population-based sample. J Epidemiol Community Health 2010; 64:269–273. [DOI] [PubMed] [Google Scholar]
- 20.Shyu CS, Lin HK, Lin CH, et al. Prevalence of attention-deficit/hyperactivity disorder in patients with pediatric allergic disorders: a nationwide, population-based study. J Microbiol Immunol Infect 2012; 45:237–242. [DOI] [PubMed] [Google Scholar]
- 21.Billeci L, Tonacci A, Tartarisco G, et al. Association between atopic dermatitis and autism spectrum disorders: a systematic review. Am J Clin Dermatol 2015; 16:371–388. [DOI] [PubMed] [Google Scholar]
- 22.van der Hulst AE, Klip H, Brand PL. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol 2007; 120:565–569. [DOI] [PubMed] [Google Scholar]
- 23.Bantz SK, Zhu Z, Zheng T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol 2014; 5:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson EL. Comorbidity in atopic dermatitis. Curr Dermatol Rep 2012; 1:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catal F, Topal E, Soylu N, et al. Psychiatric disorders and symptoms severity in preschool children with atopic eczema. Allergol Immunopathol (Madr) 2015; 44:120–124. [DOI] [PubMed] [Google Scholar]
- 26.Gee SN, Bigby M. Atopic dermatitis and attention-deficit/hyperactivity disorder: is there an association? Arch Dermatol 2011; 147:967–970. [DOI] [PubMed] [Google Scholar]
- 27.Tsai JD, Chang SN, Mou CH, et al. Association between atopic diseases and attention-deficit/hyperactivity disorder in childhood: a population-based case-control study. Ann Epidemiol 2013; 23:185–188. [DOI] [PubMed] [Google Scholar]
- 28.Chen MH, Su TP, Chen YS, et al. Attention deficit hyperactivity disorder, tic disorder, and allergy: is there a link? A nationwide population-based study. J Child Psychol Psychiatry 2013; 54:545–551. [DOI] [PubMed] [Google Scholar]
- 29.Chang HY, Seo JH, Kim HY, et al. Allergic diseases in preschoolers are associated with psychological and behavioural problems. Allergy Asthma Immunol Res 2013; 5:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen MH, Su TP, Chen YS, et al. Is atopy in early childhood a risk factor for ADHD and ASD? A longitudinal study. J Psychosom Res 2014; 77:316–321. [DOI] [PubMed] [Google Scholar]
- 31.Genuneit J, Braig S, Brandt S, et al. Infant atopic eczema and subsequent attention-deficit/hyperactivity disorder—a prospective birth cohort study. Pediatr Allergy Immunol 2014; 25:51–56. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt J, Romanos M, Schmitt NM, et al. Atopic eczema and attention-deficit/hyperactivity disorder in a population-based sample of children and adolescents. JAMA 2009; 301:724–726. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt J, Apfelbacher C, Heinrich J, et al. Association of atopic eczema and attention-deficit/hyperactivity disorder—meta-analysis of epidemiologic studies. Z Kinder Jugendpsychiatr Psychother 2013; 41:35–42.quiz 42-34. [DOI] [PubMed] [Google Scholar]
- 34.Suh DI, Koh YY. Early childhood wheezing: various natural courses and their relationship to later asthma. Korean J Pediatr 2012; 55:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeng MJ, Lee YS, Tsao PC, et al. A 10-year population-based nationwide descriptive analysis of pediatric emergency care. BMC Pediatr 2014; 14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang LY, Lai CC, Chen CJ, et al. Recent trends in prescribing antibiotics for acute tonsillitis in pediatric ambulatory care in Taiwan, 2000-2009: a nationwide population-based study. J Microbiol Immunol Infect 2015; pii: S1684-1182(15)00844-0. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 37.Jeng MJ, Lee YS, Tsao PC, et al. A longitudinal study on early hospitalized airway infections and subsequent childhood asthma. PLoS One 2014; 10:e0121906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YS, Jeng MJ, Tsao PC, et al. Prognosis and risk factors for congenital airway anomalies in children with congenital heart disease: a nationwide population-based study in Taiwan. PLoS One 2015; 10:e0137437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen CC, Tsai SJ, Perng CL, et al. Risk of Parkinson disease after depression: a nationwide population-based study. Neurology 2013; 81:1538–1544. [DOI] [PubMed] [Google Scholar]
- 40.Chang JW, Jeng MJ, Yang LY, et al. The epidemiology and prognostic factors of mortality in critically ill children with acute kidney injury in Taiwan. Kidney Int 2015; 87:632–639. [DOI] [PubMed] [Google Scholar]
- 41.Chen MH, Su TP, Chen YS, et al. Asthma and attention-deficit/hyperactivity disorder: a nationwide population-based prospective cohort study. J Child Psychol Psychiatry 2013; 54:1208–1214. [DOI] [PubMed] [Google Scholar]
- 42.Knudson CJ, Varga SM. The relationship between respiratory syncytial virus and asthma. Vet Pathol 2015; 52:97–106. [DOI] [PubMed] [Google Scholar]
- 43.Li CT, Bai YM, Huang YL, et al. Association between antidepressant resistance in unipolar depression and subsequent bipolar disorder: cohort study. Br J Psychiatry 2012; 200:45–51. [DOI] [PubMed] [Google Scholar]
- 44.Drtilkova I, Sery O, Theiner P, et al. Clinical and molecular-genetic markers of ADHD in children. Neuro Endocrinol Lett 2008; 29:320–327. [PubMed] [Google Scholar]
- 45.Oades RD, Dauvermann MR, Schimmelmann BG, et al. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: S100B, cytokines and kynurenine metabolism—effects of medication. Behav Brain Funct 2010; 6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angelidou A, Alysandratos KD, Asadi S, et al. Brief report: “allergic symptoms” in children with Autism Spectrum Disorders. More than meets the eye? J Autism Dev Disord 2011; 41:1579–1585. [DOI] [PubMed] [Google Scholar]
- 47.Wei H, Zou H, Sheikh AM, et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation 2011; 8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei H, Chadman KK, McCloskey DP, et al. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim Biophys Acta 2012; 1822:831–842. [DOI] [PubMed] [Google Scholar]
- 49.Croonenberghs J, Bosmans E, Deboutte D, et al. Activation of the inflammatory response system in autism. Neuropsychobiology 2002; 45:1–6. [DOI] [PubMed] [Google Scholar]
- 50.Yarlagadda A, Alfson E, Clayton AH. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont) 2009; 6:18–22. [PMC free article] [PubMed] [Google Scholar]


