Abstract
Background:
Depression, a prevalent psychiatric disorder, is associated with abnormality in the prefrontal cortex, particularly the left dorsolateral prefrontal cortex. In this study, we report on a patient with severe depression who showed injury of the dorsolateral prefronto-thalamic tract following mild traumatic brain injury, which was demonstrated by diffusion tensor tractography (DTT).
Methods and results:
A 63-year-old female patient suffered an in-car accident. The patient lost consciousness for approximately 10 minutes and experienced posttraumatic amnesia approximately 30 minutes from the time of the accident. Her Glasgow Coma Scale score was 15. No specific lesion was observed on the conventional brain magnetic resonance imaging. Since the onset of head trauma, she had shown continuous depression and on 32 month evaluation, she exhibited severe depression (Beck Depression Inventory-II: 42 [full score: 63 score] and Patient Health Questionnaire-9: 24 [full score: 27 score]).
Results:
On 32-month DTT, partical tearing of the dorsolateral prefronto-thalamic tract was observed in the right hemisphere and thinning in the left hemisphere.
Conclusion:
Injury of the dorsolateral prefronto-thalamic tract was demonstrated in a patient with depression following mild traumatic brain injury, using DTT. We believe that injury of the dorsolateral prefronto-thalamic tract might be a pathogenetic mechanism of depression in patients with brain injury.
Keywords: depression, diffusion tensor tractography, dorsolateral prefrontal cortex, mild traumatic brain injury, prefronto-thalamic tract
1. Introduction
Depression, which is caused by various factors including trauma, stress, and social factors, is a prevalent psychiatric disorder.[1–3] In particular, among these etiological factors, traumatic brain injury (TBI) is a frequent cause of depression and over half of patients (53%) with TBI are known to suffer depression.[4] Regarding the pathogenic mechanism of depression, association of depression with abnormality in various brain regions, including the cingulate cortex, amygdala, hippocampus, thalamus, prefrontal cortex (PFC), etc., has been reported.[2,5–7] Among these brain regions, the PFC, particularly, the medial and dorsolateral PFC is considered to have a crucial role in development of depression.[5,7] As a result, many studies have demonstrated abnormalities of the PFC in patients with depression using various brain imaging techniques including positron emission tomography, diffusion tensor imaging (DTI), and voxel-based morphometry.[7–14]
TBI is classified according to mild, moderate, and severe, based on the severity, and 70% to 90% of all cases of TBI are classified as mild TBI.[15] Diffusion tensor tractography (DTT), derived from DTI, has an advantage in estimation and detection of injury of neural tracts by measuring the diffusion of water molecules in white matter of patients with mild TBI.[16] Many studies have reported on injury of neural tracts, including the corticospinal tract, spinothalamic tract, fornix, cingulum, etc., in patients with mild TBI.[17–19] However, no study on injury of the dorsolateral prefronto-thalamic (mediodorsal nucleus) tract using DTT in patients with mild TBI has been reported so far.
In the current study, we report on a patient with severe depression who showed injury of the dorsolateral prefronto-thalamic tract following mild TBI, which was demonstrated by DTT.
2. Case report
A 63-year-old female patient suffered an in-car accident: while sitting in the seat next to the driver of a sedan, her car was struck by another sedan from behind while stopped at an intersection; her head then hit the front window during flexion-hyperextension injury. The patient lost consciousness for approximately 10 minutes and experienced posttraumatic amnesia approximately 30 minutes from the time of the accident. Her Glasgow Coma Scale score was 15. Since the onset of head trauma, she had shown continuous depression and on 32 month evaluation, she exhibited severe depression (Beck Depression Inventory-II: 42 [full score: 63 score] and Patient Health Questionnaire-9: 24 [full score: 27 score]).[20,21] Her cognitive function was as follows: Wechsler Adult Intelligence Scale: 98, and the Memory Assessment Scale (global memory: 99 (47%ile), short-term memory: 91 (28%ile), verbal memory: 89 (23%ile), and visual memory: 110 (75%ile).[22,23] No specific lesion was observed on the conventional brain magnetic resonance imaging (MRI) performed at 32 months after onset (Fig. 1A). Ten age- and sex-matched normal control subjects (mean age: 62.3 ± 3.1 years, range: 58–67 years) with no history of neurological disease were recruited for this study. The patient and all normal control subjects provided signed, informed consent, and Yeungnam University hospital institutional review board approved the study protocol.
Figure 1.

A, Brain CT images (upper row) at onset and T2-weighted brain MR images (lower row) at 32 months after onset show no abnormality. B, Regions of interest for the prefronto-thalamic tracts. C, Results of the prefronto-thalamic tracts on 32-month diffusion tensor tractography, the dorsolateral prefronto-thalamic tract (red color) shows partial tearing (green arrow) in the right hemisphere and thinning (sky-blue arrows) in the left hemisphere compared with a normal subject (60-year-old female). CT = computed tomography, DLPFC = dorsolateral prefrontal cortex, MD = mediodorsal nucleus, OFC = orbitofrontal cortex, ROI = region of interest, VLPFC = ventrolateral prefrontal cortex.
DTI data were acquired at 32 months after the onset using a 6-channel head coil on a 1.5T Philips Gyroscan Intera (Philips Ltd, Best, The Netherlands) with 32 diffusion gradients. Imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed to matrix = 192 × 192; field of view = 240 × 240 mm2; repetition time = 10,398 ms; echo time = 72 ms; parallel imaging reduction factor = 2; echo-planar imaging factor = 59; b = 1000 s/mm2; and a slice thickness of 2.5 mm. Head motion effect and image distortion due to eddy current were corrected by affine multiscale 2-dimensional registration. Fiber tracking was performed using probabilistic tractography, and applied in the default tractography option in the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Diffusion Software (5000 streamline samples, 0.5 mm step lengths, curvature thresholds = 0.2). This fiber-tracking method calculated and generated 5000 streamline samples from seed regions of interest (ROI) with reflection of both dominant and nondominant orientation of diffusion in each voxel. For reconstruction of the prefronto-thalamic tracts,[24,25] a seed ROI was placed on the known anatomical location of the mediodorsal nucleus of the thalamus on the coronal image of the b0 map and each target ROI was as follows (Fig. 1B): dorsolateral PFC as Brodmann areas (BAs) 8, 9, and 46 on the coronal image of the b0 map, ventrolateral PFC as BAs 44, 45, and 47 on the coronal image of the b0 map, and orbitofrontal cortex as BAs 47/12, 10, 11, and 13 on the axial image of the b0 map. The prefronto-thalamic tracts were determined by selection of fibers passing through a seed and each target ROI with the threshold of 2 streamlines. Values of fractional anisotropy (FA), mean diffusivity (MD), and tract volume were measured and DTT parameter values showing a deviation of more than 2 standard deviations from normal control values were defined as significant differences.
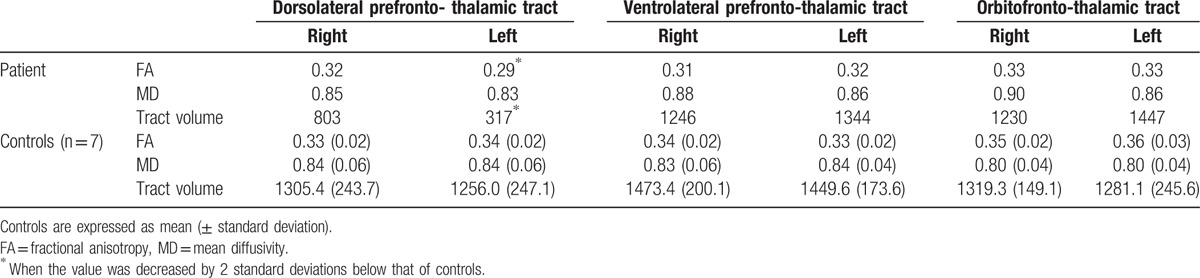
Results of the prefronto-thalamic tracts on 32-month DTT, the ventrolateral prefronto-, and orbitofronto-thalamic tracts were well reconstructed in both hemispheres. In contrast, partial tearing of the dorsolateral prefronto-thalamic tract was observed in the right hemisphere and thinning in the left hemisphere (Fig. 1C). Regarding DTT parameters, results of DTT parameters are summarized in Table 1. In the ventrolateral prefronto- and orbitofronto-thalamic tracts, no significant differences in FA, MD, and tract volume of both hemispheres were observed compared with those of normal control subjects, while in the dorsolateral prefronto-thalamic tract, FA in the left hemisphere and tract volume in both hemispheres were significantly lower compared with those of normal control subjects, respectively.
Table 1.
Results of diffusion tensor tractography parameters.
3. Discussion
In the current study, using DTT, we evaluated the prefronto-thalamic tract in a patient who showed severe depression following mild TBI using DTT and found injury of the dorsolateral prefronto-thalamic tract in both hemispheres (left side showed more severe injury). In the field of DTI, FA represents the white matter organization is influenced by axonal myelination, density, and the degree of directionality and tract volume reflects the total number of voxel in a neural tract. Therefore, decreased FA value in the left hemisphere and tract volume in both hemispheres indicated injury of the dorsolateral prefronto-thalamic tracts. Depression is functionally considered the state of disruption of mood regulation or executive function, which is controlled by the dorsolateral PFC.[14,26] Thus, although other brain regions are also involved in depression, it appears that the depression observed in this patient was ascribed, at in least part, to injury of the dorsolateral prefronto-thalamic tract (particularly the left side) by traumatic axonal injury because no definite brain lesion was observed on conventional brain MRI. Therefore, our results suggest the necessity of evaluation of the dorsolateral prefronto-thalamic tract in patients who show depression after TBI, even when no definite brain lesion is observed on conventional brain MRI.
Many studies have reported on abnormality of the dorsolateral PFC in patients with depression using various brain imaging techniques including positron emission tomography, DTI, and voxel-based morphometry.[7–14] Using positron emission tomography, Bench et al and Dolan et al reported that regional cerebral blood flow in the left dorsolateral PFC was reduced in patients with depression.[8,9] Using DTI, Taylor et al, Yang et al, and Blood reported that FA value of the dorsolateral PFC was significantly decreased in patients with depression compared with control subjects.[10,11,14] Subsequently, using voxel-based morphometry, Chang et al, Lim et al, and Grieve et al reported that the volume of gray matter of the dorsolateral PFC was reduced in patients with depression compared with control subjects.[7,12,13] They also suggested that the dorsolateral PFC has an important central role in depression. Consequently, to the best of our knowledge, this is the first study to demonstrate injury of the dorsolateral prefronto-thalamic tract in a patient with depression following mild TBI.
In summary, using DTT, injury of the dorsolateral prefronto-thalamic tract was demonstrated in a patient with depression following mild TBI. We believe that injury of the dorsolateral prefronto-thalamic tract might be a pathogenetic mechanism of depression in patients with brain injury. Because it is based on a single case report, this study is limited. In addition, because conventional MRI is difficult to find microhemorrhage particularly after mild TBI, High-resolution brain images such as susceptibility-weighted imaging could be helpful to detect microhemorrhage. Regarding DTT, several limitations should be considered; first, the fiber-tracking technique using seed masking is operator-dependent, second, results of probabilistic DTT might be affected by false-positive and negative effects due to the presence of kissing fibers in a voxel or partial volume effect throughout the brain.[27] Third, when investigating on frontal area that is vulnerable artifacts due to geometric distortion and signal drop, DTI data should be corrected. Therefore, we suggest conduct of further studies including large numbers of patients, adopting smaller voxel size with high diffusion gradients and for overcoming limitation of DTT should be encouraged.
Footnotes
Abbreviations: BA = Brodmann area, DTI = diffusion tensor imaging, DTT = diffusion tensor tractography, PFC = prefrontal cortex, ROI = region of interest, TBI = traumatic brain injury.
SHJ and JHY contributed equally to this work and should be considered co-first authors.
This work was supported by the Medical Research Center Program (2015R1A5A2009124) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning.
The authors have no conflicts of interest to disclose.
References
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289:3095–3105. [DOI] [PubMed] [Google Scholar]
- 2.Bora E, Fornito A, Pantelis C, et al. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord 2012; 138:9–18. [DOI] [PubMed] [Google Scholar]
- 3.Liao Y, Huang X, Wu Q, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci 2013; 38:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bombardier CH, Fann JR, Temkin NR, et al. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 2010; 303:1938–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry 2000; 48:813–829. [DOI] [PubMed] [Google Scholar]
- 6.Mayberg HS. Positron emission tomography imaging in depression: a neural systems perspective. Neuroimaging Clin N Am 2003; 13:805–815. [DOI] [PubMed] [Google Scholar]
- 7.Grieve SM, Korgaonkar MS, Koslow SH, et al. Widespread reductions in gray matter volume in depression. Neuroimage Clin 2013; 3:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bench CJ, Friston KJ, Brown RG, et al. The anatomy of melancholia—focal abnormalities of cerebral blood flow in major depression. Psychol Med 1992; 22:607–615. [DOI] [PubMed] [Google Scholar]
- 9.Dolan RJ, Bench CJ, Liddle PF, et al. Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity? J Neurol Neurosurg Psychiatry 1993; 56:1290–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Q, Huang X, Hong N, et al. White matter microstructural abnormalities in late-life depression. Int Psychogeriatr 2007; 19:757–766. [DOI] [PubMed] [Google Scholar]
- 11.Blood AJ, Iosifescu DV, Makris N, et al. Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS One 2010; 5:e13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CC, Yu SC, McQuoid DR, et al. Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Res 2011; 193:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim HK, Jung WS, Ahn KJ, et al. Regional cortical thickness and subcortical volume changes are associated with cognitive impairments in the drug-naive patients with late-onset depression. Neuropsychopharmacology 2012; 37:838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor WD, MacFall JR, Payne ME, et al. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry 2004; 161:1293–1296. [DOI] [PubMed] [Google Scholar]
- 15.De Kruijk JR, Twijnstra A, Leffers P. Diagnostic criteria and differential diagnosis of mild traumatic brain injury. Brain Inj 2001; 15:99–106. [DOI] [PubMed] [Google Scholar]
- 16.Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 2012; 6:137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HD, Jang SH. Injury of the corticoreticular pathway in patients with mild traumatic brain injury: a diffusion tensor tractography study. Brain Inj 2015; 29:1219–1222. [DOI] [PubMed] [Google Scholar]
- 18.Jang SH, Kwon HG. Injury of the dentato-rubro-thalamic tract in a patient with mild traumatic brain injury. Brain Inj 2015; 29:1725–1728. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Ahn SH, Cho YW, et al. The relation between injury of the spinothalamocortical tract and central pain in chronic patients with mild traumatic brain injury. J Head Trauma Rehabil 2015; 30:E40–E46. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Steer RA, Ball R, et al. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess 1996; 67:588–597. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 23.Williams JM. MAS: Memory Assessment Scales: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 24.Klein JC, Rushworth MF, Behrens TE, et al. Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage 2010; 51:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang SH, Yeo SS. Thalamocortical connections between the mediodorsal nucleus of the thalamus and prefrontal cortex in the human brain: a diffusion tensor tractographic study. Yonsei Med J 2014; 55:709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffy JD, Campbell JJ3rd. The regional prefrontal syndromes: a theoretical and clinical overview. J Neuropsychiatry Clin Neurosci 1994; 6:379–387. [DOI] [PubMed] [Google Scholar]
- 27.Yamada K, Sakai K, Akazawa K, et al. MR tractography: a review of its clinical applications. Magn Reson Med Sci 2009; 8:165–174. [DOI] [PubMed] [Google Scholar]


