Abstract
In the present study, we investigated the potential changes in the corneal nerve terminals in non–insulin-dependent diabetes mellitus of moderate duration. The dissected corneas were subjected to a protocol of ultracentrifugation to obtain synaptosomes of sensory nerve terminals. Within these nerve varicosities, 2 major mechanisms were examined, viz., alterations of the mechanosensitive channel pannexin1 and ATP release on stimulation of these terminals. We hypothesized that altered cellular location and function of the pannexin channel may contribute to altered mechanosensitivity of the cornea, which in turn may affect wound healing and primary visual function of the cornea. The chief rationale for focusing on examining the pannexin channel is due to its role in mechanosensitivity, as well as its glycosylation property. Pannexin1 remains unchanged between diabetic subjects in comparison to nondiabetic controls. However, lectin immunoassay showed that pannexin1 is significantly more glycosylated in diabetic corneal synaptosomes. Membrane biotinylation assay showed that membrane localization of pannexin1 is significantly enhanced in diabetic samples. Furthermore, S-nitrosylation of the glyco-pannexin1 is significantly decreased in comparison to pannexin1 obtained from corneal varicosities of normoglycemic subjects. The diabetic corneal synaptosomes show enhanced ATP release after potassium chloride stimulation, when compared to controls. Furthermore, we have shown that S-nitrosylation of pannexin1 actually diminishes the ability of pannexin1 to release ATP. Thus, much like the peripheral nerves, the corneal nerves also show increased hypersensitivity in diabetes of chronic duration. All of these pathological changes may cumulatively alter corneal function in diabetes.
Keywords: ATP, cornea, glycosylation, mechanosensitive, synaptosomes
1. Introduction
Chronic diabetes mellitus causes a wide variety of subtle and frank pathological changes in the eye.[1–5] These changes affect organs both in the anterior chamber and in the posterior chamber of the eye. The pathological changes may occur in a highly stochastic fashion. Also, some of these changes are potentially irreversible, including impaired epithelial healing, the formation of cataracts, wet exudates, and retinal ischemia.[6–13] A characteristic neurological complication of moderate long-standing type II diabetes mellitus and with inadequate therapeutic efficiency involves neuropathy of fine unmyelinated fibers.[14–16] Patients often have complaints of increased somatic hypersensitivity, including feelings of grit or foreign body in the eye, increased eye rubbing, and increased watering. These changes often lead to numerous clinic visits, primarily because of potential refractive errors. However, these neuropathic changes involving the corneal nerves may also be a surrogate measure of progressive pathophysiologic changes due to ineffective control of circulating blood glucose.
In the present study, we wanted to perform a basic investigation of the potential changes in the corneal nerve terminals in non–insulin-dependent diabetes mellitus of moderate duration and with less than optimal medical management. The dissected corneas were subjected to a protocol of ultracentrifugation to obtain synaptosomes of sensory nerve terminals. Within these nerve varicosities, 2 major mechanisms were examined, viz., alterations of the mechanosensitive channel pannexin1 and ATP release on stimulation of these terminals. We hypothesized that altered cellular location and function of the pannexin channel may contribute to altered mechanosensitivity of the cornea, which in turn may affect wound healing and primary visual function of the cornea. The chief rationale for focusing on examining the pannexin channel is due to its role in mechanosensitivity in multiple cells, including the retina and cornea,[17–21] as well as its propensity to get biochemical modification by glycosylation on cysteine residues.[22,23]
2. Materials and methods
2.1. Human cornea samples
Explicit permission was obtained from Institutional Review Board of Chinese PLA General Hospital, China, and all experiments were performed according to Helsinki guidelines. Men between the ages of 50 and 70 years were included in the present investigation. Age-matched samples were used for control studies. A total of 60 freshly obtained whole-thickness cornea samples from the eye bank (30 controls and 30 documented type II diabetes mellitus of >3-year duration) were used for obtaining corneal synaptosome samples. Experiments were performed by pooling samples and performing the studies in triplicates for each condition. Written consent was obtained from the subjects’ families postmortem to conduct the study.
2.2. Antibodies and chemicals
All antibodies were obtained from Santa Cruz Biotech (Santa Cruz, CA), and chemicals from either Sigma-Aldrich (Shanghai, China) or Tocris Bioscience (Avonmouth, Bristol, UK).
2.3. Corneal synaptosome preparation
Corneal tissues were homogenized in a Brinkmann homogenizer. This was thereafter cold centrifuged at increasing speeds of 1000 to 10,000 g to obtain a clear supernatant and a largely epithelial pellet. The supernatant was decanted, and overlaid on a 0.3/0.8-M sucrose gradient and centrifuged at 15,000 × g for 30 minutes. A scum-like ring was formed at the interface, which was carefully aspirated. This layer tested positive for synaptophysin, neuro-beta-tubulin III, and transient receptor potential vanilloid 4 in Western blotting (data not shown), confirming that the synaptosomal layer was isolated. This was aliquoted in microfuge tubes and stored at −20°C until further experiments. For obtaining membranes of varicosities, the synaptosomal rich layer was ultracentrifuged at 100,000 × g for 1 hour in the cold in the presence of a Mg2+-supplemented isotonic buffer. Membrane varicosities were stored for further experiments related to membrane localization of pannexin channels.
2.4. Lectin immunosorbent assays to identify glyco-pannexin
Lectin immunosorbent assay is a method akin to enzyme-linked immunosorbent assays, using lectins as probes to identify glycan structures.[24] Buffer composition was 10 mM phosphate-buffered saline (PBS) containing 150 mM sodium chloride, pH 7.2, at 4°C. The wells containing the synpatosomal lysates were washed several times with PBS containing 0.05% (w/v) Tween 20 and blocked with bovine serum albumin. A total of 100 μL of biotinylated Ulex europaeus agglutinin I lectin at 0.6 μg/mL (Vector Laboratories, Burlingame, CA) in PBS was added to each well and allowed to incubate for 1 hour. A total of 100 μL of 1:3000 dilution of 1 mg/mL streptavidin conjugated to alkaline phosphatase (Vector Laboratories) was added and signal was developed by incubation for 30 minutes with 100 μL of 1 mg/mL para-nitrophenyl phosphate substrate (Sigma-Aldrich). The reaction was stopped using 50 μL of 3 M sodium bicarbonate. Negative and positive controls were used for all of the experiments.
2.5. Biotin switch assay to assess pannexin S-nitrosylation
Basal S-nitrosylation signals of panexin1 of control as well as diabetic corneal varicosities were examined by biotin switch assay, based on protocols described earlier.[25] Briefly, varicosities were incubated with the nitric oxide (NO) donors, 100 μMS-nitrosoglutathione, 50 μM diethylamine NONOate, or untreated (diabetic/control) for 15 minutes in an incubator at 37°C. The varicosities were subsequently washed with PBS, and further incubated with radioimmune precipitation assay buffer with added protease inhibitors. Protein quantity was estimated using the Bradford technique. Proteins were pelleted by acetone and centrifugation. The unbound cysteine thiols were inhibited by N-ethylmaleimide for 30 minutes. Proteins were again precipitated to decant excess N-ethylmaleimide. Thereafter, S-nitrosylated cysteines were altered of their oxidizing potential with 1 mM ascorbate and 1 mM Cu2+ and subsequently biotinylated with N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)-propionamide for 1 hour. Biotinylated proteins were immobilized with streptavidin-agarose beads. Sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed for detection of pannexin that was S-nitrosylated. Ascorbate was not reduced to assay the specificity of the reducing controls.
2.6. Cell surface biotinylation to identify cell membrane-bound pannexin
Cell surface biotinylation was performed to assess the membrane distribution of panexin1 channels in synaptosomes, and whether they were altered in diabetic varicosities, using protocols described earlier.[26] Corneal varicosities from control (normoglycemic) and diabetic patients, with and without glycosidase, were washed with cold PBS and then treated with Dulbecco's Modified Eagle Medium and 50 μM carbenoxolone (CBX) over ice for an hour. CBX toxin prevents biotin from percolating through Panx1 channels and prevents labeling intracellular proteins. The varicosities were further incubated in cold PBS containing EZ-Link sulfosuccinimidyl-6-[biotinamido]hexanoate (1 mg/mL), including CBX (50 μM). Proteins were eluted and equal amounts of protein were incubated with streptavidin-agarose beads overnight in the cold room to pull down biotinylated proteins. Membrane-bound pannexin1 was detected by Western blotting.
2.7. In vitro ATP release assay of corneal synaptosomes
Synaptosomes from control and diabetic patients were incubated in isotonic 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid media in an incubator at 37°C for an hour, and stimulated with 100 mM potassium chloride. A total of 200 μM ARL 67156 ectonucleotidase was present in the medium. Glycosidase inhibitors, as well as NO donors, were added to the medium to assess their effects on ATP release. The medium was aspirated and cold stored. A total of 30 μL of luciferin–luciferase reagent (ATP luminescence assay, Perkin Elmers, Waltham, MA) was added to a well, followed by detection of the optical density of luminescence (Luminoskan Ascent Microplate Luminometer, Thermo Scientific, Waltham, MA, USA). This was utilized to plot the standard curve for estimation of ATP concentration. The percentage changes of ATP were used to compare ATP release between nondiabetic and diabetic corneal nerve varicosities.
2.8. Statistics
Results are presented as means ± standard error. Statistical significance was determined by P < 0.05 using analyses of variance.
3. Results
3.1. Total pannexin1 levels unchanged in diabetic as well as control, nondiabetic synaptosomes
Western blotting revealed that the total pannexin levels of type 1 isoform remained unchanged between diabetic and nondiabetic controls (Fig. 1). The band intensities were estimated from 3 independent pooled samples in each group, and did not show any significant difference (data not shown). Representative images from 2 pooled samples in each group are demonstrated in Fig. 1.
Figure 1.
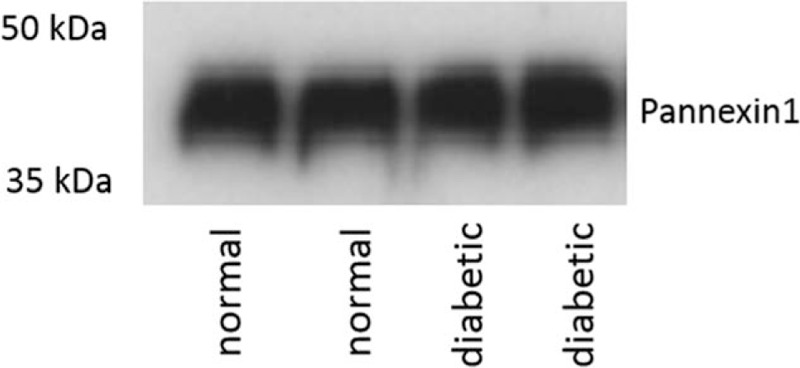
Total pannexin1 levels unchanged in diabetic as well as control, nondiabetic synaptosomes. Western blot showing unchanged total pannexin1 between diabetic and nondiabetic controls. Two representative lanes are shown for each group, which are pooled samples from 10 cornea samples in each lane.
3.2. Lectin immunoassays reveal increased glycosylation of pannexin1 in diabetic corneal synaptosomes
Quantitative lectin immunoassays revealed increased glycosylated pannexin1 levels in corneal nerves obtained from patients with long-standing diabetes, when compared to age-matched controls. When the synaptosomal samples of diabetic synaptosomes were incubated in glycosidase, these increased glycosylated pannexin1 levels decreased significantly. The distribution of these values, performed in triplicates to reveal the quantitative data, is shown in Fig. 2.
Figure 2.

Lectin immunoassays reveal increased glycosylation of pannexin1 in diabetic corneal synaptosomes. Quantitative lectin immunoassays revealed increased glycosylated pannexin1 levels in corneal nerves obtained from patients with long-standing diabetes, when compared to age-matched controls. When the synaptosomal samples of diabetic synaptosomes were incubated with glycosidase, the increased levels of glycosylated pannexin1 levels decreased significantly. The distribution of these values from pooled samples is shown. The Y-axis represents arbitrary units of normalized values with respect to controls. LISA = lectin immunosorbent assay.
3.3. S-Nitroso-pannexin1 in diabetic corneal synaptosomes
Figure 3 shows the reduction of S-nitroso-pannexin1 in synaptosomes obtained from diabetic nerve terminal in the biotin switch assay. Normal control varicosities showed significantly increased S-nitrosylation signals, which were also enhanced by external stimulation with the NO donors. Synaptosomal treatment with the reducing agent dithiothreitol (1 mM) immediately after S-nitrosoglutathione or diethylamine NONOate treatment to reduce S-nitrosothiols inhibited biotinylation. In addition, S-nitrosylation of Panx1 was not seen when ascorbic acid was not added to prevent the availability of free S-nitrosylated cysteines for subsequent biotinylation.
Figure 3.
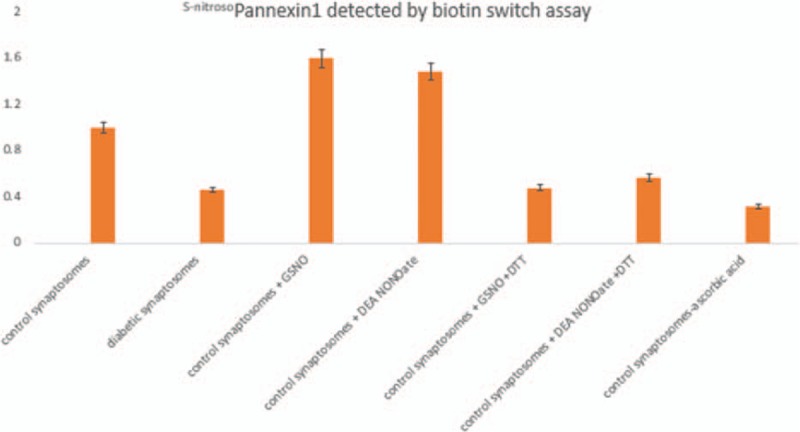
S-Nitroso-pannexin1 in diabetic corneal synaptosomes. Biotin switch assay revealed diminution of S-nitroso-pannexin1 in synaptosomes obtained from diabetic nerve terminals. Normal control varicosities showed significantly increased S-nitrosylation signals, which were also enhanced by external stimulation with the nitric oxide donors GSNO and DEA NONOate. Synaptosomal treatment with the reducing agent DTT (1 mM) immediately after GSNO or DEA NONOate treatment to reduce S-nitrosothiols prevented the biotinylation reaction. In addition, S-nitrosylation of Panx1 was not seen when ascorbic acid was not added to prevent the availability of free S-nitrosylated cysteines for subsequent biotinylation. DEA NONOate = diethylamine NONOate, DTT = dithiothreitol, GSNO = S-nitrosoglutathione.
3.4. Increase in membrane pannexin1 in diabetes
Membrane biotinylation assay revealed signals of enhanced intensity in diabetic synaptosomes, indicating that glycosylated pannexin1 is located at the membranes in comparison to controls (Fig. 4A). When diabetic synaptosomes were incubated with a glycosidase, these membrane-bound signals were decreased. Representative blots of the western blotting signals of the membrane-bound pannexins are also shown in Fig. 4B.
Figure 4.
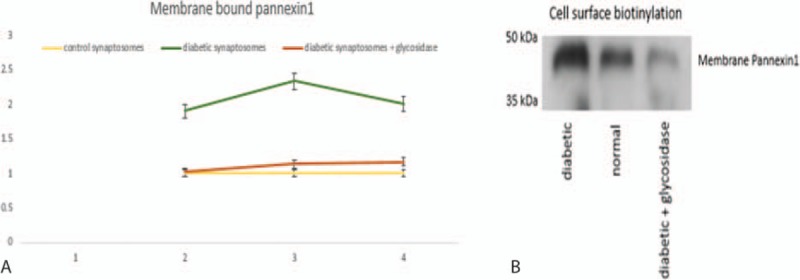
Increase in pannexin1 in corneal synaptosomal membrane in diabetes. Membrane biotinylation assay revealed signals of enhanced intensity in diabetic synaptosomes, indicating that glycosylated pannexin1 is located at the membranes in comparison to controls (A). When diabetic synaptosomes were incubated with a glycosidase, these membrane-bound signals were decreased, as shown in “B.”
3.5. Increase in ATP release in diabetic corneal synaptosomes
Potassium stimulation enhanced ATP release of diabetic synaptosomes, in comparison to controls (Fig. 5). NO-donor–incubated control synaptosomes, as well as glycosidase-treated diabetic synaptosomes, showed minimum ATP release on nerve stimulation by potassium chloride.
Figure 5.
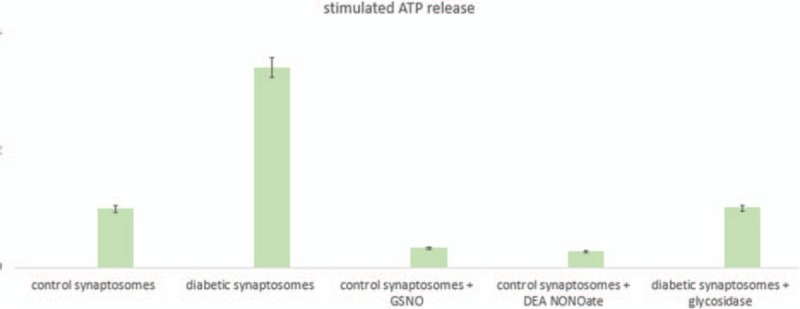
Increase in ATP release in diabetic corneal synaptosomes. Potassium chloride stimulation enhanced ATP release of diabetic synaptosomes, in comparison to controls. NO-donor–incubated control synaptosomes, as well as glycosidase-treated diabetic synaptosomes, showed minimum ATP release on nerve stimulation by potassium chloride. DEA NONOate = diethylamine NONOate, GSNO = S-nitrosoglutathione, NO = nitric oxide.
4. Discussion
The results of the present study reveal unique mechanistic changes in the mechanosensitive pannexin1 channel in human corneal synaptosomes in subjects with long-standing type II diabetes mellitus. Namely, using a combination of relevant molecular techniques to track intracytologic trafficking, evidence has been provided that although the levels of pannexin1 remain unchanged between diabetic subjects in comparison to nondiabetic controls, pannexin1 is significantly more glycosylated in diabetic corneal synaptosomes, and its membrane localization is significantly enhanced in comparison to controls. Furthermore, we have demonstrated that S-nitrosylation of the glyco-pannexin1 is significantly decreased in comparison to pannexin1 obtained from corneal varicosities of normoglycemic subjects. The S-nitrosylation of pannexin1 may be a significant mechanism in gating the channel for its mechanochemical activity.
Namely, pannexin1 plays a significant role in ATP release in response to external mechanical cues.[19–21] We have also demonstrated in the present study that diabetic corneal synaptosomes show enhanced ATP release, when compared to controls. Furthermore, we have shown that S-nitrosylation of pannexin1 actually diminishes the ability of pannexin1 to release ATP. Thus, much like the peripheral nerves, the corneal nerves also show increased hypersensitivity in diabetes of chronic duration. This altered mechanosensitivity of the corneal nerves may affect visual refraction of the cornea and epithelial healing, as well as affect aqueous humor flow, functions that are directly affected by mechanosensitive pannexin channels in the cornea.[6–8,27–30] The reduction in S-nitrosylation may have resulted from alterations of neuronal nitric oxide synthase in diabetes. All of these pathological changes may cumulatively alter corneal function in diabetes. These functional aspects remain our future scope of investigation.
Footnotes
Abbreviations: CBX = carbenoxolone, NO = nitric oxide, PBS = phosphate-buffered saline.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Calvo-Maroto AM, Perez-Cambrodí RJ, Albarán-Diego C, et al. Optical quality of the diabetic eye: a review. Eye (Lond) 2014;28:1271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abcouwer SF, Gardner TW. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann N Y Acad Sci 2014;1311:174–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reynolds AL, Kent D, Kennedy BN. Current and emerging therapies for ocular neovascularisation. Adv Exp Med Biol 2014;801:797–804. [DOI] [PubMed] [Google Scholar]
- [4].Santiago JG, Walia S, Sun JK, et al. Influence of diabetes and diabetes type on anatomic and visual outcomes following central rein vein occlusion. Eye (Lond) 2014;28:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lutty GA. Effects of diabetes on the eye. Invest Ophthalmol Vis Sci 2013;54:ORSF81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhivov A, Winter K, Hovakimyan M, et al. Imaging and quantification of subbasal nerve plexus in healthy volunteers and diabetic patients with or without retinopathy. PLoS One 2013;8:e52157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].He J, Bazan HE. Mapping the nerve architecture of diabetic human corneas. Ophthalmology 2012;119:956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Midena E, Brugin E, Ghirlando A, et al. Corneal diabetic neuropathy: a confocal microscopy study. J Refract Surg 2006;22:S1047–52. [DOI] [PubMed] [Google Scholar]
- [9].Chikama T, Wakuta M, Liu Y, et al. Deviated mechanism of wound healing in diabetic corneas. Cornea 2007;26:S75–81. [DOI] [PubMed] [Google Scholar]
- [10].Kabosova A, Kramerov AA, Aoki AM, et al. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp Eye Res 2003;77:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res 2007;2007:95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].D’Amore PA. Mechanisms of retinal and choroidal neovascularization. Invest Ophthalmol Vis Sci 1994;35:3974–9. [PubMed] [Google Scholar]
- [13].Nukada H. Ischemia and diabetic neuropathy. Handb Clin Neurol 2014;126:469–87. [DOI] [PubMed] [Google Scholar]
- [14].Verrotti A, Prezioso G, Scattoni R, et al. Autonomic neuropathy in diabetes mellitus. Front Endocrinol (Lausanne) 2014;5:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zochodne DW. Diabetes and the plasticity of sensory neurons. Neurosci Lett 2014;596:60–5. [DOI] [PubMed] [Google Scholar]
- [16].Schmidt RE. Autonomic neuropathy in experimental models of diabetes mellitus. Handb Clin Neurol 2014;126:579–602. [DOI] [PubMed] [Google Scholar]
- [17].D’hondt C, Himpens B, Bultynck G. Mechanical stimulation-induced calcium wave propagation in cell monolayers: the example of bovine corneal endothelial cells. J Vis Exp 2013;77:e50443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Križaj D, Ryskamp DA, Tian N, et al. From mechanosensitivity to inflammatory responses: new players in the pathology of glaucoma. Curr Eye Res 2014;39:105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xia J, Lim JC, Lu W, et al. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J Physiol 2012;590(pt 10):2285–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li A, Banerjee J, Leung CT, et al. Mechanisms of ATP release, the enabling step in purinergic dynamics. Cell Physiol Biochem 2011;28:1135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li A, Leung CT, Peterson-Yantorno K, et al. Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow. J Cell Physiol 2012;227:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Penuela S, Simek J, Thompson RJ. Regulation of pannexin channels by post-translational modifications. FEBS Lett 2014;588:1411–5. [DOI] [PubMed] [Google Scholar]
- [23].Penuela S, Bhalla R, Nag K, et al. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell 2009;20:4313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu AM, Lisowska E, Duk M, et al. Lectins as tools in glycoconjugate research. Glycoconj J 2009;26:899–913. [DOI] [PubMed] [Google Scholar]
- [25].Burgoyne JR, Eaton P. A rapid approach for the detection, quantification, and discovery of novel sulfenic acid or S-nitrosothiol modified proteins using a biotin-switch method. Methods Enzymol 2010;473:281–303. [DOI] [PubMed] [Google Scholar]
- [26].Elia G. Biotinylation reagents for the study of cell surface proteins. Proteomics 2008;8:4012–24. [DOI] [PubMed] [Google Scholar]
- [27].Sanderson J, Dartt DA, Trinkaus-Randall V, et al. Purines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Müller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp Eye Res 2014;127:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mayo C, Ren R, Rich C, et al. Regulation by P2X7: epithelial migration and stromal organization in the cornea. Invest Ophthalmol Vis Sci 2008;49:4384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Parra A, Gonzalez-Gonzalez O, Gallar J, et al. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain 2014;155:1481–91. [DOI] [PubMed] [Google Scholar]
- [30].Redbrake C, Becker J, Salla S, et al. The influence of the cause of death and age on human corneal metabolism. Invest Ophthalmol Vis Sci 1994;35:3553–6. [PubMed] [Google Scholar]


