Abstract
The maximum rate of pressure rise (dP/dtmax) in radial artery has been proposed as a noninvasive surrogate of aortic dp/dtmax, reflecting left ventricular (LV) contractility in children. The aim of this study was to investigate relationship between aortic and radial dp/dtmax at weaning from cardiopulmonary bypass (CPB) and usefulness of these indices for estimating postoperative outcomes in pediatric congenital heart surgery.
Aortic and radial arterial pressure waveforms were analyzed simultaneously during weaning from CPB in 29 congenital heart surgery. The maximum first derivatives of aortic and radial arterial waveforms were calculated and averaged from 3 consecutive respiratory cycles. We obtained the maximum vasoactive inotropic score during the first 36 postoperative hours, LV ejection fraction, and fractional shortening on transthoracic echocardiography performed within postoperative day 7.
A significant difference between aortic and radial dP/dtmax was observed (mean difference 356 mm Hg/s, 44% of averages), and radial dP/dtmax was weakly correlated with aortic dP/dtmax (r =0.373, P = 0.047). Aortic dP/dtmax was significantly associated with the maximum vasoactive inotropic score (P < 0.001), postoperative LV ejection fraction (P = 0.018), and fractional shortening (P = 0.015); however, radial dP/dtmax was not. On Receiver operating characteristic analysis, aortic dP/dtmax had a greater area under the curve than radial dP/dtmax in predicting higher vasoactive inotropic score (0.827 vs 0.673).
Immediately after CPB in pediatric congenital heart surgery, radial dP/dtmax may not replace aortic dP/dtmax because of a discrepancy between central and peripheral arterial waveforms. In this critical period, aortic dP/dtmax can be useful to estimate postoperative ventricular function rather than peripherally derived dP/dtmax.
Keywords: aortic maximum rate of pressure rise, congenital heart surgery, postoperative ventricular function, radial maximum rate of pressure rise
1. Introduction
The maximum rate of the left ventricular pressure rise (LV dP/dtmax) has been known to reflect the LV contractility, which is not influenced by wall motion abnormalities, afterload or variations in ventricular anatomy and morphology.[1–4] However, the measurement of LV dP/dtmax is undoubtedly invasive monitoring, which is required intraventricular catheterization, which is limited as a bedside clinical feasibility particularly in small children.
As a less invasive index, it has been suggested that the maximum rate of aortic pressure rise (Ao dP/dtmax) can accurately predict the LV dP/dtmax in pediatric patients with congenital and acquired cardiac disease.[5] Recently, the dp/dtmax tonometrically measured at the brachial and radial arteries has also been found to show excellent correlation with the LV dP/dtmax in children underwent cardiac catheterization for various cardiovascular abnormalities.[6] Therefore, the peripheral dP/dtmax derived from pressure waveform analysis seems to be useful to assess ventricular contractility as a noninvasive intraoperative monitoring, particularly in pediatric cardiac surgery.
However, immediately after patients are weaned from CPB, there is a discrepancy in the pressure waveform between central and peripheral arteries because of changes in vascular characteristics.[7,8] Moreover, the relationship between the Ao dP/dtmax and peripheral arterial dP/dtmax during cardiac surgery in children remains uncertain, especially during the period of weaning from CPB. Therefore, we investigated the relationship between the aortic and radial dP/dtmax immediately after weaning from CPB in pediatric congenital heart surgery. In addition, we also evaluated whether these parameters are associated with immediate postoperative LV function.
2. Materials and methods
2.1. Study population
This retrospective analysis of prospectively collected data was approved by the Institutional Review Board of Asan Medical Center, Seoul, Korea (No 2014-0773). We investigated the electrical medical records of 34 consecutive children who underwent corrective cardiac surgery at our single tertiary care hospital between January 2013 and February 2014 and who were assigned to Risk Adjustment in Congenital Heart Surgery (RACHS-1) category 1–2.[9] All operations were performed by the same pediatric cardiac surgeon (C.-S. Park). We excluded 5 patients who did not require CPB, incomplete data collection, or poor signal quality of arterial waveforms, and thus 29 patients were available for final analysis.
2.2. General anesthesia and clinical practice
Patients arrived at the operating room lightly sedated following premedication with 0.05 to 0.075 mg/kg of intravenous midazolam. Patients received routine monitoring using 5-lead electrocardiography, pulse oximetry, noninvasive blood pressure, and capnography. In accordance with the standard protocol at our institution,[10,11] anesthesia was induced with midazolam (0.1–0.2 mg/kg), thiopental sodium (1.5–2.0 mg/kg), or ketamine (1–2 mg/kg), as appropriate. After the patient received vecuronium (0.15 mg/kg) and fentanyl (1–3 μg/kg), tracheal intubation was performed. Anesthesia was maintained with boluses of midazolam (0.1–0.2 mg/kg), rocuronium (0.5 mg/kg), and fentanyl (3–5 μg/kg) given every 30 to 40 minutes. Direct arterial catheterization (24 G; Becton Dickinson Infusion Therapy Systems Inc., Sandy, Utah) of the radial artery was performed to continuously monitor the arterial pressure. The central venous pressure was also monitored using ultrasound-guided placement of a pediatric multi-lumen central venous catheter (4–5.5 Fr; Arrow International Inc., Reading, PA). Cerebral oxygen saturation was also continuously monitored using near-infrared spectroscopy (INVOS 5100, Somanetics, Troy, MI). CPB was started with aortic cannulation (Medtronic, Minneapolis, MN) and bicaval venous drainage, and systemic cooling (25–28°C) was obtained uniformly under 2.4 L/min/m2 of the perfusion index. Modified ultrafiltration was used for all patients immediately after separation from CPB. To achieve hemodynamic stability, dopamine (3–10 μg/kg/min), milrinone (0.375–0.75 μg/kg/min), or epinephrine (0.02–0.1 μg/kg/min) were infused continuously, as appropriate. After CPB, transfusion of filtered red blood cells was used to maintain the hematocrit greater than 30%. Before aortic decannulation, protamine (1 mg/100 units of heparin) was administered to reverse heparin.
2.3. Hemodynamic variables and measurements of the Ao dP/dtmax and radial dP/dtmax
Baseline systolic and diastolic noninvasive blood pressure (SBP and DBP), heart rate and arterial oxygen saturation (SpO2) were recorded before anesthesia. Following anesthetic induction, we also obtained pre-CPB hemodynamic variables; radial SBP and DBP, heart rate, SpO2, radial dP/dtmax and cerebral oxygen saturation. After completion of CPB, a pressure monitoring line was connected into the ascending aorta cannula to continuously measure the aortic pressure. Data on the continuous arterial pressure waveform of the ascending aorta and radial artery were simultaneously collected using a personal computer interfaced with a Windaq analog/digital converter (DATAQ Instruments Inc., Akron, OH). For each patient, aortic and radial artery pressure waveforms were abstracted during the first 5-minute period immediately after cessation of CPB. The Ao dP/dtmax and Radial dP/dtmax were obtained by calculation of the maximum first derivatives of the aortic and radial arterial pressure waveforms using a signal processing software program (CODAS, DATAQ; DADiSP/Adv DSP; DSP Development, Cambridge, MA) (Fig. 1). We also recorded aortic and radial SBP and DBP, and all pressure-derived data were averaged for 3 consecutive respiratory cycles.
Figure 1.
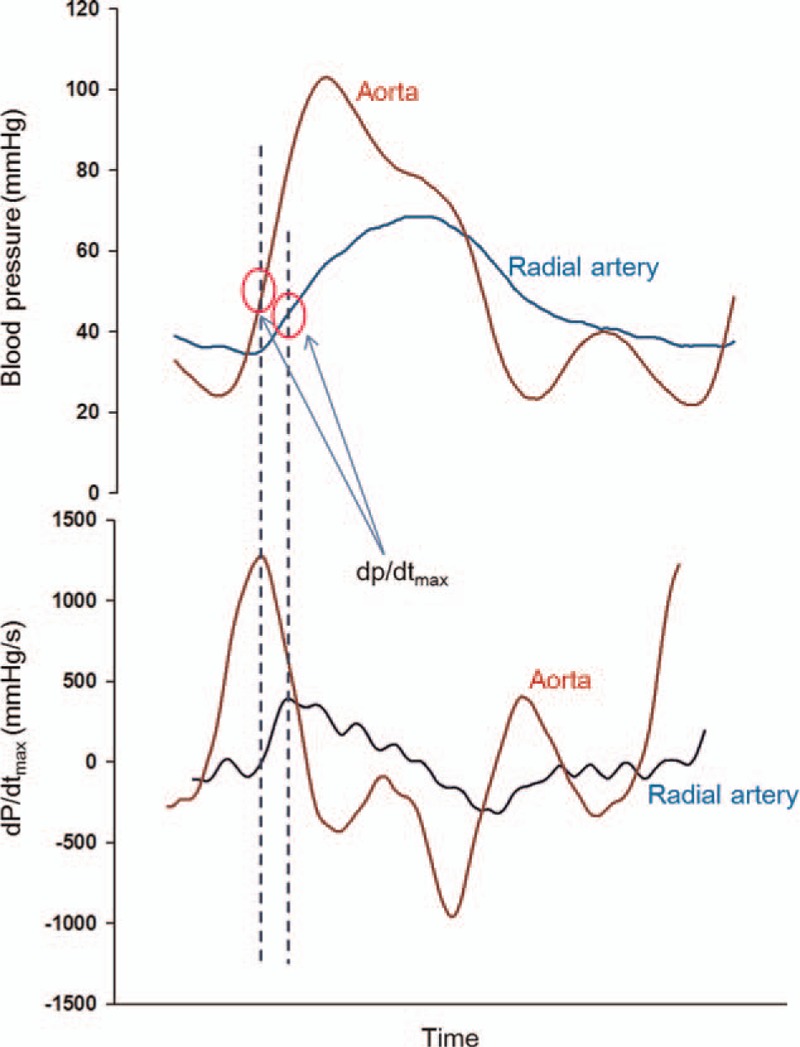
Example tracings of arterial pressure waveforms (upper panel) and the first derivatives (dP/dt) recorded at aorta and radial artery. The circle indicates the measuring point of dP/dtmax.
2.4. Assessment of early postoperative LV function and outcomes
To quantify the requirement for inotropic and vasoactive drugs, we calculated the maximum vasoactive inotropic score (VISmax) for each patient during the first 36 hours after surgery.[12,13] The inotropic score was calculated as follows: (dopamine [μg/kg/min]) + (dobutamine [μg/kg/min]) + (10000 × vasopressin [U/kg/min]) + (10 × milrinone [μg/kg/min]) + (100 × epinephrine [μg/kg/min]) + (100 × norepinephrine [μg/kg/min]).[12] Transthoracic echocardiography was performed in all patients within 7 postoperative days before discharge from the hospital. The 2D- echocardiographic images from the apical 4- and 2-chamber views showing the LV measurements were used with the modified Simpson's method to estimate the LV ejection fraction (EF). LV fractional shortening (FS) was measured from an M-mode trace in the parasternal long-axis view. We also obtained outcome data, including the need for inhaled nitric oxide, duration of mechanical ventilation, and length of postoperative hospital stay.
2.5. Statistical analysis
Data are expressed as mean with standard deviation or median with the interquartile range, as appropriate. The Bland–Altman method was used to assess of agreement between variables derived from aortic and radial arterial pressure waveforms. The correlation between the Ao dP/dtmax and radial dP/dtmax was tested with Pearson's correlation coefficient. We also performed Spearman's rank correlation between dP/dtmax and the early postoperative outcomes. Receiver operating characteristics (ROC) analysis was used to assess the ability of the dp/dtmax of the aorta and radial artery to predict decreased ventricular function (EF <50% and FS<24%), greater requirement of inotropic and vasoactive drugs, prolonged mechanical ventilation, or postoperative hospital stay, defined as upper quartile in distribution. We calculated assuming that the area under the ROC curve representing the ability of dP/dtmax to determine the postoperative outcome was 0.8, and that correlation coefficient between aortic and radial dP/dtmax was 0.5. It would be required to include 28 patients, based on an α significance of 0.05 and a β error of 0.2. A P-value less than or equal to 0.05 was considered significant. Analyses were performed using SPSS version 21.0 software (IBM SPSS Inc., Chicago, IL) and SigmaPlot version 12.0 (Systat Inc., San Jose, CA).
3. Results
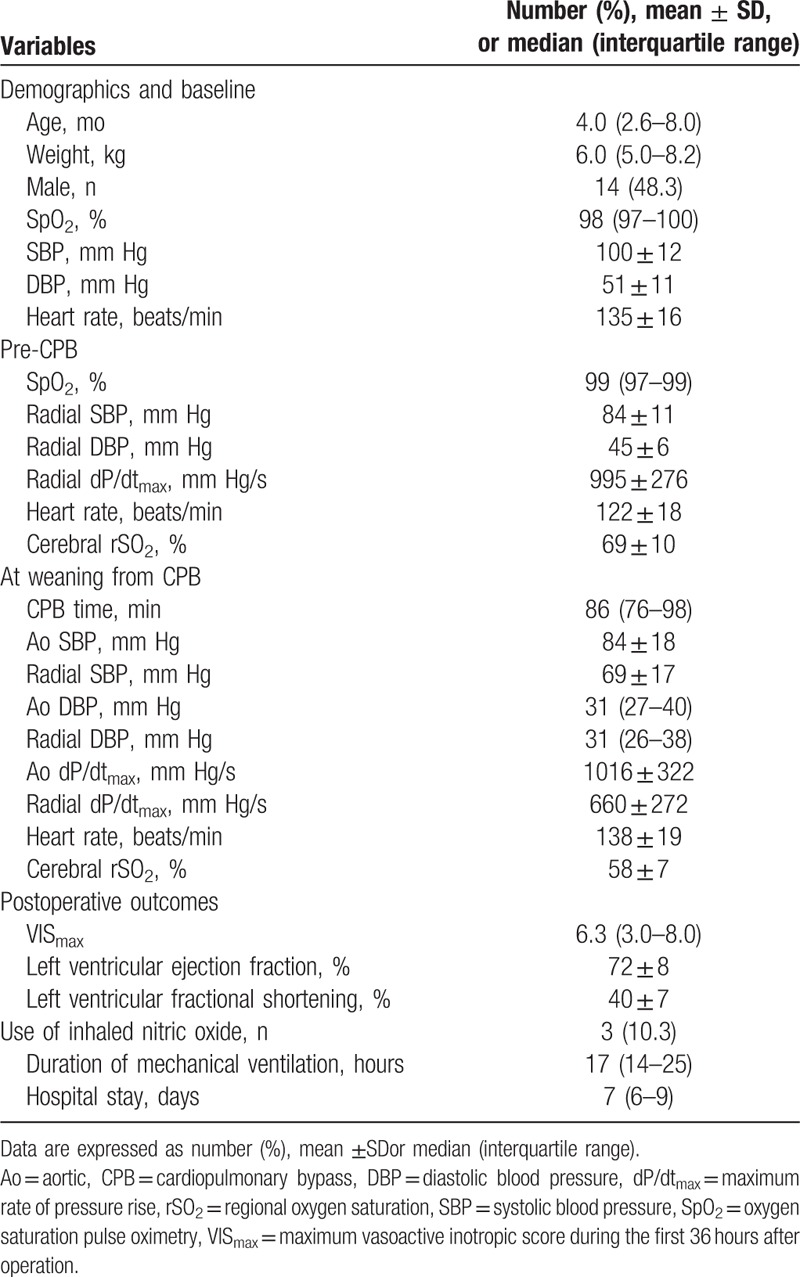
Twenty-nine patients were included in final analyses. Five patients were excluded because they had an incomplete continuous arterial waveform, such as missing data or poor signal quality. None of the 29 children in our current study had a residual shunt or died during their period of hospitalization. Eighteen of these patients had a ventricular septal defect, 8 were had an atrial septal defect, 1 had ventricular septal defect with concomitant pulmonary stenosis, and 2 had Tetralogy of Fallot. Patient demographic data and perioperative clinical variables are summarized in Table 1.
Table 1.
Patient demographic data and perioperative clinical variables.
Immediately after weaning from CPB, radial SBP showed lower value than aortic SBP (mean difference 15.6 mm Hg; 95% limits of agreement –6.9, 38.1), whereas DBP was similar between aorta and radial artery (mean difference; 95% limits of agreement –6.1, 8.1) (Table 1). The mean difference was 356 mm Hg/s (44% of averages) between Ao and radial dP/dtmax (Fig. 2). The radial dP/dtmax weakly but significantly correlated with the Ao dp/dtmax immediately after CPB weaning (r = 0.373, P = 0.047; Fig. 3).
Figure 2.
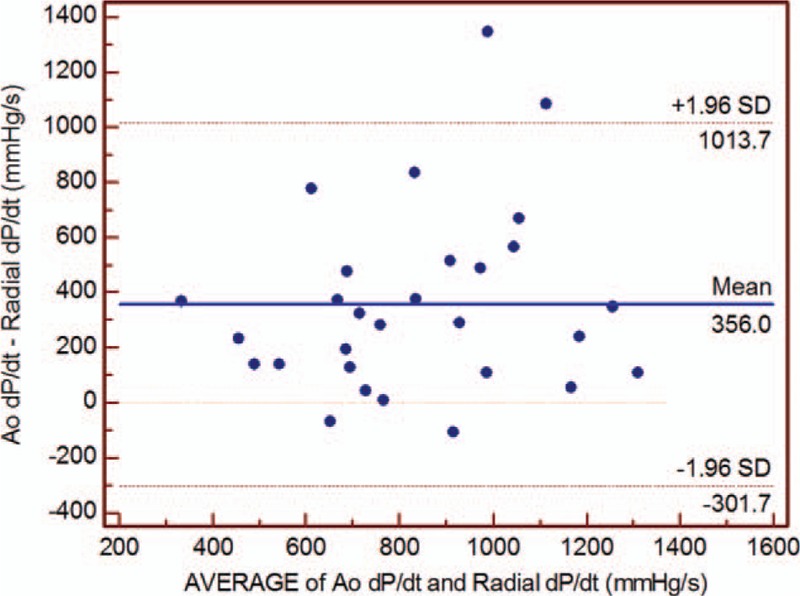
The Bland–Altman plot for the maximum pressure rise in the aorta (Ao dP/dtmax) and radial artery (radial dP/dtmax). Ao = aortic, dP/dtmax = maximum rate of pressure rise.
Figure 3.
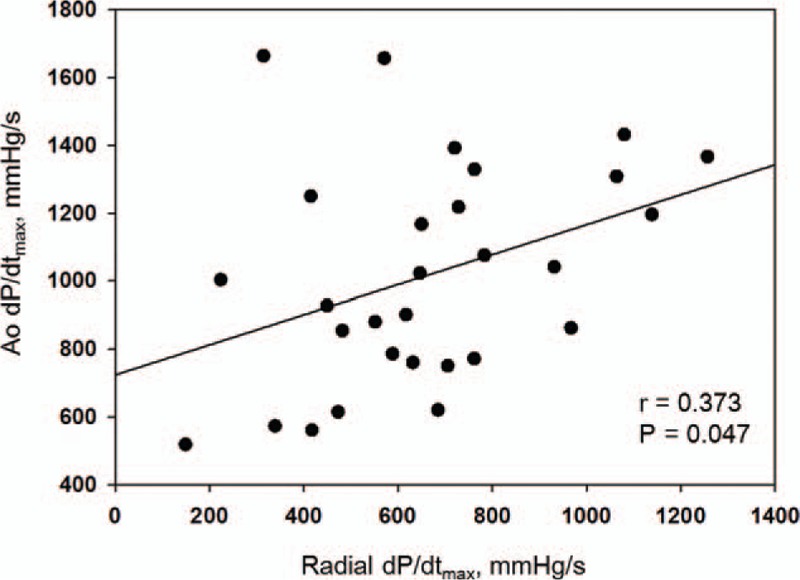
Correlation between the maximum pressure rise in the aorta (Ao dP/dtmax) and radial artery (radial dP/dtmax). Ao = aortic, dP/dtmax = maximum rate of pressure rise.
There was an inverse correlation between the Ao dP/dtmax and requirement for postoperative vasoactive inotropic use (r = −0.625, P < 0.001). The radial dP/dtmax was negatively associated with the postoperative VISmax, but statistically insignificant (r = −0.366, P = 0.051). The Ao dP/dtmax also significantly correlated with the postoperative LVEF (r = 0.453, P = 0.018) and LVFS (r = 0.470, P = 0.015), unlike the radial dP/dtmax (P = 0.118 and P = 0.126, respectively). The duration of mechanical ventilation, length of postoperative hospital stay, and need for postoperative inhaled nitric oxide did not significantly correlate with the Ao dP/dtmax or radial dP/dtmax. In ROC curve analysis, the area under the curve (AUC) of the Ao dP/dtmax, predicting high requirement of vasoactive inotropic drugs was greater than the AUC of the radial dP/dtmax (Table 2, Fig. 4). A post-CPB Ao dP/dtmax ≤ 785 mm Hg/s could discriminate VISmax greater than 7 (sensitivity, 75.0%; specificity, 85.7%). We also observed that the Ao dP/dtmax also had greater AUCs to predict decreased LV function and prolonged mechanical ventilation than those of the radial dP/dtmax (Table 2).
Table 2.
Receiver operating characteristic curves of aortic and radial dP/dtmax to predict poor postoperative outcomes.
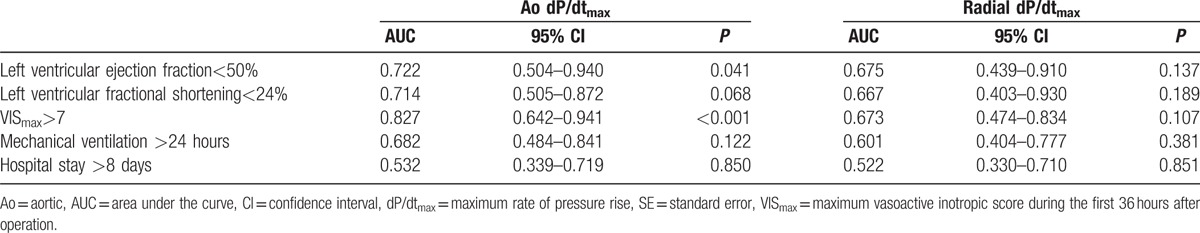
Figure 4.
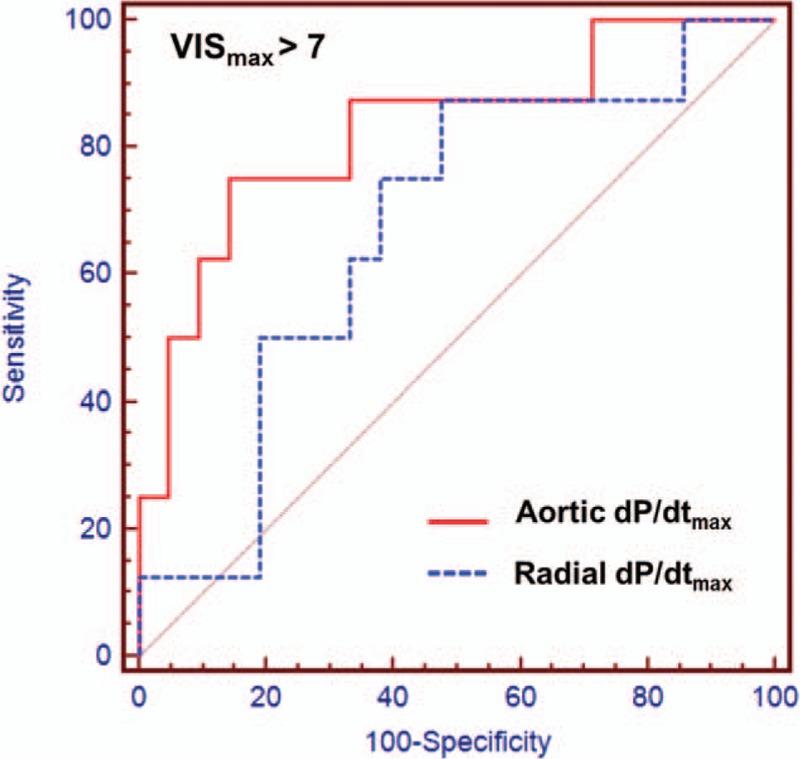
Receiver operating characteristic curves comparing the maximum pressure rise in the aorta (Ao dP/dtmax) and radial artery (radial dP/dtmax) to predict the maximum vasoactive inotropic score (VISmax) >7. The area under the curve of Ao dP/dtmax (0.827) is higher than that of radial dP/dtmax (0.673). Ao = aortic, dP/dtmax = maximum rate of pressure rise, VISmax = maximum vasoactive inotropic score.
4. Discussion
Immediately after weaning from CPB in pediatric congenital heart surgery, this study showed that the radial dP/dtmax was consistently lower than the Ao dP/dtmax, and weakly correlated with the Ao dP/dtmax. In addition, the Ao dP/dtmax had higher AUC for predicting decrease in LVEF and LVFS, greater requirement of vasoactive inotropic drugs, prolonged mechanical ventilation, and postoperative hospital stay than the radial dP/dtmax during this period.
Children with congenital heart disease have distorted ventricular geometry and volume- or pressure-overloaded ventricles. Therefore, it has been known to be difficult to assess ventricular contractility using the conventional echocardiographic technique.[14,15] The LV dP/dtmax may be reliable and accurate to quantify LV contractility in patients with congenital heart disease, because it has been known that The LV dP/dtmax is not influenced by wall motion abnormalities or variations in ventricular anatomy and morphology.[16] Regarding the utility of dP/dtmax as a bedside monitoring, it has been suggested that the Ao dP/dtmax is closely correlated with the LV dP/dtmax, which means Ao dP/dtmax may be potential as a less invasive method to determine LV contractility especially in patients with congenital heart disease.[5] Recently, Kawasaki et al[6] reported that the LV dP/dtmax can be estimated noninvasively from brachial or radial arterial pressure waveforms using a tonometry. They also showed that dP/dtmax derived from peripheral arteries have significant linear relationships with Ao dP/dtmax, despite pressure differences between the measured sites.
In infants and children, postoperative ventricular failure has been considered as a major cause of death and important predictor of clinical outcome after congenital heart surgery.[17–19] At the time of completion of operation during congenital heart surgery, accurate quantification of ventricular contractility may help to decide which treatment will be effective in the critical period. Although it has been investigated the usefulness of noninvasive bedside monitoring of ventricular contractility,[20] it remains uncertain to determine ventricular contractility and cardiac output in patients who have defective or distorted heart. During the cardiac surgery, transesophageal echocardiography has been most commonly used to evaluate the surgically corrected structures and function. However, echocardiographic derived parameters may be invalid resulting from abnormal geometry and load- and age-dependent properties.[21] Although Doppler myocardial imaging, strain rate, and backscatter were introduced,[22–25] these methods have been challenged in ensuring data standardization and inter- and intra-individual variability requiring a highly specialized echocardiographic technique.[26] Particularly during the weaning period, unrecovered myocardium of small children is vulnerable to little changes in volume or pressure loading, resulting in hemodynamic instability. Additionally, Ao dP/dtmax can also be measured without additional invasive procedure, because we can obtain Ao pressure waveforms via the pressure monitoring line connected to Ao root cannulation of CPB circuit before discontinuation from CPB. In this regard, our study adds value in the sense that continuous measurement of dP/dtmax may provide beat-to-beat information about ventricular contractility.
Many types of noninvasive hemodynamic monitoring devices have been used in children, such as arterial pulse contour analysis, ultrasound, and bioreactance technique.[27–29] In adults undergoing coronary artery bypass surgery, femoral dP/dtmax obtained from arterial waveform analysis using the transpulmonary thermodilution monitor (PiCCOplus; Pulsion, Munich, Germany) has shown to be closely related to LV dP/dtmax during inotropic administration.[30] Although disappointing results have been reported following application of these noninvasive hemodynamic monitoring for neonates and small children,[31–33] evidence has been growing to show the applicability of dP/dtmax. Recently, it has been suggested that the dP/dtmax, which is provided using a device embedded arterial pulse contour analysis (Mostcare/PRAM; Vytech, Padova, Italy) can be used as an index of ventricular contractility.[34] In a study for critically ill children, the dP/dtmax derived from PRAM has been used as a parameter representing ventricular contractility and shown to track the change according to inotropic use.[35]
However, our study findings indicated a relatively low correlation coefficient between the Ao and radial dP/dtmax. We speculated that vascular properties may be different according to measuring sites in the post-CPB period, although it remains uncertain why a central-to-peripheral arterial pressure gradient occurs after CPB.[36,37] It is thought that marked arterial constriction may contribute to the damped transmission of the pressure pulse to the radial artery and intensifies discrepancy of the pressure.[38] In addition, Kanazawa et al[7] reported that central-to-peripheral arterial pressure gradient may be caused by the differences in pulse wave velocity and arterial elasticity between central and peripheral arteries. Even given that children may have age-related differences in vascular mechanical characteristics,[39,40] systolic arterial pressure measured in peripheral artery is still displayed lower value than central arterial pressure in the weaning period.[8] Collectively, the decrease in pulse wave velocity caused by decreased arterial elasticity may induce discrepancies of pressure rise and peak pressure between aortic and radial arterial waveforms related to CPB. In consequence, we can observe that the radial dP/dtmax was lower than the Ao dP/dtmax, and there was a weak correlation between the 2 values immediately after CPB.
There were some limitations to this study. First, it was a retrospective study from a single medical center with a small sample size. In addition, it should be considered that patients with congenital heart disease may have varying vascular properties. It has been known that arterial elastance is decreased or increased depending on pathologies of cardiovascular anatomic lesion,[41,42] and that elastic characteristics of aorta are also impaired in congenital anomaly involved aorta even after corrective surgery.[43,44] In this study, we only included pediatric patients with uncomplicated congenital heart disease without significant cyanosis, and undergoing complete correction of the congenital heart defect. To extend the application of this approach, further studies are needed in children with complicated congenital heart disease such as functional single ventricle. In addition, children have age-related differences in vascular mechanical characteristics.[40] Therefore, it is also needed to investigate whether radial and Ao dP/dtmax relationship after CPB weaning is different according to different age groups although we included patients whose ages ranged from 13 days to 6 years. Second, we could not measure simultaneously LV dP/dtmax via direct catheterization. Instead of direct catheterization, noninvasive method to estimate LV dP/dtmax using Doppler signals of mitral insufficiency has been widely used,[45,46] although this measurement has some shortcomings that it can be only used in presence of mitral insufficiency. In a previous report, the radial dP/dtmax has been found to be closely correlated with the dP/dtmax derived on mitral regurgitation in patients with heart failure.[47] Therefore, confirmation study is needed to validate the relationship between peripheral dP/dtmax and the dP/dtmax from Doppler measurement of mitral regurgitation during the weaning period. Third, we did not perform femoral arterial catheterization. Radial artery is the first choice of cannulation site for arterial pressure monitoring during congenital heart surgery in our institution, however, radial catheterization must be not easy to perform in small children. It has been reported that radial-to-femoral systolic pressure gradient was observed during the first 10 minutes after CPB in pediatric cardiac surgery.[48] Therefore, it is expected that femoral dP/dtmax may more reliably reflect the dP/dtmax of central artery, and relationship between femoral and Ao dP/dtmax may be different from radial-Ao dP/dtmax relationship. In addition, we could not examine changes in the relationship between Ao and radial dP/dtmax before and after CPB, because we have recorded only radial arterial waveforms before CPB. Finally, LVEF and FS that we employed as measure of postoperative ventricular performance still may be questionable for accurate assessment of LV contractility due to their load-sensitive properties. In this respect, we thought that VISmax may be a more valuable index as a postoperative outcomes reflecting ventricular function. In pediatric cardiac surgery, VISmax has been known to be associated with LV systolic function, postoperative morbidity and mortality.[49–51] Therefore, we suggest that monitoring of Ao dP/dtmax may be useful to predict greater requirement of vasoactive inotropic drugs, reflecting early postoperative ventricular function.
5. Conclusions
Immediately after children are weaned from CPB, the radial dP/dtmax was weakly correlated with Ao dP/dtmax. However, the radial dP/dtmax was not interchangeable with Ao dP/dtmax, because vascular properties might be changed differently between central and peripheral arteries, resulting in a disparity of pressure waveforms. The Ao dP/dtmax was still closely associated with early postoperative ventricular function in congenital heart surgery. Therefore, intraoperative measurement of Ao dP/dtmax via aortic cannula may provide information about beat-to-beat ventricular contractility during immediate post-CPB period. However, further studies are needed for patients with complicated congenital heart disease including functional single ventricle, and for different age groups to extend the usefulness of Ao dP/dtmax to predict postoperative ventricular function.
Footnotes
Abbreviations: Ao = aortic, AUC = area under the curve, CPB = cardiopulmonary bypass, DBP = diastolic blood pressure, dP/dtmax = maximum rate of pressure rise, EF = ejection fraction, FS = fractional shortening, LV = left ventricle, RACHS-1 = Risk Adjustment in Congenital Heart Surgery category 1, ROC = Receiver operating characteristics, SBP = systolic blood pressure, SpO2 = arterial oxygen saturation, VISmax = maximum vasoactive inotropic score.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Wallace AG, Skinner NS, Jr, Mitchell JH. Hemodynamic determinants of the maximal rate of rise of left ventricular pressure. Am J Physiol 1963;205:30–6. [DOI] [PubMed] [Google Scholar]
- [2].Quinones MA, Gaasch WH, Alexander JK. Influence of acute changes in preload, afterload, contractile state and heart rate on ejection and isovolumic indices of myocardial contractility in man. Circulation 1976;53:293–302. [DOI] [PubMed] [Google Scholar]
- [3].Kass DA, Maughan WL, Guo ZM, et al. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure–volume relationships. Circulation 1987;76:1422–36. [DOI] [PubMed] [Google Scholar]
- [4].Little WC, Rassi A, Jr, Freeman GL. Comparison of effects of dobutamine and ouabain on left ventricular contraction and relaxation in closed-chest dogs. J Clin Invest 1987;80:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Masutani S, Iwamoto Y, Ishido H, et al. Relationship of maximum rate of pressure rise between aorta and left ventricle in pediatric patients. Implication for ventricular-vascular interaction with the potential for noninvasive determination of left ventricular contractility. Circ J 2009;73:1698–704. [DOI] [PubMed] [Google Scholar]
- [6].Kawasaki H, Seki M, Saiki H, et al. Noninvasive assessment of left ventricular contractility in pediatric patients using the maximum rate of pressure rise in peripheral arteries. Heart Vessels 2012;27:384–90. [DOI] [PubMed] [Google Scholar]
- [7].Kanazawa M, Fukuyama H, Kinefuchi Y, et al. Relationship between aortic-to-radial arterial pressure gradient after cardiopulmonary bypass and changes in arterial elasticity. Anesthesiology 2003;99:48–53. [DOI] [PubMed] [Google Scholar]
- [8].Cetin S, Pirat A, Kundakci A, et al. Radial mean arterial pressure reliably reflects femoral mean arterial pressure in uncomplicated pediatric cardiac surgery. J Cardiothorac Vasc Anesth 2014;28:76–83. [DOI] [PubMed] [Google Scholar]
- [9].Jenkins KJ, Gauvreau K, Newburger JW, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 2002;123:110–8. [DOI] [PubMed] [Google Scholar]
- [10].Kim JW, Shin WJ, Park I, et al. Splanchnic oxygen saturation immediately after weaning from cardiopulmonary bypass can predict early postoperative outcomes in children undergoing congenital heart surgery. Pediatr Cardiol 2014;35:587–95. [DOI] [PubMed] [Google Scholar]
- [11].Kim JW, Gwak M, Shin WJ, et al. Preoperative factors as a predictor for early postoperative outcomes after repair of congenital transposition of the great arteries. Pediatr Cardiol 2015;36:537–42. [DOI] [PubMed] [Google Scholar]
- [12].Butts RJ, Scheurer MA, Atz AM, et al. Comparison of maximum vasoactive inotropic score and low cardiac output syndrome as markers of early postoperative outcomes after neonatal cardiac surgery. Pediatr Cardiol 2012;33:633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hoffman TM, Wernovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 2003;107:996–1002. [DOI] [PubMed] [Google Scholar]
- [14].Mor-Avi V, Sugeng L, Weinert L, et al. Fast measurement of left ventricular mass with real-time three-dimensional echocardiography: comparison with magnetic resonance imaging. Circulation 2004;110:1814–8. [DOI] [PubMed] [Google Scholar]
- [15].Riehle TJ, Mahle WT, Parks WJ, et al. Real-time three-dimensional echocardiographic acquisition and quantification of left ventricular indices in children and young adults with congenital heart disease: comparison with magnetic resonance imaging. J Am Soc Echocardiogr 2008;21:78–83. [DOI] [PubMed] [Google Scholar]
- [16].Rhodes J, Udelson JE, Marx GR, et al. A new noninvasive method for the estimation of peak dP/dt. Circulation 1993;88:2693–9. [DOI] [PubMed] [Google Scholar]
- [17].Parr GV, Blackstone EH, Kirklin JW. Cardiac performance and mortality early after intracardiac surgery in infants and young children. Circulation 1975;51:867–74. [DOI] [PubMed] [Google Scholar]
- [18].Carrel T, Pfammatter JP. Complete transposition of the great arteries: surgical concepts for patients with systemic right ventricular failure following intraatrial repair. Thorac Cardiovasc Surg 2000;48:224–7. [DOI] [PubMed] [Google Scholar]
- [19].Ma M, Gauvreau K, Allan CK, et al. Causes of death after congenital heart surgery. Ann Thorac Surg 2007;83:1438–45. [DOI] [PubMed] [Google Scholar]
- [20].Smith BE, Madigan VM. Non-invasive method for rapid bedside estimation of inotropy: theory and preliminary clinical validation. Brit J Anaesth 2013;111:580–8. [DOI] [PubMed] [Google Scholar]
- [21].Pacileo G, Di Salvo G, Limongelli G, et al. Echocardiography in congenital heart disease: usefulness, limits and new techniques. J Cardiovasc Med (Hagerstown) 2007;8:17–22. [DOI] [PubMed] [Google Scholar]
- [22].Simmons LA, Weidemann F, Sutherland GR, et al. Doppler tissue velocity, strain, and strain rate imaging with transesophageal echocardiography in the operating room: a feasibility study. J Am Soc Echocardiogr 2002;15:768–76. [DOI] [PubMed] [Google Scholar]
- [23].Weidemann F, Eyskens B, Jamal F, et al. Quantification of regional left and right ventricular radial and longitudinal function in healthy children using ultrasound-based strain rate and strain imaging. J Am Soc Echocardiogr 2002;15:20–8. [DOI] [PubMed] [Google Scholar]
- [24].Weidemann F, Eyskens B, Mertens L, et al. Quantification of regional right and left ventricular function by ultrasonic strain rate and strain indexes after surgical repair of tetralogy of Fallot. Am J Cardiol 2002;90:133–8. [DOI] [PubMed] [Google Scholar]
- [25].Di Salvo G, Drago M, Pacileo G, et al. Comparison of strain rate imaging for quantitative evaluation of regional left and right ventricular function after surgical versus percutaneous closure of atrial septal defect. Am J Cardiol 2005;96:299–302. [DOI] [PubMed] [Google Scholar]
- [26].Friedberg MK, Mertens L. Tissue velocities, strain, and strain rate for echocardiographic assessment of ventricular function in congenital heart disease. Eur J Echocardiogr 2009;10:585–93. [DOI] [PubMed] [Google Scholar]
- [27].Mahajan A, Shabanie A, Turner J, et al. Pulse contour analysis for cardiac output monitoring in cardiac surgery for congenital heart disease. Anesth Analg 2003;97:1283–8. [DOI] [PubMed] [Google Scholar]
- [28].Knirsch W, Kretschmar O, Tomaske M, et al. Cardiac output measurement in children: comparison of the Ultrasound Cardiac Output Monitor with thermodilution cardiac output measurement. Intensive Care Med 2008;34:1060–4. [DOI] [PubMed] [Google Scholar]
- [29].Ballestero Y, Lopez-Herce J, Urbano J, et al. Measurement of cardiac output in children by bioreactance. Pediatr Cardiol 2011;32:469–72. [DOI] [PubMed] [Google Scholar]
- [30].De Hert SG, Robert D, Cromheecke S, et al. Evaluation of left ventricular function in anesthetized patients using femoral artery dP/dt(max). J Thorac Cardiovasc Surg 2006;20:325–30. [DOI] [PubMed] [Google Scholar]
- [31].Taylor K, Manlhiot C, McCrindle B, et al. Poor accuracy of noninvasive cardiac output monitoring using bioimpedance cardiography [PhysioFlow(R)] compared to magnetic resonance imaging in pediatric patients. Anesth Analg 2012;114:771–5. [DOI] [PubMed] [Google Scholar]
- [32].Saxena R, Durward A, Puppala NK, et al. Pressure recording analytical method for measuring cardiac output in critically ill children: a validation study. Brit J Anaesth 2013;110:425–31. [DOI] [PubMed] [Google Scholar]
- [33].Vergnaud E, Vidal C, Verchere JM, et al. Noninvasive cardiac output measurement using bioreactance in postoperative pediatric patients. Paediatr Anaesth 2015;25:160–6. [DOI] [PubMed] [Google Scholar]
- [34].Urbano J, Lopez J, Gonzalez R, et al. Measurement of cardiac output in children by pressure-recording analytical method. Pediatr Cardiol 2015;36:358–64. [DOI] [PubMed] [Google Scholar]
- [35].Saxena R, Durward A, Steeley S, et al. Predicting fluid responsiveness in 100 critically ill children: the effect of baseline contractility. Intensive Care Med 2015;41:2161–9. [DOI] [PubMed] [Google Scholar]
- [36].Rich GF, Lubanski RE, Jr, McLoughlin TM. Differences between aortic and radial artery pressure associated with cardiopulmonary bypass. Anesthesiology 1992;77:63–6. [DOI] [PubMed] [Google Scholar]
- [37].De Hert SG, Vermeyen KM, Moens MM, et al. Central-to-peripheral arterial pressure gradient during cardiopulmonary bypass: relation to pre- and intra-operative data and effects of vasoactive agents. Acta Anaesthesiol Scand 1994;38:479–85. [DOI] [PubMed] [Google Scholar]
- [38].Nakayama R, Goto T, Kukita I, et al. Sustained effects of plasma norepinephrine levels on femoral-radial pressure gradient after cardiopulmonary bypass. J Anesth 1993;7:8–16. [DOI] [PubMed] [Google Scholar]
- [39].Sharp MK, Pantalos GM, Minich L, et al. Aortic input impedance in infants and children. J Appl Physiol 2000;88:2227–39. [DOI] [PubMed] [Google Scholar]
- [40].Senzaki H, Akagi M, Hishi T, et al. Age-associated changes in arterial elastic properties in children. Eur J Pediatr 2002;161:547–51. [DOI] [PubMed] [Google Scholar]
- [41].Senzaki H, Chen CH, Masutani S, et al. Assessment of cardiovascular dynamics by pressure-area relations in pediatric patients with congenital heart disease. J Thorac Cardiovasc Surg 2001;122:535–47. [DOI] [PubMed] [Google Scholar]
- [42].Schlangen J, Fischer G, Petko C, et al. Arterial elastance and its impact on intrinsic right ventricular function in palliated hypoplastic left heart syndrome. Int J Cardiol 2013;168:5385–9. [DOI] [PubMed] [Google Scholar]
- [43].Vogt M, Kuhn A, Baumgartner D, et al. Impaired elastic properties of the ascending aorta in newborns before and early after successful coarctation repair: proof of a systemic vascular disease of the prestenotic arteries? Circulation 2005;111:3269–73. [DOI] [PubMed] [Google Scholar]
- [44].Grotenhuis HB, de Roos A. Structure and function of the aorta in inherited and congenital heart disease and the role of MRI. Heart 2011;97:66–74. [DOI] [PubMed] [Google Scholar]
- [45].Chen C, Rodriguez L, Guerrero JL, et al. Noninvasive estimation of the instantaneous first derivative of left ventricular pressure using continuous-wave Doppler echocardiography. Circulation 1991;83:2101–10. [DOI] [PubMed] [Google Scholar]
- [46].Garcia-Lledo A, Moya JL, Balaguer J, et al. Sensitivity of the Doppler rate of pressure rise to changes in the inotropic state: an experimental comparison with invasively obtained dP/dt. Eur J Echocardiogr 2000;1:271–6. [DOI] [PubMed] [Google Scholar]
- [47].Tartiere JM, Logeart D, Beauvais F, et al. Non-invasive radial pulse wave assessment for the evaluation of left ventricular systolic performance in heart failure. Eur J Heart Fail 2007;9:477–83. [DOI] [PubMed] [Google Scholar]
- [48].Cetin S, Mustafa G, Keskin G, et al. Infliximab, an anti-TNF-alpha agent, improves left atrial abnormalities in patients with rheumatoid arthritis: preliminary results. Cardiovasc J Afr 2014;25:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Critic Care Med 2010;11:234–8. [DOI] [PubMed] [Google Scholar]
- [50].Gaies MG, Jeffries HE, Niebler RA, et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med 2014;15:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Conlon TW, Falkensammer CB, Hammond RS, et al. Association of left ventricular systolic function and vasopressor support with survival following pediatric out-of-hospital cardiac arrest. Pediatr Crit Care Med 2015;16:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]


