Abstract
The proportion of alcohol-induced osteonecrosis of the femoral head (ONFH) in all ONFH patients was 30.7%, with males prevailing among the ONFH patients in mainland China (70.1%). Matrix metalloproteinase 2 (MMP2), a member of the MMP gene family, encodes the enzyme MMP2, which can promote osteoclast migration, attachment, and bone matrix degradation. In this case–control study, we aimed to investigate the association between MMP2 and the alcohol-induced ONFH in Chinese males.
In total, 299 patients with alcohol-induced ONFH and 396 healthy controls were recruited for a case–control association study. Five single-nucleotide polymorphisms within the MMP2 locus were genotyped and examined for their correlation with the risk of alcohol-induced ONFH and treatment response using Pearson χ2 test and unconditional logistic regression analysis. We identified 3 risk alleles for carriers: the allele “T” of rs243849 increased the risk of alcohol-induced ONFH in the allele model, the log-additive model without adjustment, and the log-additive model with adjustment for age. Conversely, the genotypes “CC” in rs7201 and “CC” in rs243832 decreased the risk of alcohol-induced ONFH, as revealed by the recessive model. After the Bonferroni multiple adjustment, no significant association was found. Furthermore, the haplotype analysis showed that the “TT” haplotype of MMP2 was more frequent among patients with alcohol-induced ONFH by unconditional logistic regression analysis adjusted for age.
In conclusion, there may be an association between MMP2 and the risk of alcohol-induced ONFH in North-Chinese males. However, studies on larger populations are needed to confirm this hypothesis; these data may provide a theoretical foundation for future studies.
Keywords: alcohol-induced osteonecrosis of the femoral head, Chinese males, MMP2, single-nucleotide polymorphism
1. Introduction
Osteonecrosis of the femoral head (ONFH) is an intractable and complex orthopedic condition that is characterized by osteocyte apoptosis and deterioration and collapse of the bone structure of the femoral head, which ultimately lead to femoral head ischemia and death.[1–3] Study has reported that the following revision is an outstanding problem in the ONFH treatment procedure.[4] Two types of ONFH exist: the traumatic and nontraumatic type.
Alcohol-induced ONFH is a type of nontraumatic ONFH caused by chronic and excessive alcohol consumption. The incidence of this condition is increasing in China; data show that males dominate with a 70.1% in mainland China, and that the proportion of alcohol-induced ONFH among all ONFH patients is 30.7%.[5] One previous study reported that when alcohol intake was higher than 400 mL/wk, there was a clear increase in the risk of ONFH.[6] Several pathogenic mechanisms associated with the risk of alcohol-induced ONFH include environmental factors, alcohol intake, dyslipidemia, blood coagulation disorders, and ossification dysfunction of mesenchymal stem cells; however, the exact pathogenesis of this condition is still controversial and unclear. Genetic researches have offered the potential insight into the occurrence and development of alcohol-induced ONFH. Zhang et al[7] confirmed the association between ABCB1 polymorphism and increased ONFH risk. In addition, the association of ONFH with angiogenesis- and hypoxia-related gene polymorphisms, such as IGFBP3, VEGFC, and ACE, has been confirmed[8]. Recent studies suggest that MMP2 may play a key role in the process of bone metabolism and influence the proliferation and/or differentiation of bone marrow stem cells in bone and joint diseases.[9,10]
MMP2 is a member of the MMP gene family, which encodes zinc-dependent enzymes capable of cleaving components of the extracellular matrix and molecules involved in signal transduction. The MMP/tissue inhibitor of MMP (MMP/TIMP) pathway has also been found to impact human bone metabolism,[11] while polymorphisms of several genes in the pathway have been related to orthopedic diseases.[12–14] Some single-nucleotide polymorphisms (SNPs) in MMP2 have been associated with several diseases: for example, rs1053605 is associated with stroke outcome,[15] but not with abdominal aortic aneurysm[16]; rs243849 is related to ischemic stroke outcome[15]; the rs243847 variant may increase intracranial aneurysms in Japanese male patients[17]; 2 SNPs, rs243832 and rs7201, that have a strong linkage disequilibrium (LD), have been associated with endometriosis.[18] However, whether these 5 MMP2 SNPs are associated with alcohol-induced ONFH is still unknown. Therefore, in this case–control study, we aimed to investigate whether the genetic aspect of MMP2 had a protective or risk-increasing nature in the context of alcohol-induced ONFH in Chinese males.
2. Methods
2.1. Ethics committee statement
This case–control study was performed in compliance with the principles of the Declaration of Helsinki of the World Medical Association and obtained the permission from the Ethics Committee of Xi’an Hong-Hui Hospital, Xi’an Jiaotong University, and Northwest University. All of the participants were informed of the case–control study, and their consents were obtained.
2.2. Research subjects
This case–control study was conducted in Xi’an Hong-Hui Hospital, and a total of 695 males pathologically confirmed alcohol-induced ONFH patients were enrolled from September 2014 to January 2016, including 299 patients and 396 healthy controls. ONFH was diagnosed by clinical examination and radiographic analysis. Patients underwent anteroposterior and frog view X-ray tests; magnetic resonance imaging was needed to further confirm the ONFH in cases when the patients had no changes observable by X-ray. According to Lee et al[19], alcohol-induced ONFH patients were those who consumed more than 400 mL of pure ethanol per week. Some patients were excluded from the study for reasons such as disagreeing with the study, consumption of drugs that have an effect on the patients’ liver function and lipid metabolism, not meeting the diagnostic criteria of alcohol-induced ONFH, and suffering from chronic diseases synchronously. The person needing steroid treatment is also excluded from this case–control study. All healthy controls had never been diagnosed with alcohol-induced ONFH and were interviewed by professional interviewers for their age and alcohol exposure.
2.3. Genotyping
MMPs play a key role in the process of bone metabolism. A number of studies have revealed that some genes in the MMP/TIMP pathway affect the risk of ONFH. Thus, we selected 5 SNPs of the MMP2 gene based on the minor allele frequencies of more than 5% in the HapMap Chinese Han population: rs1053605, rs243849, rs243847, rs243832, and rs7201.
Whole blood samples of each participant were extracted and placed in anticoagulant tubes. The extraction kit (GoldMag Co. Ltd., Xi’an, China) was used to isolate genomic DNA from the whole blood, and the genomic DNA concentration was then measured by spectrometry (DU530 UV/VIS spectrophotometer, Beckman Instruments, Fullerton, CA). The DNA was subsequently stored at −20 °C until detection. According to the manufacturer agreement, the Sequenom MassARRAY® RS1000 system (Agena Bioscience Inc., San Diego, CA) was used to perform genotyping, and the Sequence MassARRAY Assay Design 4.0 (Agena Bioscience Inc.) software was used to design a Multiplexed SNP mass-EXTEND assay.[20] Data analyses and management were conducted by Sequenom Typer 4.0 Software (Agena Bioscience Inc.).[21]
2.4. Statistical analysis
Microsoft Excel and SPSS 16.0 (SPSS, Chicago, IL) were used to perform statistical analyses. Throughout the document, P values were 2-sided; furthermore, P values less than 0.05 were considered statistically significant. The age difference of cases and controls was calculated by Welch t tests, and the exact test was used to determine whether the SNPs departed from the Hardy–Weinberg equilibrium (HWE). The association between the minor allele and the risk of alcohol-induced ONFH was evaluated by Pearson χ2 test. To assess the association between each genotype and the risk of alcohol-induced ONFH, 5 models were used, including the codominant, dominant, recessive, overdominant, and log-additive model. Finally, the SHEsis software platform[22] and Haploview software package (version 4.2) (Broad Institute, Cambridge, MA) were used to analyze the patterns of LD, haplotype construction, and the genetic association at polymorphic loci.[23] Odds ratios (ORs) and 95% confidence intervals (95% CIs) could show whether the frequencies between cases and controls had a significant difference. Unconditional logistic regression analysis was adjusted for age. In order to eliminate the probability of false-positive results, Bonferroni multiple adjustment was applied to the level of significance, which was set at P < 0.0017 (0.05/30).
3. Results
In this case–control study, all participants were males. The age characteristic is presented in Table 1. We selected 695 individuals, including 299 alcohol-induced ONFH patients (age: 43.24 ± 13.07) and 396 healthy controls (age: 47.62 ± 10.28). A total of 5 SNPs were analyzed in the study. Gene, chromosomal position, the minor/major allele frequency, HWE test, and Pearson χ2 test results for all the SNPs are presented in Table 2. The HWE test was used to check whether the surveyed population approached the genetic equilibrium and whether random sampling requirements were achieved. P > 0.05 showed that the SNPs met the HWE, so none of the SNPs was excluded. The differences in the frequency distribution of alleles between cases and controls were compared by Pearson χ2 test. We found that 1 significant SNP of the MMP2 gene was associated with the risk of alcohol-induced ONFH at a level of 5% (rs243849 OR = 1.33, 95% CI = 1.02–1.75, P = 0.037).
Table 1.
Characteristics of the male individuals in controls and alcohol-induced ONFH patients.

Table 2.
Summary of the basic information on candidate SNPs examined in the MMP2 gene among the cases and controls, and the odds ratio estimates for alcohol-induced ONFH.
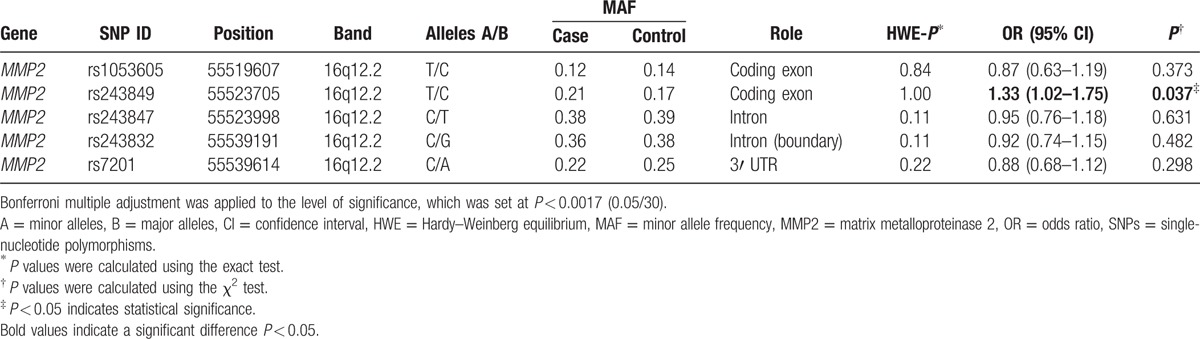
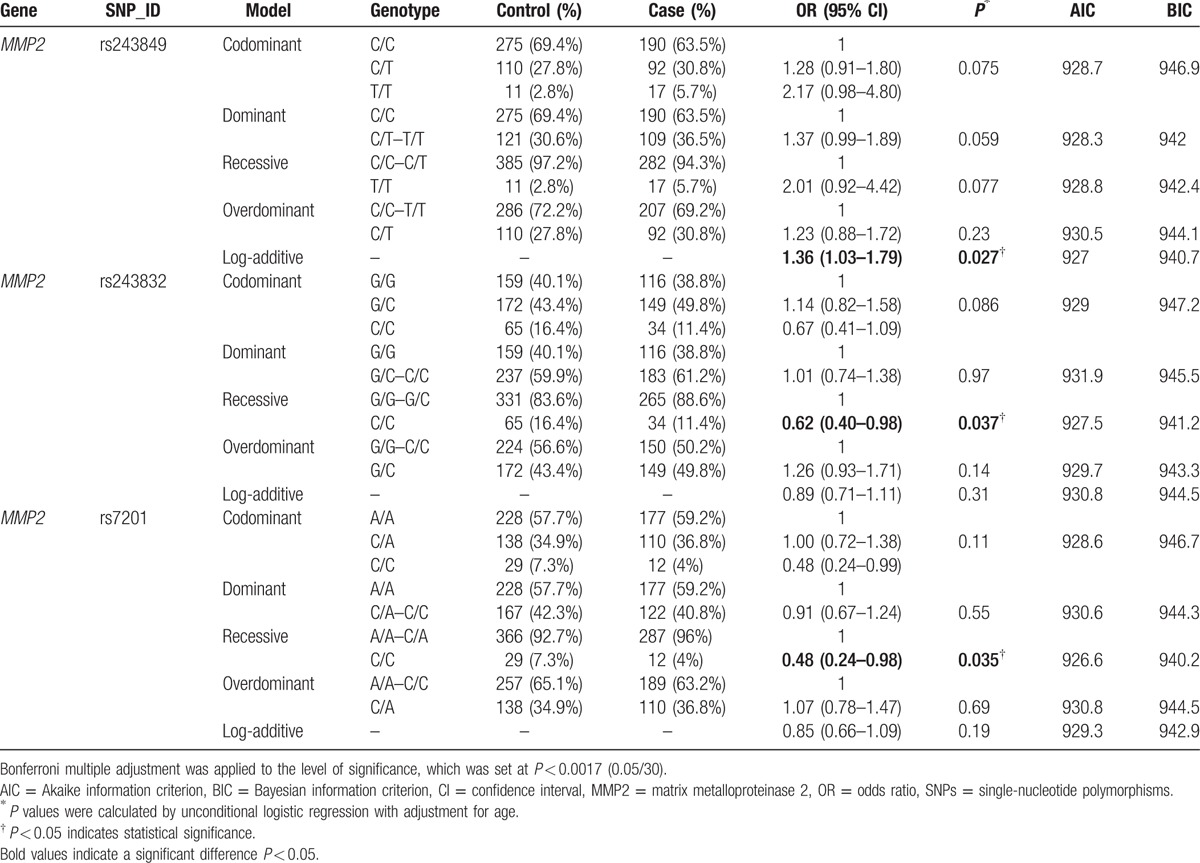
Next, we assumed that the minor allele of each SNP was a risk factor and analyzed the association between each variant and the risk of alcohol-induced ONFH under 5 genetic models (codominant, dominant, recessive, overdominant, and log-addictive) (Tables 3 and 4). The Akaike information criterion and the Bayesian information criterion (BIC) were used to select the optimal model among candidate models, as discussed in the study by Vrieze.[24] The “T” allele of rs243849 was found to increase the risk of alcohol-induced ONFH under the log-addictive model by unconditional logistic regression analysis without adjustments (OR = 1.32, 95% CI = 1.01–1.73, P = 0.041) (Table 3). Moreover, unconditional logistic regression analysis adjusted for age was used for further analysis of the association of each SNP with the risk of alcohol-induced ONFH (Table 4). Similarly, we found that rs243849 was associated with increased odds of developing alcohol-induced ONFH in the log-addictive model in the North-Chinese male population (OR = 1.36, 95% CI = 1.03–1.79, P = 0.027). Conversely, the recessive model revealed that the genotype “CC” in rs243832 and the genotype “CC” in rs7201 decreased the risk of alcohol-induced ONFH (rs243832 OR = 0.62, 95% CI = 0.40–0.98, P = 0.037; rs7201 OR = 0.48, 95% CI = 0.24–0.98, P = 0.035). We did not find any statistically significant association between the risk of alcohol-induced ONFH and the loci rs1053605 and rs243847 using the 5 models. However, after Bonferroni multiple adjustment was applied to our data, no SNP in our study was significantly related to alcohol-induced ONFH.
Table 3.
Genotypes of rs243849 and the risk of alcohol-induced ONFH were not adjusted for age.
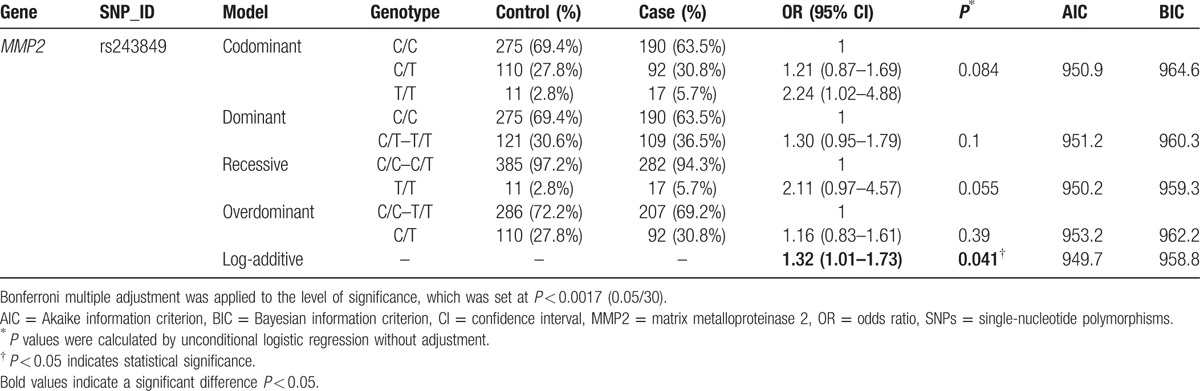
Table 4.
Genotypes of dominant SNPs in the MMP2 gene and the risk of alcohol-induced ONFH adjusted for age.
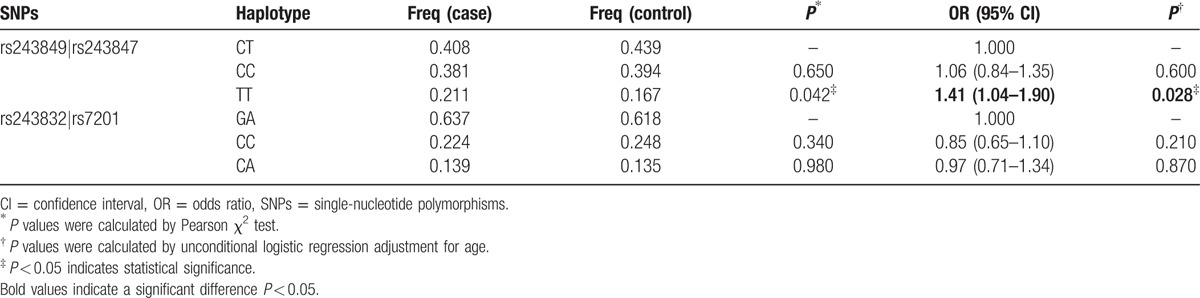
Finally, haplotype analysis results showed that 2 blocks have the LD, rs243849–rs243847 and rs243832–rs7201, and the D’ values were 1 and 0.99, respectively (Fig. 1). The haplotype “TT” of the rs243849 and rs243847 block was found to be associated with an increased risk of alcohol-induced ONFH by Pearson χ2 test (P < 0.05) (Table 5). Furthermore, under unconditional logistic regression analysis adjusted for age, the “TT” haplotype equally augmented the risk (OR = 1.41, 95% CI = 1.04–1.90, P = 0.028). However, no significant haplotype was identified in the rs243832–rs7201 block.
Figure 1.
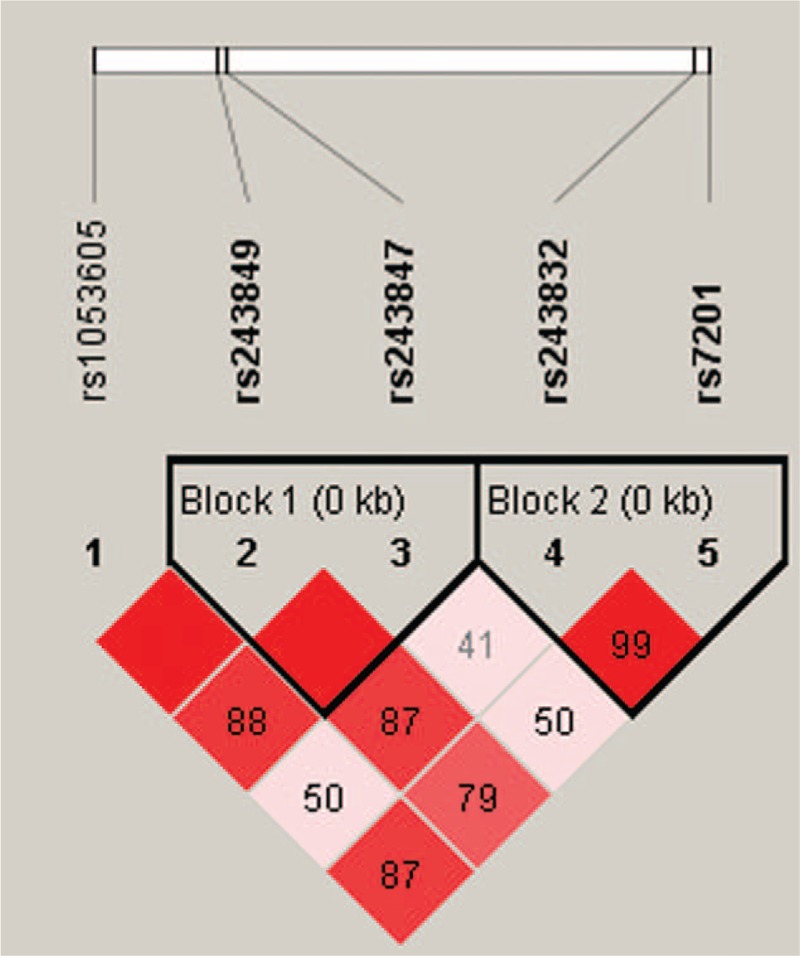
Haplotype block map for part of the single-nucleotide polymorphisms (SNPs) in the MMP2 gene. Linkage disequilibrium (LD) plots containing 5 SNPs of 16q12.2. Standard color frame is used to show LD pattern. Two blocks in the figure showed higher LD. Darker shades of red indicate higher D’ and display statistically significant associations between a pair of SNPs.
Table 5.
MMP2 haplotype frequencies and their association with alcohol-induced ONFH among the cases and controls adjusted for age.
4. Discussion and conclusion
Epidemiologic studies found that SNPs in several genes might be associated with patients’ susceptibility to orthopedic diseases,[25,26] including alcohol-induced ONFH.[27] Investigation of the relationship between different genes and diseases can improve the treatment, prevention, and prognosis. Furthermore, several genetic markers for alcohol-induced ONFH had already been found.[27,28] In our study, we identified the association of 5 SNPs in the MMP2 gene with the risk of alcohol-induced ONFH in the male Chinese Han population from the area of Shanxi. Our result showed that MMP2, found on chromosome 16, might be associated with the risk of alcohol-induced ONFH. The haplotype “TT” was associated with a 1.41-fold increase in the risk of alcohol-induced ONFH.
Alcohol-induced ONFH is a serious and complex condition characterized by the apoptosis of osteocytes and an imbalance in bone metabolism, caused by a disruption in the dynamic equilibrium of bone formation and breakdown and in the prosoplasia of bone marrow stromal cells.[3,29] Patients present with pain and limited hip-joint movement, caused by bone trabecula breakage and collapse of the subchondral bone, or with abnormal lipid metabolism and intravascular coagulation, which result in necrosis of the femoral head.[30,31] Some studies report that normal repair mechanisms might be disrupted after bone death, and the repair process probably takes place in the form of bone remodeling coupled with bone resorption and bone formation.[32] Generally, cellular responses in the physiologic cycle of bone remodeling involve a series of highly regulated steps, containing “resting,” “activation,” “resorption,” “reversal,“ and “formation” phases.[33] Both osteoblasts and osteoclasts are the primary functional cells involved in the process of bone remodeling. Osteoclasts attach to the mineralized bone surface and initiate bone resorption by secreting hydrogen ions and by digesting bone collagen. Afterward, osteoblasts migrate to the bone resorption site and secrete bone matrix, which then mineralizes and forms new bone. The balance of the bone remodeling process plays a key role in maintaining normal bone mass.
MMPs are not only directly involved in bone matrix degradation but can also promote osteoclast migration and attachment. In fact, it has been demonstrated that osteoblasts have the ability to secrete MMPs.[34,35] MMPs are maintained at a normal level under the regulation of a variety of factors. During inflammation, MMPs regulate the differentiation of osteoblasts and bone resorption by osteoclasts to complete the repair of the necrotic bone. Moreover, it has been suggested that MMP2, which is encoded by the MMP2 gene located in 16q12.2, contributes to bone remodeling.[36] According to Keiichi Inoue, it plays a crucial role in forming and maintaining the osteocyte canalicular network, whose formation is a determining factor of bone remodeling and mineralization.[37,38] A report suggested that the abnormal expression of MMP2 causes osteolysis.[39] The upregulation of MMP2 induces the MMP/TIMP pathway, which in turn activates osteoclast bone resorption and accelerates osteoblast apoptosis and bone matrix degradation. The balance between bone resorption and bone formation is thus disrupted, and this imbalance could be the most important mechanism of collapse in ONFH.[32] Therefore, MMP2 is an important regulatory gene in alcohol-induced ONFH patients. We have not found any evidence for the role of heredity between MMP2 and the risk of alcohol-induced ONFH in Chinese males. In this case–control study, carriers of the rs243849 “T” allele exhibited a 1.33-, 1.32-, and 1.36-fold, statistically significant increase in the susceptibility of alcohol-induced ONFH by the allele model, log-additive model without adjustments, and log-additive model adjusted for age, respectively. The patients who had the genotypes “CC” of rs243832 and “CC” of rs7201 had a decreased susceptibility of alcohol-induced ONFH. After Bonferroni multiple adjustment was applied, no significant association was found. However, the rs243849 risk allele “T” and rs243847 wild type allele “T” constituted the haplotype “TT”, which, compared to the wild type “CT”, increased the risk of alcohol-induced ONFH. Because Bonferroni correction is the most conservative approach, the variability of MMP2 may be a risk factor in the Chinese males.
In summary, we identified 5 SNPs of the MMP2 gene and found that the variability of MMP2 could be a biomarker for the risk of alcohol-induced ONFH. It is important to note that there were several limitations in our case–control study, such as limited sample size and lack of corresponding clinical information. Thus, further research is needed to verify our results, especially due to the lack of previous research on this topic. It is possible that MMP2 polymorphisms are related to the risk of alcohol-induced ONFH, and these data provide a theoretical foundation for future studies of this correlation in the Chinese population or in other populations.
Acknowledgments
We thank all the patients and individuals for their participation, as well as all the physicians and nurses of Xi’an Hong-Hui Hospital for offering their blood samples.
Footnotes
Abbreviations: CIs = confidence intervals, HWE = Hardy–Weinberg equilibrium, LD = linkage disequilibrium, MMP = matrix metalloproteinase, ONFH = osteonecrosis of the femoral head, OR = odds ratios, SNP = single-nucleotide polymorphism, TIMP = tissue inhibitor of matrix metalloproteinases.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Aaron RK. Importance of the early diagnosis of hip pain: new approaches to hip preservation in osteonecrosis. Med Health R I 1998;81:157–61. [PubMed] [Google Scholar]
- [2].Zalavras CG, Vartholomatos G, Dokou E, et al. Genetic background of osteonecrosis: associated with thrombophilic mutations? Clin Orthop Relat Res 2004;422:251–5. [PubMed] [Google Scholar]
- [3].Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am 1995;77:459–74. [DOI] [PubMed] [Google Scholar]
- [4].Scaglione M, Fabbri L, Celli F, et al. Hip replacement in femoral head osteonecrosis: current concepts. Clin Cases Miner Bone Metab 2015;12suppl 1:51–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cui L, Zhuang Q, Lin J, et al. Multicentric epidemiologic study on six thousand three hundred and ninety five cases of femoral head osteonecrosis in China. Int Orthop 2016;40:267–76. [DOI] [PubMed] [Google Scholar]
- [6].Matsuo K, Hirohata T, Sugioka Y, et al. Influence of alcohol intake, cigarette smoking, and occupational status on idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res 1988;115–23. [PubMed] [Google Scholar]
- [7].Zhang Z, Li Y, Liu H, et al. ABCB1 polymorphisms associated with osteonecrosis of the femeral head. Int J Clin Exp Pahtol 2015;8:15240–4. [PMC free article] [PubMed] [Google Scholar]
- [8].Hong JM, Kim TH, Kim HJ, et al. Genetic association of angiogenesis- and hypoxia-related gene polymorphisms with osteonecrosis of the femoral head. Exp Mol Med 2010;42:376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sires UI, Schmid TM, Fliszar CJ, et al. Complete degradation of type X collagen requires the combined action of interstitial collagenase and osteoclast-derived cathepsin-B. J Clin Invest 1995;95:2089–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998;93:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Geoffroy V, Marty-Morieux C, Le Goupil N, et al. In vivo inhibition of osteoblastic metalloproteinases leads to increased trabecular bone mass. J Bone Miner Res 2004;19:811–22. [DOI] [PubMed] [Google Scholar]
- [12].Wang W, Wang L, Xu Z, et al. Effects of estradiol on reduction of osteoarthritis in rabbits through effect on matrix metalloproteinase proteins. Iran J Basic Med Sci 2016;19:310–5. [PMC free article] [PubMed] [Google Scholar]
- [13].Uemura Y, Hayashi H, Takahashi T, et al. MMP-3 as a biomarker of disease activity of rheumatoid arthritis. Rinsho Byori 2015;63:1357–64. [PubMed] [Google Scholar]
- [14].Ho LJ, Lin LC, Hung LF, et al. Retinoic acid blocks pro-inflammatory cytokine-induced matrix metalloproteinase production by down-regulating JNK-AP-1 signaling in human chondrocytes. Biochem Pharmacol 2005;70:200–8. [DOI] [PubMed] [Google Scholar]
- [15].Manso H, Krug T, Sobral J, et al. Variants of the matrix metalloproteinase-2 but not the matrix metalloproteinase-9 genes significantly influence functional outcome after stroke. BMC Med Genet 2009;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Smallwood L, Warrington N, Allcock R, et al. Matrix metalloproteinase-2 gene variants and abdominal aortic aneurysm. Eur J Vascr Endovasc 2009;38:169–71. [DOI] [PubMed] [Google Scholar]
- [17].Low SK, Zembutsu H, Takahashi A, et al. Impact of LIMK1, MMP2 and TNF-α variations for intracranial aneurysm in Japanese population. J Hum Genet 2011;56:211–6. [DOI] [PubMed] [Google Scholar]
- [18].Tsai EM, Wang YS, Lin CS, et al. A microRNA-520 mirSNP at the MMP2 gene influences susceptibility to endometriosis in Chinese women. J Hum Genet 2013;58:202–9. [DOI] [PubMed] [Google Scholar]
- [19].Lee HJ, Choi SJ, Hong J, et al. Association of a polymorphism in the intron 7 of the SREBF1 gene with osteonecrosis of the femoral head in Koreans. Ann Hum Genet 2009;73:34–41. [DOI] [PubMed] [Google Scholar]
- [20].Trembizki E, Smith H, Lahra MM, et al. High-throughput informative single nucleotide polymorphism-based typing of Neisseria gonorrhoeae using the Sequenom MassARRAY iPLEX platform. J Antimicrob Chemot 2014;69:1526–32. [DOI] [PubMed] [Google Scholar]
- [21].Thomas RK, Baker AC, DeBiasi RM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet 2007;39:347–51. [DOI] [PubMed] [Google Scholar]
- [22].Li Z, Zhang Z, He Z, et al. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis (http://analysis.bio-x.cn). Cell Res 2009;19:519–23. [DOI] [PubMed] [Google Scholar]
- [23].Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005;21:263–5. [DOI] [PubMed] [Google Scholar]
- [24].Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods 2012;17:228–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yin JM, Liu Z, Zhao SC, et al. Relationship between the apolipoprotein AI, B gene polymorphism and the risk of non-traumatic osteonecrosis. Lipids Health Dis 2014;13:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim TH, Baek JI, Hong JM, et al. Significant association of SREBP-2 genetic polymorphisms with avascular necrosis in the Korean population. BMC Med Genet 2008;9:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang Y, Cao Y, Li Y, et al. Genetic association of the ApoB and ApoA1 gene polymorphisms with the risk for alcohol-induced osteonecrosis of femoral head. Int J Clin Exp Pahtol 2015;8:11332–9. [PMC free article] [PubMed] [Google Scholar]
- [28].Okazaki S, Nagoya S, Tateda K, et al. Experimental rat model for alcohol-induced osteonecrosis of the femoral head. Int J Exp Pathol 2013;94:312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am 2006;88:1117–32. [DOI] [PubMed] [Google Scholar]
- [30].Wang Y, Li Y, Mao K, et al. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin Orthop Relat Res 2003;410:213–24. [DOI] [PubMed] [Google Scholar]
- [31].Jones JP., Jr Intravascular coagulation and osteonecrosis. Clin Orthop Relat Res 1992;277:41–53. [PubMed] [Google Scholar]
- [32].Gou W, Lu Q, Wang X, et al. Key pathway to prevent the collapse of femoral head in osteonecrosis. Eur Rev Med Pharmacol Sci 2015;19:2766–74. [PubMed] [Google Scholar]
- [33].Raisz LG. Physiology and pathophysiology of bone remodeling. Clin Chem 1999;45:1353–8. [PubMed] [Google Scholar]
- [34].Andersen TL, del Carmen Ovejero M, Kirkegaard T, et al. A scrutiny of matrix metalloproteinases in osteoclasts: evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone 2004;35:1107–19. [DOI] [PubMed] [Google Scholar]
- [35].Tezuka K-I, Tezuka Y, Maejima A, et al. Molecular cloning of a possible cysteine proteinase predominantly expressed in osteoclasts. J Biol Chem 1994;269:1106–9. [PubMed] [Google Scholar]
- [36].Delaissé J-M, Engsig MT, Everts V, et al. Proteinases in bone resorption: obvious and less obvious roles. Clin Chim Acta 2000;291:223–34. [DOI] [PubMed] [Google Scholar]
- [37].Inoue K, Mikuni-Takagaki Y, Oikawa K, et al. A crucial role for matrix metalloproteinase 2 in osteocytic canalicular formation and bone metabolism. J Biol Chem 2006;281:33814–24. [DOI] [PubMed] [Google Scholar]
- [38].Grässel S, Beckmann J, Rath B, et al. Expression profile of matrix metalloproteinase-2 and -9 and their endogenous tissue inhibitors in osteonecrotic femoral heads. Int J Mol Med 2010;26:127–33. [DOI] [PubMed] [Google Scholar]
- [39].Martignetti JA, Al Aqeel A, Al Sewairi W, et al. Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat Genet 2001;28:261–5. [DOI] [PubMed] [Google Scholar]


