Abstract
Delayed encephalopathy after carbon monoxide (CO) poisoning (DEACMP) is still a clinical challenge. This study aimed to investigate the efficacy of combined therapy of mesenchymal stem cell (MSC) transplantation and butylphthalide in DEACMP patients.
Forty-two DEACMP patients were treated with 1 of the 3 therapies: combined therapy of MSC transplantation and butylphthalide; MSC transplantation alone; or hyperbaric oxygen therapy. The MSCs were alternatively injected into the subarachnoid space and the carotid artery using a self-made high-pressure injector. The Mini-Mental State Examination and the Barthel index of activities of daily living were administered before the treatment, and at 1 month, 3 months, and 6 months after the treatment. Computed tomography and magnetic resonance imaging results before and after the treatment were compared.
At 1 month, 3 months, and 6 months after the treatment, the Mini-Mental State Examination scores and the Barthl scores were significantly higher in patients with the combined therapy of MSC transplantation and butylphthalide than those in patients with MSC transplantation alone or hyperbaric oxygen therapy (all P < 0.0001). No significant adverse events occurred.
The combination of MSC transplantation and butylphthalide is safe and effective in treating DEACMP.
Keywords: butylphthalide, carbon monoxide poisoning, delayed encephalopathy, stem cell transplantation
1. Introduction
Carbon monoxide (CO) poisoning is not uncommon. Approximately 10% to 30% patients develop the delayed encephalopathy after carbon monoxide poisoning (DEACMP) despite successful treatment of the initial symptoms. After a period of pseudo-recovery for around 1 month, these patients show acute symptoms of dementia and bladder/bowel dysfunction, and even disturbance of intelligence.[1,2]
The specific mechanisms of DEACMP are still unclear. Multiple factors have been found to be associated with the development of this condition, such as ischemia, hypoxia, cytotoxic injury, reperfusion injury, immune dysfunction, and neurotransmitter dysregulation.[3] CO can rapidly bind to hemoglobin, and then form carboxyhemoglobin. The binding ability of CO to hemoglobin is 300 times higher than that of oxygen, and the dissociation of CO from carboxyhemoglobin is 3600 times slower than that of oxygen. Thus the carboxyhemoglobin loses the ability of carrying oxygen, thereby leading to hypoxia. In addition, CO can also bind to the ferrous iron in the reduced cytochrome oxidase, which exaggerates the hypoxia. Although the carboxyhemoglobin concentration is lowered to the normal level, the residual intercellular CO continues to be a toxic factor by inhibiting the mitochondria, and finally causing the symptoms of DEACMP. The intermittent hypoxia after CO poisoning causes pathological changes in the brain similar to that of reperfusion. The reperfusion process produces large amounts of free radicals, which enhances the lipid oxidation of the cell membrane and causes mitochondrial dysfunction. In this process, the reperfusion process causes calcium ion overload, thereby leading to cell membrane hydrolysis by the membrane phospholipid, cellular organelle damage, and irreversible cell damage. Various therapies such as hyperbaric oxygen and free radical scavengers have failed in the management of DEACMP.[2,4–6] Therefore, it is necessary to search for more effective treatments for DEACMP.
It is well-known that DEACMP is caused by cerebral dysfunction, and may be associated with central nervous system injury,[7,8] which indicates that the recovery of central nervous system function may be a potential treatment for DEACMP. Notably, stem cell transplantation has been shown with great potential in repairing the nervous tissue by replacing the damaged cells.[9] Mesenchymal stem cells (MSCs) can be induced to differentiate into neurons or glial cells, which has been used to treat nervous system diseases.[10] However, few studies have reported the therapeutic potency of MSC transplantation on DEACMP. In addition, butylphthalide is a lipid-soluble drug with good blood–brain barrier permeability.[11] This novel drug has been approved for the treatment of cerebral ischemia in China. The treatment mechanisms of butylphthalide include mobilization of circulating endothelial progenitor cells[12] and promoting neurogenesis and neuroplasticity in the brain.[13] In addition, previous study has shown that butylphthalide has a neuroprotective effect in rats after acute CO poisoning.[14] We speculated that a combined therapy of MSC transplantation and butylphthalide might have a promising treatment outcome for DEACMP.
In this study, we prospectively treated 42 patients with either of 3 therapies: combined therapy of MSC transplantation and butylphthalide; MSC transplantation alone; or hyperbaric oxygen therapy. The efficacies and safety profiles of these treatments were examined.
2. Materials and methods
2.1. Patients
This prospective study was carried out from October 2012 to December 2015. Patients were included if they met the following criteria: had a history of CO exposure; had a period of pseudo-recovery; with symptoms of dementia and bladder/bowel dysfunction, and even disturbance of intelligence and conscious; neuroimaging evidences. The exclusion criteria were as follows: history of severe allergy; shock; systemic infection or severe local infection; major organ dysfunction; coagulation disorders; positive of human immunodeficiency virus (HIV), hepatitis B virus (HBV), or syphilis; genetic disorders. This study was approved by the Ethics Committee of our hospital. Informed consent was obtained from each patient.
2.2. MSC culture and characterization
A 8 to 10-cm long fresh umbilical cord was rinsed using normal saline.[15,16] The cord lining, umbilical artery, and umbilical vein were dissected. The Wharton Jelly was obtained and cut into 1-mm3 blocks. The Wharton Jelly blocks were washed using normal saline and then centrifuged at 2000 rpm for 5 minutes. After another washing and centrifugation, the supernatant was transferred to a culture flask, and 30-mL MSC culture medium (Biowit, BW110013, China) was added. The MSCs were incubated with 5% CO2 at 37°C. The culture medium was replenished every 5 to 6 days. The cells were passaged every 10 days. As a result, the third passage of the cells was used for transplantation.
For MSC characterization, the third passage of the cells were digested using 0.25% trypsin and then incubated with the following antibodies: rabbit-anti human CD106-PE, CD45-PE, CD34-PE, CD29-FITC, CD44-FITC, CD-14-PE, CD31-PE, HL-DR-FITC (BD Biosciences) at room temperature for 30 minutes. The cells were examined using a flow cytometer (Beckman).
2.3. Treatment protocol
The patients were randomized into 3 groups. The combined-therapy group (n = 14) received both MSC transplantation and butylphthalide, the MSC group (n = 15) received MSC transplantation alone, and the control group (n = 13) was managed with hyperbaric oxygen therapy and symptomatic treatments.
The MSCs were alternatively injected into the subarachnoid space and the carotid artery using a self-made high-pressure injector. The interval between each injection was 5 to 7 days. A total of 1 to 2 × 107 MSCs were injected each time. A therapy cycle included 4 injections. A second cycle was used if the first cycle did not achieve adequate efficacy. In the combined-therapy group, 100 mL butylphthalide was intravenously infused twice every day. A cycle of butylphthalide treatment was 14 days.
2.4. Outcome evaluation
All patients were evaluated using the Mini-Mental State Examination (MMSE)[17] and the Barthel index of activities of daily living[18] before the treatment and at 1 month, 3 months, and 6 months after the treatment. Computed tomography (CT) and magnetic resonance imaging (MRI) results before and after the treatment were compared.
2.5. Statistical analysis
Continuous data are presented as mean ± standard deviation (SD). Categorical data are presented as percentages. Comparisons were made using the analysis of variance (ANOVA) test or the chi-square test. All statistical analyses were performed using the SPSS 16.0 software (SPSS, Chicago, IL). A P value less than 0.05 was considered statistically significant.
3. Results
3.1. Basic patient information
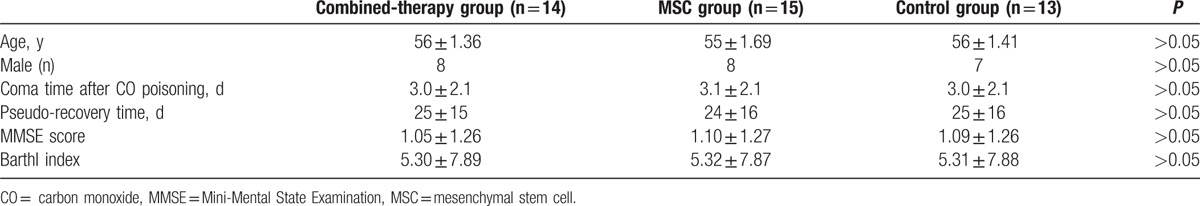
The basic patient information is shown in Table 1. No significant difference was found in basic information among the combined therapy, MSC, and control groups. All patients showed acute neurologic symptoms of dementia, bladder/bowel dysfunction, and disturbance of intelligence and conscious after the pseudo-recovery period.
Table 1.
Basic patient information.
3.2. MSC characterization
Over 90% of the MSCs were positive for CD44, CD73, and CD90. The MSCs were negative for CD34 and CD45.
3.3. Neuroimages
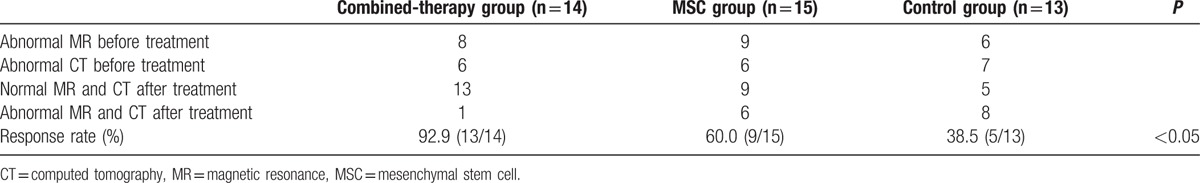
The CT images were obtained from 25 patients. The results showed that 6, 6, and 7 cases of abnormal CT, including mild, intermediate, and severe changes, respectively, were found in the combined therapy, MSC, and control groups before treatment (Table 2). Mild changes in the CT images were defined as unilateral or bilateral low-density lesions in the basal ganglia, and the globus pallidus was primarily involved. Intermediate to severe changes in the CT images were considered as broad low-density lesions in the subcortex white matter, and symmetrical or unsymmetrical low-density patches with unclear borders in the frontal white matter, globus pallidus, lentiform nucleus, and genu of internal capsule. MRI images were obtained from 17 patients. Before treatment, abnormal MR, including mild, intermediate, and severe changes, were observed in 8 cases of combined-therapy group, 9 cases of MSC group, and 6 cases of control group (Table 2). Mild changes in the MRI images were long T1 and long T2 oval abnormal signals in the bilateral globus pallidus. Intermediate to severe changes in the CT images were defined as lesions in the periventricular matter, and lesions symmetrical to the centrum ovale. The lesions were T2-weighted hyperintense and T1-weighted hypointense, with signs of chronic ischemia in the basal ganglia or the globuspallidus. After treatment, we found that only 1 case of abnormal MR and CT was found in combined-therapy group, whereas 6 and 8 cases of abnormal MR and CT were, respectively, observed in the MSC group and the control group (Table 2). As a result, radiological response rate in combined-therapy group (92.9%, 13/14) was significantly higher than that in the MSC (60.0%, 9/15) and control groups (38.5%, 5/13). There were significant differences in the radiological response rate among the 3 groups (P < 0.05; Table 2).
Table 2.
Radiological responses after the treatment.
3.4. Treatment efficacy
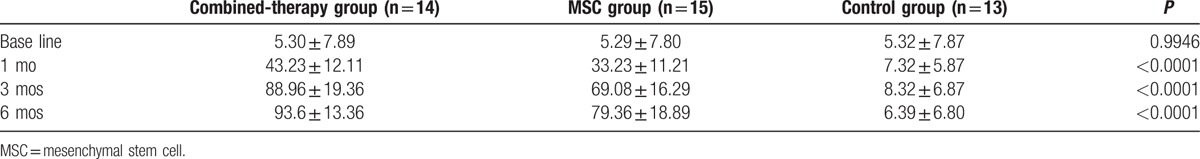
At 1 month, 3 months, and 6 months after the treatment, the MMSE scores (Table 3) were all significantly higher in the combined-therapy group than those in the MSC and control groups, respectively (7.57 ± 5.86 vs 4.25 ± 4.23 and 1.58 ± 1.78; 22.38 ± 7.21 vs 16.36 ± 9.35 and 4.25 ± 3.35; 25.08 ± 4.23 vs 18.42 ± 7.82 and 4.28 ± 2.32; all P < 0.0001). In addition, compared with the MSC and control groups, the Barthl scores (Table 4) were also remarkably increased in the combined-therapy group at 1 month (43.23 ± 12.11 vs 33.23 ± 11.21 and 7.32 ± 5.87), 3 months (88.96 ± 19.36 vs 69.08 ± 16.29 and 8.32 ± 6.87), and 6 months (93.6 ± 13.36 vs 79.36 ± 18.89 and 6.39 ± 6.80) after the treatment (all P < 0.0001).
Table 3.
The MMSE scores of the 3 groups.
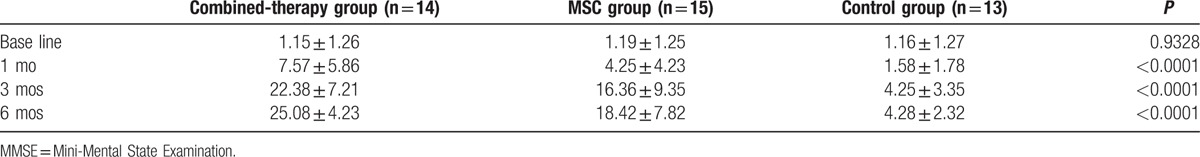
Table 4.
The Barthl scores of the 3 groups.
3.5. Safety profile
In the combined-therapy group, a few patients ran a low fever. No other complications were seen in our patients such as puncture site bleeding or infection.
4. Discussion
Our study showed that the combined therapy of MSC transplantation and butylphthalide significantly improved the MMSE scores and the Barthl scores in comparison with MSC transplantation alone or hyperbaric oxygen therapy.
Stem cells were undifferentiated cells capable of self-renewal and multiple lineage differentiation. The stem cells could be induced in vitro or in vivo to differentiate into certain tissue cells, thereby achieving the aim of treating diseases. It had been shown that the MSCs could be induced into neurons or glial cells under certain conditions.[10] The underlying mechanism was that the MSCs located and surrounded the diseased tissue, then formed a network to repair the damaged neural tissue, enabling the recovery of the lost neural functions.[19] In the microenvironment of the central nervous system, MSCs could promote the repair of the injured tissue and decreasing cell necrosis by secreting brain-derived neurotrophic factor and basic fibroblast growth factor, or stimulating the injured brain area to produce endogenous factors. Previous study revealed that the MSCs were transplanted into the brain and differentiated into neuron-like cells or astrocytes, and then the MSC-derived cells survived around the injured brain area and even migrated to the whole brain.[20] MSCs could differentiate into vascular endothelial cells, thereby protecting the neurons and promoting angiogenesis.[21] MSCs could secret multiple neurotrophic factors and active the endogenous neural stem cells, and also differentiate into neural cells to replace the damaged cells, which was the endogenous route of neural repair.
Several theories had been proposed to explain the mechanisms of stem cells passing through the blood–brain barrier.[22–24] Adhesion molecules might mediate the migration of MSCs. Brain damage could cause a series of inflammatory reactions in the brain, and then the inflammatory mediators induced the endothelial cells to express intercellular adhesion molecules and vascular cell adhesion molecules. The weak point of the blood–brain barrier might be involved in the migration of MSCs. It had been reported that a special transport means might exist. The local microvascular system expressed a certain adhesion molecule, which adhered to the MSCs and mediated its migration through the blood–brain barrier.[25]
Most present treatment methods for DEACMP were designed to target the damaged but sill survived brain cells by providing neurotrophic and functional supports. In DEACMP, necrosis and apoptosis occurred in large amounts of neurons, and demyelination was severe. It was impossible to recover the lost neural functions and mitigate the symptoms merely by reviving the survived neural cells. In our study, MSCs were alternatively injected into the subarachnoid space and the carotid artery to increase the contact volume and contact chance of the MSCs with the injured brain tissue. A self-made high-pressure injector was used to inject the MSCs directly into the carotid artery. In addition, butylphthalide induced the MSCs in vivo into new neurons and glial cells, replacing the dead brain cells and forming new myelin sheath. Butylphthalide was lipid-soluble and could pass the blood–brain barrier, which might help the MSCs pass the blood–brain barrier. The nerve tracts were re-established and the neural functions were repaired. Thus, the neurologic and psychiatric symptoms of DEACMP were improved.
4.1. Limitations
Undoubtedly, our study has several limitations. The first limitation is the small sample of this study; more prospective studies with larger sample size should be carried out to confirm the results of this study. Second, longer longitudinal follow-up is required to confirm the treatment effect of combined therapy of butylphthalide and MSC transplantation in DEACMP.
5. Conclusions
In conclusion, the combined therapy of butylphthalide and MSC transplantation might be safe and effective in treating DEACMP. This treatment method showed superior efficacy in comparison with MSC transplantation alone and hyperbaric oxygen therapy.
Footnotes
Abbreviations: ANOVA = analysis of variance, CO = carbon monoxide, CT = computed tomography, DEACMP = delayed encephalopathy after carbon monoxide poisoning, HBV = hepatitis B virus, HIV = human immunodeficiency virus, MMSE = Mini-Mental State Examination, MRI = magnetic resonance imaging, MSC = mesenchymal stem cell.
The authors report no conflicts of interest.
References
- [1].Harper A, Croft-Baker J. Carbon monoxide poisoning: undetected by both patients and their doctors. Age Ageing 2004;33:105–9. [DOI] [PubMed] [Google Scholar]
- [2].Hsiao CL, Kuo HC, Huang CC. Delayed encephalopathy after carbon monoxide intoxication: long-term prognosis and correlation of clinical manifestations and neuroimages. Acta Neurol Taiwan 2004;13:64–70. [PubMed] [Google Scholar]
- [3].Thom SR, Bhopale VM, Fisher D, et al. Delayed neuropathology after carbon monoxide poisoning is immune-mediated. Proc Natl Acad Sci U S A 2004;101:13660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tofighi R, Tillmark N, Dare E, et al. Hypoxia-independent apoptosis in neural cells exposed to carbon monoxide in vitro. Brain Res 2006;1098:1–8. [DOI] [PubMed] [Google Scholar]
- [5].Gorman D, Drewry A, Huang YL, et al. The clinical toxicology of carbon monoxide. Toxicology 2003;187:25–38. [DOI] [PubMed] [Google Scholar]
- [6].Thom SR, Ohnishi ST, Ischiropoulos H. Nitric oxide released by platelets inhibits neutrophil B2 integrin function following acute carbon monoxide poisoning. Toxicol Appl Pharmacol 1994;128:105–10. [DOI] [PubMed] [Google Scholar]
- [7].Hsiao CL, Kuo HC, Huang CC. Delayed encephalopathy after carbon monoxide intoxication--long-term prognosis and correlation of clinical manifestations and neuroimages. Acta Neurologica Taiwanica 2004;13:64–70. [PubMed] [Google Scholar]
- [8].Huang D, Wujek J, Kidd G, et al. Chronic expression of monocyte chemoattractant protein-1 in the central nervous system causes delayed encephalopathy and impaired microglial function in mice. FASEB J 2005;19:761–72. [DOI] [PubMed] [Google Scholar]
- [9].Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science 1999;284:1168–70. [DOI] [PubMed] [Google Scholar]
- [10].Jeong JA, Gang EJ, Hong SH, et al. Rapid neural differentiation of human cord blood-derived mesenchymal stem cells. Neuroreport 2004;15:1731–4. [DOI] [PubMed] [Google Scholar]
- [11].Diao XX, Zhong K, Li XL, et al. Isomer-selective distribution of 3-n-butylphthalide (NBP) hydroxylated metabolites, 3-hydroxy-NBP and 10-hydroxy-NBP, across the rat blood-brain barrier. Acta Pharmacol Sin 2015;36:1520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhao H, Yun W, Zhang Q, et al. Mobilization of circulating endothelial progenitor cells by dl-3-n-butylphthalide in acute ischemic stroke patients. J Stroke Cerebrovasc Dis 2016;25:752–60. [DOI] [PubMed] [Google Scholar]
- [13].Yang LC, Li J, Xu SF, et al. L-3-n-butylphthalide promotes neurogenesis and neuroplasticity in cerebral ischemic rats. CNS Neurosci Ther 2015;21:733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li Q, Cheng Y, Bi M, et al. Effects of N-butylphthalide on the activation of Keap1/Nrf-2 signal pathway in rats after carbon monoxide poisoning. Environ Toxicol Pharmacol 2015;40:22–9. [DOI] [PubMed] [Google Scholar]
- [15].Phuc PV, Ngoc VB, Lam DH, et al. Isolation of three important types of stem cells from the same samples of banked umbilical cord blood. Cell Tissue Bank 2012;13:341–51. [DOI] [PubMed] [Google Scholar]
- [16].Bieback K, Kern S, Kluter H, et al. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004;22:625–34. [DOI] [PubMed] [Google Scholar]
- [17].Pangman VC, Sloan J, Guse L. An examination of psychometric properties of the mini-mental state examination and the standardized mini-mental state examination: implications for clinical practice. Appl Nurs Res 2000;13:209–13. [DOI] [PubMed] [Google Scholar]
- [18].Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- [19].Crigler L, Robey RC, Asawachaicharn A, et al. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol 2006;198:54–64. [DOI] [PubMed] [Google Scholar]
- [20].Jin GZ, Cho SJ, Choi EG, et al. Rat mesenchymal stem cells increase tyrosine hydroxylase expression and dopamine content in ventral mesencephalic cells in vitro. Cell Biol Int 2008;32:1433–8. [DOI] [PubMed] [Google Scholar]
- [21].Kim YJ, Park HJ, Lee G, et al. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia 2009;57:13–23. [DOI] [PubMed] [Google Scholar]
- [22].Park WS, Sung SI, Ahn SY, et al. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS One 2015;10:e0120893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhu LH, Bai X, Zhang N, et al. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res 2014;1563:13–21. [DOI] [PubMed] [Google Scholar]
- [24].Zhang X, Zhang Q, Li W, et al. Therapeutic effect of human umbilical cord mesenchymal stem cells on neonatal rat hypoxic-ischemic encephalopathy. J Neurosci Res 2014;92:35–45. [DOI] [PubMed] [Google Scholar]
- [25].Stanimirovic DB, Wong J, Shapiro A, et al. Increase in surface expression of ICAM-1, VCAM-1 and E-selectin in human cerebromicrovascular endothelial cells subjected to ischemia-like insults. Acta Neurochir Suppl 1997;70:12–6. [DOI] [PubMed] [Google Scholar]


