Abstract
Background:
Both anterior cervical discectomy and fusion (ACDF) and anterior cervical corpectomy and fusion (ACCF) are used to treat multilevel cervical spondylotic myelopathy (mCSM); however, which one is better treatment for mCSM remains considerable controversy. A meta-analysis was performed to compare clinical outcomes, radiographic outcomes, and surgical outcomes between ACDF and ACCF in treatment for mCSM.
Methods:
An extensive search of literature was performed in Pubmed/MEDLINE, Embase, the Cochrane library, CNKI, and WANFANG databases on ACDF versus ACCF treatment for mCSM from January 2011 to August 2016. The following variables were extracted: length of hospital stay, blood loss, operation time, Japanese Orthopedic Association (JOA) scores, Neck Disability Index (NDI) score, fusion rate, Cobb angles of C2 to C7, dysphagia, hoarseness, C5 palsy, infection, cerebral fluid leakage, donor site pain, epidural hematoma, graft subsidence, graft dislodgment, pseudoarthrosis, and total complications. Data analysis was conducted with RevMan 5.3 and STATA 12.0.
Results:
A total of 8 studies containing 878 patients were included in our study. The results showed that ACDF is better than ACCF in the angle of C2 to C7 at the final follow-up (P < 0.00001, standardized mean difference = 4.76 [3.48, 6.03]; heterogeneity: P = 0.17, I2 = 43%), C5 plasy (P = 0.02, odds ratio [OR] 0.42, 95% confidence interval [CI] 0.21, 0.86; heterogeneity: P = 0.52, I2 = 0%), blood loss (P < 0.00001, standardized mean difference = −53.12, 95% CI −64.61, −41.64; heterogeneity: P = 0.29, I2 = 20%), fusion rate (P = 0.04, OR 2.54, 95% CI 1.05, 6.11; heterogeneity: P = 0.29, I2 = 20%), graft subsidence (P = 0.004, OR 0.11, 95% CI 0.02, 0.48; heterogeneity: P = 0.94, I2 = 0%), and total complications (P = 0.0009, OR 0.56, 95% CI 0.40, 0.79; heterogeneity: P = 0.29, I2 = 18%).However, there are no significant differences in length of hospital stay, operation time, JOA scores, NDI scores, preoperative angle of C2 to C7, dysphagia, hoarseness, infection, cerebral fluid leakage, donor site pain, epidural hematoma, graft dislodgment, and pseudoarthrosis (all P > 0.05).
Conclusions:
Based on our meta-analysis, our results suggest that both ACDF and ACCF are good plans in clinical outcomes; however, ACDF is a better choice in radiographic outcomes and total complications for the treatment of multilevel CSM.
Keywords: anterior cervical corpectomy and fusion, anterior cervical discectomy and fusion, clinical outcomes, multilevel cervical spondylotic myelopathy, radiographic outcomes, surgical outcomes
1. Introduction
Cervical spondylotic myelopathy (CSM), a common clinical degenerative disease, seriously influences quality of life and even leads to disability for the old population.[1–3] CSM is usually caused by narrowing of the cervical spinal canal due to degenerative and congenital changes.[3–6] The selection of optimal surgical treatment for CSM, especially for multilevel cervical spondylotic myelopathy (mCSM), remains controversial.[1–9] Surgeries mainly involved anterior and posterior approaches, including anterior cervical discectomy and fusion (ACDF),[10–12] anterior cervical corpectomy and fusion (ACCF),[11–15] laminoplasty,[16–20] laminectomy,[12,18–22] and laminectomy with fusion.[20–23] ACDF for treating CSM was firstly introduced by Smith and Robinson[24] and Cloward[25]; the anterior procedure has become the most widely used surgical choice.[26] Among the anterior approaches, ACDF can decompress the anterior spinal cord and preserve the stability of the spinal column[27–33]; however, ACDF may have a high risk of incomplete decompression, limited visual exposure, and injury to the cord.[30–36] ACCF also provides a more extensive decompression and serves as a source for autografting.[37–41] Unfortunately, ACCF is a more difficult spinal surgery to perform and also has a higher incidence of complications, such as injury to the spinal cord or nerve roots, excessive bleeding, graft displacement, or extrusion.[40–45]
Previous meta-analyses[46,47] reviewed mainly focused on the comparison between ACDF and ACCF for 1-level or 2-level CSM, few variables, or included studies from 1980s or1990s. However, the clinical efficacy and complications of ACDF compared with ACCF in patients with mCSM still remain controversial. The purpose of this meta-analysis is to compare clinical outcomes, radiographic outcomes, and surgical outcomes of ACDF compared with ACCF in treatment for mCSM.
2. Materials and methods
2.1. Ethics statement
There is no need to seek informed consent from patients, since this is a meta-analysis based on the published data, without any potential harm to the patients; this is approved by Ethics Committee of The Third Hospital of HeBei Medical University.
2.2. Search strategy
An extensive search of literature was performed in PubMed, Embase, the Cochrane library, CNKI, and WANFANG databases. The following key words were used for search: “anterior cervical discectomy and fusion,”, “anterior cervical corpectomy and fusion,” “multilevel cervical spondylotic myelopathy,” from January 2011 to August 2016, with various combinations of the operators “AND” and “OR”. Language was restricted to Chinese and English.
2.3. Inclusion criteria
Studies were included if they met the following criteria: randomized or nonrandomized controlled study; age greater than or equal to 18 years; studies compared ACDF with ACCF for treatment of CSM; 3 or 4 levels cervical spondylotic myelopathy; follow-up more than 2 years.
2.4. Exclusion criteria
Studies were excluded if they met the following criteria: dealt only with combined ACDF and ACCF surgery versus ACDF or ACCF alone for treatment of CSM; had an average follow-up time of less than 2 years; had repeated data; did not report outcomes of interest; in vitro human cadaveric biomechanical studies; earlier trial, reviews, and case-reports; have ossification of posterior longitudinal ligament.
2.5. Selection of studies
Two reviewers independently reviewed all subjects, abstracts, and the full text of articles. Then the eligible trials were selected according to the inclusion criteria. When consensus could not be reached, a third reviewer was consulted to resolve the disagreement.
2.6. Data extraction and management
Two reviewers extracted data independently. The data extracted included the following categories: study ID; study design; study location; total patients; follow-up; mean age; sex, clinical outcomes—length of hospital stay, preoperative and the final follow-up Japanese Orthopedic Association (JOA) scores, preoperative and the final follow-up Neck Disability Index (NDI) scores; radiographic outcomes—preoperative and the final follow-up Cobb angles of C2 to C7, fusion rate, graft subsidence, graft dislodgment; and surgical outcomes—blood loss, operation time, dysphagia, hoarseness, C5 palsy, infection, cerebral fluid leakage, donor site pain, epidural hematoma, and pseudoarthrosis.
2.7. Statistical analysis
We analyzed data by RevMan 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) and STATA 12.0 (Stata Corporation, College Station, TX). Odds ratio (OR), as a summary statistic, was applied to analyze dichotomous variables, and continuous variables were analyzed by standardized mean difference (SMD). Both were reported with 95% confidence intervals (CIs), and P value <0.05 presented statistical significance. We assessed statistical heterogeneity by the I2 tests, which described the proportion of the total variation from 0% to 100% in meta-analysis assessments. If I2 was >50%, which implies an obvious heterogeneity, we chose random-effects model using for the analysis If I2 was ≤50%, which implies no significant heterogeneity, fixed-effects model was used to analyze.[48,49]
2.8. Test for risk of publication bias
Funnel plot as a visual inspection was used to assess publication bias. If there is publication bias, funnel plot should be asymmetric, but if there is no publication bias, funnel plot should be symmetric. Egger and Begg tests were used to evaluate the funnel plot asymmetry; if P < 0.05, we considered it as a significance level.
3. Results
3.1. Search results
We had searched 206 English studies in MEDLINE and Embase, and 86 Chinese studies in WANFANG and CNKI databases. Of these, 50 English articles and 51 Chinese articles after duplicates were removed; 128 English articles and 27 Chinese articles were excluded due to unrelated studies. Twenty-two English articles and 6 Chinese articles were excluded due to eligibility criteria. As a result, a total of 8 studies were identified for this meta-analysis. The literature search procedure is shown in Fig. 1.
Figure 1.

Flow diagram of study selection.
3.2. Baseline characteristics and quality assessment
In all, 878 patients with mCSM from 8 studies were included in our study. Table 1 shows the baseline characteristics of included articles.
Table 1.
Characteristics of included studies.

All included studies were retrospective studies. Newcastle Ottawa Quality Assessment Scale (NOQAS) was applied to estimate the quality of each study. We used NOQAS, the maximum of 9 points, to assess quality of selection for nonrandomized case-controlled studies and cohort studies in term of comparability, exposure, and outcomes. Among these studies, 5 studies scored 8 points and 3 studies scored 7 points. Therefore, each study had relatively high quality (Table 2).
Table 2.
The quality assessment according to the Newcastle Ottawa Quality Assessment Scale (NOQAS) of each study.

3.3. Clinical outcomes
3.3.1. JOA score
Seven studies (813 of 878 patients)[50–56] reported preoperative and the final follow-up JOA scores between ACDF and ACCF. The meta-analysis showed that there is no significant difference between ACDF and ACCF in preoperative and the final follow-up JOA scores (P = 0.29, SMD = 0.13 [−0.11, 0.37]; heterogeneity: P = 0.63, I2 = 0%, fixed-effects model, Fig. 2; P = 0.62, SMD = 0.06 [−0.18, 0.30]; heterogeneity: P = 0.23, I2 = 26%, fixed-effects model, Fig. 3).
Figure 2.
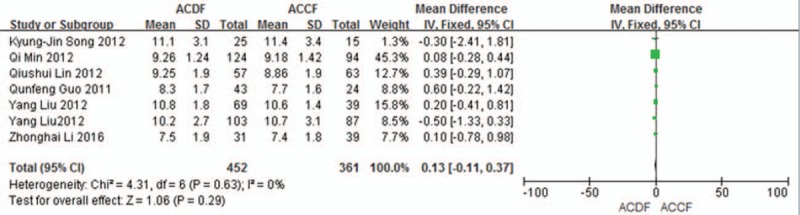
The standardized mean difference (SMD) estimate preoperative JOA score in 2 groups. CI = confidence interval, df = degrees of freedom, JOA = Japanese Orthopedic Association, M-H = Mantel–Haenszel.
Figure 3.

Forest plot showing at the final follow-up JOA score in 2 groups. CI = confidence interval, df = degrees of freedom, JOA = Japanese Orthopedic Association, M-H = Mantel–Haenszel.
3.3.2. NDI score
Three studies (368 of 878 patients)[50,51,55] reported preoperative and the final follow-up NDI scores between ACDF and ACCF. The meta-analysis showed that there is no significant difference between ACDF and ACCF in preoperative and the final follow-up NDI scores (P = 0.38, SMD = 0.28 [−0.35, 0.91]; heterogeneity: P = 0.59, I2 = 0%, fixed-effects model, Fig. 4; P = 0.36, SMD = −0.49 [−1.54, 0.56]; heterogeneity: P = 0.07, I2 = 61%, random-effects model, Fig. 5).
Figure 4.

The standardized mean difference (SMD) estimate preoperative NDI score in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel, NDI = Neck Disability Index.
Figure 5.

The standardized mean difference (SMD) estimate at the final follow-up NDI score in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel, NDI = Neck Disability Index.
3.3.3. Hospital stay
Two studies (110 of 878 patients)[52,55] reported hospital stay between ACDF and ACCF. The meta-analysis showed that there is no significant difference between ACDF and ACCF in hospital stay (P = 0.40, SMD = −3.40 [−11.31, 4.51]; heterogeneity: P = 0.00004, I2 = 92%, random-effects model, Fig. 6).
Figure 6.
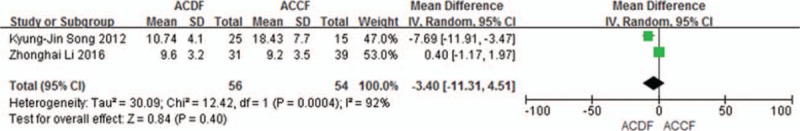
The standardized mean difference (SMD) estimate hospital stay in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.4. Radiographic outcomes
3.4.1. The angle of C2 to C7
Three studies (243 of 878 patients)[50,55,57] reported preoperative and the final follow-up angle of C2 to C7 between ACDF and ACCF. The meta-analysis showed that there is no difference between ACDF and ACCF in preoperative angle of C2 to C7 (P = 0.33, SMD = −0.42 [−1.27, 0.43]; heterogeneity: P = 0.67, I2 = 0%, fixed-effects model, Fig. 7), but significant difference in the final follow-up angle of C2 to C7 (P < 0.00001, SMD = 4.76 [3.48, 6.03]; heterogeneity: P = 0.17, I2 = 43%, fixed-effects model, Fig. 8).
Figure 7.
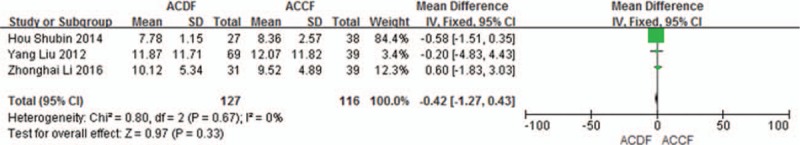
The standardized mean difference (SMD) estimate preoperative the angle of C2 to C7 in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
Figure 8.

The standardized mean difference (SMD) estimate at the final follow-up of the angle of C2 to C7 in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.4.2. Fusion rate
Five studies (350 of 878 patients)[50,52,54–55,57] reported fusion rate between ACDF and ACCF. The meta-analysis showed that ACDF have a better result of fusion rate than that of ACCF (P = 0.04, OR 2.54, 95% CI 1.05, 6.11; heterogeneity: P = 0.29, I2 = 20%, fixed-effects model, Fig. 9).
Figure 9.

Forest plot showing fusion rate in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.4.3. Graft subsidence
Four studies (365 of 878 patients)[50,53–55] reported incidence of graft subsidence between ACDF and ACCF. The meta-analysis showed that ACDF is less than ACCF in incidence of graft subsidence (P = 0.004, OR 0.11, 95% CI 0.02, 0.48; heterogeneity: P = 0.94, I2 = 0%, fixed-effects model, Fig. 10).
Figure 10.

Forest plot showing graft subsidence in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.4.4. Graft dislodgment
Three studies (298 of 878 patients)[50,53,55] reported incidence of graft dislodgment between ACDF and ACCF. The meta-analysis showed that there is no significant difference (P = 0.27, OR 0.46, 95% CI 0.12, 1.83; heterogeneity: P = 0.45, I2 = 0%, fixed-effects model, Fig. 11).
Figure 11.

Forest plot showing graft dislodgment in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.5. Surgical outcomes
3.5.1. Blood loss
Five studies (430 of 878 patients)[50,53–55,57] reported blood loss between ACDF and ACCF. The meta-analysis showed that ACDF is less than ACCF in blood loss (P < 0.00001, SMD = −53.12 [−64.61, −41.64]; heterogeneity: P = 0.29, I2 = 20%, fixed-effects model, Fig. 12).
Figure 12.

The standardized mean difference (SMD) estimate blood loss in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.5.2. Operation time
Six studies (470 of 878 patients)[50,52–55,57] reported operation time between ACDF and ACCF. The meta-analysis showed that there is no significant difference between ACDF and ACCF in operation time [(P = 0.40, SMD = −8.99 [−29.76, 11.79]; heterogeneity: P < 0.00001, I2 = 93%, random-effects model, Fig. 13).
Figure 13.

The standardized mean difference (SMD) operation time in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.5.3. Total complications
Seven studies (838 of 878 patients)[50–51,53–57] reported incidence of total complications between ACDF and ACCF. The meta-analysis showed that ACDF is less than ACCF in incidence of total complications (P = 0.0009, OR 0.56, 95% CI 0.40, 0.79; heterogeneity: P = 0.29, I2 = 18%, fixed-effects model, Fig. 14).
Figure 14.

Forest plot showing number of total complications in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.5.4. C5 plasy
Six studies (773 of 878 patients)[50–51,53–56] reported incidence of C5 plasy between ACDF and ACCF. The meta-analysis showed that ACDF is less than ACCF in incidence of C5 plasy (P = 0.02, OR 0.42, 95% CI 0.21, 0.86; heterogeneity: P = 0.52, I2 = 0%, fixed-effects model, Fig. 15).
Figure 15.

Forest plot showing C5 plasy in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.5.5. Infection
Four studies (581 of 878 patients)[50–51,56–57] reported incidence of infection between ACDF and ACCF. The meta-analysis showed that there is no significant difference (P = 0.12, OR 0.28, 95% CI 0.06, 1.39; heterogeneity: P = 0.98, I2 = 0%, fixed-effects model, Fig. 16).
Figure 16.
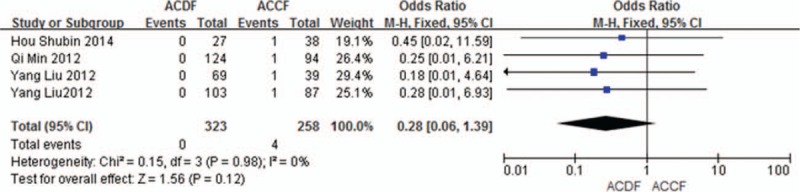
Forest plot showing infection in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.5.6. Cerebral fluid leakage
Seven studies (838 of 878 patients)[50–51,53–57] reported incidence of cerebral fluid leakage between ACDF and ACCF. The meta-analysis showed that there is no significant difference (P = 0.29, OR 1.67, 95% CI 0.65, 4.29; heterogeneity: P = 0.81, I2 = 0%, fixed-effects model, Fig. 17).
Figure 17.

Forest plot showing cerebral fluid leakage in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.5.7. Hoarseness
Seven studies (811 of 878 patients)[50–53,55–57] reported incidence of hoarseness between ACDF and ACCF. The meta-analysis showed that there is no significant difference (P = 0.71, OR 0.88, 95% CI 0.45, 1.73; heterogeneity: P = 1.00, I2 = 0%, fixed-effects model, Fig. 18).
Figure 18.

Forest plot showing hoarseness in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.5.8. Dysphagia
Seven studies (811 of 878 patients)[50–53,55–57] reported incidence of dysphagia between ACDF and ACCF. The meta-analysis showed that there is no significant difference (P = 0.83, OR 1.06, 95% CI 0.63, 1.78; heterogeneity: P = 0.91, I2 = 0%, fixed-effects model, Fig. 19).
Figure 19.

Forest plot showing dysphagia in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.5.9. Epidural hematoma
Four studies (365 of 878 patients)[50,53–55] reported incidence of epidural hematoma between ACDF and ACCF. The meta-analysis showed that there is no significant difference (P = 0.22, OR 0.41, 95% CI 0.10, 1.69; heterogeneity: P = 0.95, I2 = 0%, fixed-effects model, Fig. 20).
Figure 20.

Forest plot showing epidural hematoma in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.5.10. Donor site pain
Two studies (110 of 878 patients)[52,55] reported incidence of donor site pain between ACDF and ACCF. The meta-analysis showed that there is no significant difference (P = 0.14, OR 0.29, 95% CI 0.06, 1.50; heterogeneity: P = 0.20, I2 = 40%, fixed-effects model, Fig. 21).
Figure 21.

Forest plot showing donor site pain in two groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.5.11. Pseudoarthrosis
Two studies (107 of 878 patients)[52,54] reported incidence of pseudoarthrosis between ACDF and ACCF. The meta-analysis showed that there is no significant difference (P = 0.53, OR 1.84, 95% CI 0.27, 12.43; heterogeneity: P = 0.96, I2 = 0%, fixed-effects model, Fig. 22).
Figure 22.

Forest plot showing pseudoarthrosis in 2 groups. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel.
3.6. Publication bias
After a detection of publication bias by STATA 12.0, there was no publication bias found for all included studies (all P > 0.05). The funnel plot did not indicate any publication bias in C5 plasy (Begg, P = 0.086; Egger, P = 0.14); infection (Begg, P = 0.734; Egger, P = 0.427); pseudoarthrosis (Begg, P = 0.296; Egger, P = 0.093); cerebral fluid leakage (Begg, P = 1.00; Egger, P = 0.534); fusion rate (Begg, P = 0.296; Egger, P = 0.240); graft subsidence (Begg, P = 1.00; Egger, P = 0.930); graft dislodgment (Begg, P = 1.00); hoarseness (Begg, P = 1.00); donor site pain (Begg, P = 1.00); dysphagia (Begg, P = 1.00); total complications (Begg, P = 1.00); epidural hematoma (Begg, P = 1.00;); the angle of C2 to C7 before surgery (Begg, P = 0.296; Egger, P = 0.228); the angle of C2 to C7 at final follow-up (Begg, P = 0.296; Egger, P = 0.228); JOA score before surgery (Begg, P = 1.000; Egger, P = 0.443); JOA score at final follow-up (Begg, P = 0.764; Egger, P = 0.723); NDI score before surgery (Begg, P = 1.000; Egger, P = 0.997); NDI score at final follow-up (Begg, P = 0.308; Egger, P = 0.619); blood loss (Begg, P = 0.462; Egger, P = 0.558); operation time (Begg, P = 0.06; Egger, P = 0.055); hospital stay (Begg, P = 1.00).
4. Discussion
Recently, some studies[50–57] reported on the surgical plan for mCSM; however, as for mCSM, the option of surgical approach remains debated.[23,52–55] The common operative options included anterior, posterior, and combined anteroposterior approaches. In the 1960s, posterior approaches including laminectomy and laminoplasty were widely used in the treatment of mCSM.[24–26,58,59] But recently the anterior approaches are extensively applied for surgical treatment of mCSM, which can directly decompress the spinal cord and nerve root due to discs herniation or ossification.[3–7,60] Everything has double-edged sword. Complications, such as graft migration, collapse or displacement, hoarseness, dysphagia, C5 palsy, cerebral fluid leakage and infection, of anterior approach are difficult to avoid and these are worth our attention.[61,62]
Recently, Liu et al[50] reported the comparison of 3 reconstructive techniques in the treatment for mCSM. In terms of clinical outcomes, radiological parameters, and complication incidence, Liu et al believed that the hybrid surgery (1-level corpectomy plus 1-level discectomy) was the best alternative compared with ACDF and ACCF. Shamji et al[63] reviewed studies on the same topic, but concluded that all 3 operative approaches are effective strategies for the anterior surgical option of mCSM. However, which surgery is a better option in the treatment of mCSM remains unclear. Wen et al[46] and Han et al[47] performed a meta-analysis on comparison of surgical treatment for mCSM between ACDF and ACCF. And they had the same conclusion that both ACDF and ACCF are effective option in treatment for mCSM. Nevertheless, some included studies reported on 1 or 2-level CSM, and some published in 1980s or 1990s influenced accuracy and rigor of the results. So, we collected 8 articles including 878 cases with 3 or 4-level CSM using ACDF and ACCF from January 2011 to August 2016 to compare which one is better for mCSM.
In this meta-analysis, we carried on strict eligibility criteria. Although no randomized controlled trial (RCT) studies were included in our study, all included studies were of high quality according to the NOQAS, and the baseline variables were similar. Thus, we considered the included reports suitable for meta-analysis. We assessed clinical outcomes (length of hospital stay, and JOA and NDI scores), radiographic outcomes (Cobb angles of C2–C7, fusion rate, graft subsidence, and graft dislodgment), and surgical outcomes (blood loss, operation time, dysphagia, hoarseness, C5 palsy, infection, cerebral fluid leakage, donor site pain, epidural hematoma, and pseudoarthrosis) in the meta-analysis. The results showed that there was no marked difference in clinical outcomes (length of hospital stay, and JOA and NDI scores) between ACDF and ACCF. In terms of radiographic outcomes, preoperative Cobb angles of C2 to C7 and incidence of graft dislodgment were similar in the 2 groups. However, in the final follow-up, Cobb angles of C2 to C7, fusion rate, and incidence of graft subsidence ACDF had better results. Although in total complications and blood loss, ACDF are better than these of ACCF, both ACDF and ACCF were similar in operation time, dysphagia, hoarseness, C5 palsy, infection, cerebral fluid leakage, donor site pain, epidural hematoma, and pseudoarthrosis.
In our meta-analysis of preoperative and the final follow-up, JOA and NDI scores were similar in the 2 groups. However, compared with preoperative JOA and NDI, both groups demonstrated a significant increase in final follow-up JOA scores and decrease in final follow-up NDI scores, indicating both ACDF and ACCF can effectively decompress spinal cord by directly removing the anterior pathogenic structures, and in the long term the clinical outcomes were similar in the 2 groups. Besides, in terms of length of hospital stay, the 2 groups were similar, which was different from the result of the study by Han et al.[47] Spinal surgeons can master skillfully surgical techniques, and more than half of Han et al's old included articles may cause difference.
Regarding radiographic outcomes, we found that ACDF and ACCF were similar in preoperative Cobb angles of C2 to C7, and Cobb angles of C2 to C7 at the final follow-up was significantly increased in the 2 groups, but the increase was better in the ACDF group. ACDF can provide more points of distraction and fixation except for the graft and interbody space shaping than these of ACCF. Besides, ACDF can also restore alignment by pulling the involved vertebral bodies toward the lordotic ventral plate.[22–28,64–66] However, ACCF grafts may straighten the cervical spinal column between the remaining vertebral bodies and have fewer force fulcrum, leading to imbalanced force distribution.[36–42] As for graft dislodgment, both the groups had a similar result. Nevertheless, ACDF produced more satisfactory results in incidence of graft subsidence. Obviously, ACDF can offer more fixation points to hold the construct rigidly in place, but ACCF provides only 2 points of fixation, which can explain the reason that more graft-related problems occur in the ACCF group. Previous meta-analyses[46,47] showed that fusion rate between the 2 groups was not significantly different, which is in contrast to our result. Considering some flaws mentioned above in previous meta-analyses,[46,47] we regard the fusion rate was better in ACDF.
We selected blood loss, operation time, and complication-related outcomes to evaluate surgical outcomes and found that ACDF have better results in blood loss, C5plasy, and total complications, whereas other variables including operation time, dysphagia, hoarseness, infection, cerebral fluid leakage, donor site pain, epidural hematoma, and pseudoarthrosis were similar between the 2 groups. C5 palsy is considered as an important complication after cervical decompression surgery. Sakaura et al[67] reported the average incidence was 4.6% (range from 0% to 30%), but pathogenesis of C5 palsy remains unclear till now; multilevel corpectomy may lead to a significant drift of spinal cord away from the ventral side. There were similar rates of dysphagia and hoarseness in both the groups. Dysphagia and hoarseness were common complications after multilevel anterior cervical surgery,[68] which may be caused by trachea and esophagus traction.[69] There is no marked difference in pseudarthrosis, indicating that both ACDF and ACCF can be able to establish a solid arthrodesis and provide inherent mechanical stability of the postdecompressed cervical spine.
There are several limitations of this study. First, there was no RCT comparing the outcomes between ACDF and ACCF; we need RCT for performing further study. Second, the statistical power could be improved in the future by including more studies. Due to the small number of included studies, some parameters could not be analyzed by subgroups to avoid a high heterogeneity, which may exert instability on the consistency of the outcomes. Third, the follow-up of all included article was up to 2 years, which was not enough to observe the long-term recovery and complications. Fourth, the searching strategy was restricted to articles published in English and Chinese languages. Articles with potentially high-quality data that were published in other languages were not included because of anticipated difficulties in obtaining accurate medical translations.
In summary, our meta-analysis showed that both ACDF and ACCF for multilevel CSM have effective results in clinical outcomes (length of hospital stay, and JOA and NDI scores). Because ACDF offers more fixation points and ACCF provides only 2 points of fixation, making ACDF hold the construct rigidly in place. So in radiographic outcomes (at the final follow-up Cobb angles of C2 to C7, fusion rate, and graft subsidence), ACDF had more advantages. Although almost single complication was similar between two groups, but in terms of number of total complications, ACDF produced more satisfactory efficacy. Further studies with high methodological quality and long-term follow-up periods are needed to evaluate the 2 procedures for mCSM treatment.
Footnotes
Abbreviations: ACDF = anterior cervical discectomy and fusion; ACCF = anterior cervical corpectomy and fusion; CI = confidence interval; CSM = cervical spondylotic myelopathy; JOA = Japanese Orthopedic Association; mCSM = multilevel cervical spondylotic myelopathy; NDI = Neck Disability Index; OR = odds ratio; SMD = standardized mean difference.
Authors’ contributions: Conceived and designed the study: DWY; collected data: TW and HW; analyzed the data: TW, SL, and FYL; wrote the paper: TW and HW.
The authors declare that they have no conflicts of interest regarding this study.
References
- [1].Goffin J, Van LJ, Van CF, et al. A clinical analysis of 4- and 6-year follow-up results after cervical disc replacement surgery using the Bryan Cervical Disc Prosthesis. J Neurosurg Spine 2010;12:261–9. [DOI] [PubMed] [Google Scholar]
- [2].Lee SB, Cho KS, Kim JY, et al. Hybrid surgery of multilevel cervical degenerative disc disease: review of literature and clinical results. J Korean Neurosurg Soc 2012;52:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kang L, Lin D, Ding Z, et al. Artificial disk replacement combined with midlevel ACDF versus multilevel fusion for cervical disk disease involving 3 levels. Orthopedics 2013;36:e88–94. [DOI] [PubMed] [Google Scholar]
- [4].Hey HWD, Hong CC, Long AS, et al. Is hybrid surgery of the cervical spine a good balance between fusion and arthroplasty? Pilot results from a single surgeon series. Eur Spine J 2013;22:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shen C, Shen Y, Ding W, et al. Contrastive analysis of neck axial symptoms after hybrid surgery or traditional anterior cervical discectomy and fusion fortreatment of two-level cervical disease. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2013;27:58–61. [PubMed] [Google Scholar]
- [6].Shin DA, Yi S, Yoon DH, et al. Artificial disc replacement combined with fusion versus two-level fusion in cervical two-level disc disease. Spine (Phila Pa 1976) 2009;34:1153–9. discussion [1160–1]. [DOI] [PubMed] [Google Scholar]
- [7].Grasso G. Clinical and radiological features of hybrid surgery in multilevel cervical degenerative disc disease. Eur Spine J 2015;24suppl 7:842–8. [DOI] [PubMed] [Google Scholar]
- [8].Mao N, Wu J, Zhang Y, et al. A comparison of anterior cervical corpectomy and fusion combined with artificial disc replacement and cage fusion in patients with multilevel cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2015;40:1277–83. [DOI] [PubMed] [Google Scholar]
- [9].Ding F, Jia Z, Wu Y, et al. Fusion-nonfusion hybrid construct versus anterior cervical hybrid decompression and fusion: a comparative study for 3-level cervical degenerative disc diseases. Spine (Phila Pa1976) 2014;39:1934–42. [DOI] [PubMed] [Google Scholar]
- [10].Cho BY, Lim J, Sim HB, et al. Biomechanical analysis of the range of motion after placement of a two-level cervical ProDisc-C versus hybrid construct. Spine (Phila Pa 1976) 2010;35:1769–76. [DOI] [PubMed] [Google Scholar]
- [11].Lee MJ, Dumonski M, Phillips FM, et al. Disc replacement adjacent to cervical fusion: a biomechanical comparison of hybrid construct versus two-level fusion. Spine (Phila Pa 1976) 2011;36:1932–9. [DOI] [PubMed] [Google Scholar]
- [12].Barrey C, Campana S, Persohn S, et al. Cervical disc prosthesis versus arthrodesis using one-level, hybrid and two-level constructs: an in vitro investigation. Eur Spine J 2012;21:432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Park J, Shin JJ, Lim J. Biomechanical analysis of disc pressure and facet contact force following simulated two-level cervical surgeries (fusion and arthroplasty) and hybrid surgery. World Neurosurg 2014;82:1388–93. [DOI] [PubMed] [Google Scholar]
- [14].Jacobs W, Anderson PG, Limbeek JV, et al. Single or double-level anteriorinterbody fusion techniques for cervical degenerative disc disease. Cochrane Database Syst Rev 2004;18:CD004958. [DOI] [PubMed] [Google Scholar]
- [15].Vicario C, Lopez-Oliva F, Sanchez-Lorente T, et al. Anterior cervical fusion with tantalum interbody implants. Clinical and radiological results in a prospective study. Neurocirugia (Astur) 2006;17:132–9. [discussion 139]. [PubMed] [Google Scholar]
- [16].Burkus JK, Traynelis VC, Haid RW, et al. Clinical and radiographic analysis of an artificial cervical disc: 7-year follow-up from the Prestige prospective randomized controlled clinical trial. J Neurosurg Spine 2014;21:516–28. [DOI] [PubMed] [Google Scholar]
- [17].Dan X, Ma XL, Ma JX, et al. A meta-analysis of cervical arthroplasty compared to anterior cervical discectomy and fusion for single-level cervical disc disease. J Clin Neurosci 2013;20:970–8. [DOI] [PubMed] [Google Scholar]
- [18].Fallah A, Akl EA, Ebrahim S, et al. Anterior cervical discectomy with arthroplasty versus arthrodesis for single-level cervical spondylosis: a systematic review and meta-analysis. PLoS One 2012;7:e43407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [20].Lian XF, Xu JG, Zeng BF, et al. Noncontiguous anterior decompression and fusion for multilevel cervical spondylotic myelopathy: a prospective randomized control clinical study. Eur Spine J 2010;19:713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lawrence BD, Shamji MF, Traynelis VC, et al. Surgical management of degenerative cervical myelopathy: a consensus statement. Spine (Phila Pa 1976) 2013;38(22 suppl 1):S171–2. [DOI] [PubMed] [Google Scholar]
- [22].Lawrence BD, Jacobs WB, Norvell DC, et al. Anterior versus posterior approach for treatment of cervical spondylotic myelopathy: a systematic review. Spine (Phila Pa 1976) 2013;38:S173–82. [DOI] [PubMed] [Google Scholar]
- [23].Muthukumar N. Surgical management of cervical spondylotic myelopathy. Neurol India 2012;60:201–9. [DOI] [PubMed] [Google Scholar]
- [24].Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am 1958;40-A:607–24. [PubMed] [Google Scholar]
- [25].Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg 2007;6:496–511. [DOI] [PubMed] [Google Scholar]
- [26].Lawrence BD, Brodke DS. Posterior surgery for cervical myelopathy: indications, techniques, and outcomes. Orthop Clin North Am 2012;43:29–40. vii–viii. [DOI] [PubMed] [Google Scholar]
- [27].Fengbin Y, Jinhao M, Xinyuan L, et al. Evaluation of a new type of titanium mesh cage versus the traditional titanium mesh cage for single-level, anterior cervical corpectomy and fusion. Eur Spine J 2013;22:2891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gao R, Yang L, Chen H, et al. Long term results of anterior corpectomy and fusion for cervical spondylotic myelopathy. PLoS One 2012;7:e34811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Miyamoto H, Maeno K, Uno K, et al. Outcomes of surgical intervention for cervical spondylotic myelopathy accompanying local kyphosis (comparison between laminoplasty alone and posterior reconstruction surgery using the screw-rod system). Eur Spine J 2013;23:341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sah S, Wang L, Dahal M, et al. Surgical management of cervical spondylotic myelopathy. JNMA J Nepal Med Assoc 2012;52:172–7. [PubMed] [Google Scholar]
- [31].Umeda M, Sasai K, Kushida T, et al. A less-invasive cervical laminoplasty for spondylotic myelopathy that preserves the semispinalis cervicis muscles and nuchal ligament. J Neurosurg Spine 2013;18:545–52. [DOI] [PubMed] [Google Scholar]
- [32].Uehara M, Takahashi J, Ogihara N, et al. Cervical pedicle screw fixation combined with laminoplasty for cervical spondylotic myelopathy with instability. Asian Spine J 2012;6:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang HL, Chen GD, Zhang HT, et al. Open-door laminoplasty with plate fixation at alternating levels for treatment of multilevel degenerative cervical disease. J Spinal Disord Tech 2013;26:E13–8. [DOI] [PubMed] [Google Scholar]
- [34].Kode S, Gandhi AA, Fredericks DC, et al. Effect of multilevel open-door laminoplasty and laminectomy on flexibility of the cervical spine: an experimental investigation. Spine (Phila Pa 1976) 2012;37:E1165–70. [DOI] [PubMed] [Google Scholar]
- [35].Mitsunaga LK, Klineberg EO, Gupta MC. Laminoplasty techniques for the treatment of multilevel cervical stenosis. Adv Orthop 2012;2012:307916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hardman J, Graf O, Kouloumberis PE, et al. Clinical and functional outcomes of laminoplasty and laminectomy. Neurol Res 2010;32:416–20. [DOI] [PubMed] [Google Scholar]
- [37].Radcliff KE, Limthongkul W, Kepler CK, et al. Cervical laminectomy width and spinal cord drift are risk factors for postoperative C5 palsy. J Spinal Disord Tech 2012;27:86–92. [DOI] [PubMed] [Google Scholar]
- [38].Ryken TC, Heary RF, Matz PG, et al. Cervical laminectomy for the treatment of cervical degenerative myelopathy. J Neurosurg Spine 2009;11:142–9. [DOI] [PubMed] [Google Scholar]
- [39].Kristof RA, Kiefer T, Thudium M, et al. Comparison of ventral corpectomy and plate-screw-instrumented fusion with dorsal laminectomy and rod-screw-instrumented fusion for treatment of at least two vertebral-level spondylotic cervicamyelopathy. Eur Spine J 2009;18:1951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hwang SL, Lee KS, Su YF, et al. Anterior corpectomy with iliac bone fusion or discectomy with interbody titanium cage fusion for multilevel cervical degenerated disc disease. J Spinal Disord 2007;20:565–70. [DOI] [PubMed] [Google Scholar]
- [41].Ashkenazi E, Smorgick Y, Rand N, et al. Anterior decompression combined with corpectomies and discectomies in the management of multilevel cervical myelopathy: a hybrid decompression and fixation technique. J Neurosurg Spine 2005;3:205–9. [DOI] [PubMed] [Google Scholar]
- [42].Zhang J, Meng F, Ding Y, et al. Hybrid surgery versus anterior cervical discectomy and fusion in multilevel cervical disc diseases: a meta-analysis. Medicine (Baltimore) 2016;95:e3621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [43].Wei BX, Wun JS, Gang L, et al. Reconstructive techniques study after anterior decompression of multilevel cervical spondylotic myelopathy. J Spinal Disord Tech 2009;22:511–5. [DOI] [PubMed] [Google Scholar]
- [44].Yuan W, Xu SM, Wang XW, et al. Segmental anterior cervical decompression with fusion for treating multilevel cervical myelopathy: analysis of the clinical effects. Chin J Spine Spinal Cord 2006;16:95–8. [Google Scholar]
- [45].Goffin J, Geusens E, Vantomme N, et al. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech 2004;17:79–85. [DOI] [PubMed] [Google Scholar]
- [46].Wen ZQ, Du JY, Ling ZH, et al. Anterior cervical discectomy and fusion versus anterior cervical corpectomy and fusion in the treatment of multilevel cervical spondylotic myelopathy: systematic review and a meta-analysis. Ther Clin Risk Manag 2015;11:161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Han YC, Liu ZQ, Wang SJ, et al. Is anterior cervical discectomy and fusion superior to corpectomy and fusion for treatment of multilevel cervical spondylotic myelopathy? A systemic review and meta-analysis. PLoS One 2014;9:e87191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [50].Liu Y, Hou Y, Yang L, et al. Comparison of 3 reconstructive techniques in the surgical management of multilevel cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2012;37:E1450–8. [DOI] [PubMed] [Google Scholar]
- [51].Liu Y, Qi M, Chen H, et al. Comparative analysis of complications of different reconstructive techniques following anterior decompression for multilevel cervical spondylotic myelopathy. Eur Spine J 2012;21:2428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Song KJ, Lee KB, Song JH. Efficacy of multilevel anterior cervical discectomy and fusion versus corpectomy and fusion for multilevel cervical spondylotic myelopathy: a minimum 5-year follow-up study. Eur Spine J 2012;21:1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lin Q, Zhou X, Wang X, et al. A comparison of anterior cervical discectomy and corpectomy in patients with multilevel cervical spondylotic myelopathy. Eur Spine J 2012;21:474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Guo Q, Bi X, Ni B, et al. Outcomes of three anterior decompression and fusion techniques in the treatment of three-level cervical spondylosis. Eur Spine J 2011;20:1539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li Z, Huang J, Zhang Z, et al. A comparison of multilevel anterior cervical discectomy and corpectomy in patients with 4-level cervical spondylotic myelopathy: a minimum 2-year follow-up study. Clin Spine Surg 2016;Jun 20 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [56].Min Q, Xinwei W, Yang L, et al. Comparative analysis of complications of different anterior decompression procedures for treating multilevel cervical spondylotic myelopathy. Chin J Spine Spinal Cord 2012;22:963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hou SB, Shen Y, Wang LF, et al. A follow- up study of two anterior surgical interventions for multi-segmental cervical spondylotic myelopath. Orthoped J China 2014;22:594–600. [Google Scholar]
- [58].Papadopoulos EC, Huang RC, Girardi FP, et al. Three-level anterior cervical discectomy and fusion with plate fixation: radiographic and clinical results. Spine 2006;31:897–902. [DOI] [PubMed] [Google Scholar]
- [59].Chang SW, Kakarla UK, Maughan PH, et al. Four-level anterior cervical discectomy and fusion with plate fixation: radiographic and clinical results. Neurosurgery 2010;66:639–46. [discussion 646–7]. [DOI] [PubMed] [Google Scholar]
- [60].Bapat MR, Chaudhary K, Sharma A, et al. Surgical approach to cervical spondylotic myelopathy on the basis of radiological patterns of compression: prospective analysis of 129 cases. Eur Spine J 2008;17:1651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Anakwenze OA, Auerbach JD, Milby AH, et al. Sagittal cervical alignment after cervical disc arthroplasty and anterior cervical discectomy and fusion: results of a prospective, randomized, con-trolled trial. Spine (Phila Pa 1976) 2009;34:2001–7. [DOI] [PubMed] [Google Scholar]
- [62].Liu T, Xu W, Cheng T, et al. Anterior versus posterior surgery for multilevel cervical myelopathy, which one is better? A systematic review. Eur Spine J 2011;20:224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shamji MF, Massicotte EM, Traynelis VC, et al. Comparison of anterior surgical options for the treatment of multilevel cervical spondylotic myelopathy: a systematic review. Spine (Phila Pa 1976) 2013;38(22 suppl 1):S195–209. [DOI] [PubMed] [Google Scholar]
- [64].Park Y, Maeda T, Cho W, et al. Comparison of anterior cervical fusion after two-level discectomy or single-level corpectomy: sagittal alignment, cervical lordosis, graft collapse, and adjacent-level ossification. Spine J 2010;10:193–9. [DOI] [PubMed] [Google Scholar]
- [65].Uribe JS, Sangala JR, Duckworth EA, et al. Comparison between anterior cervical discectomy fusion and cervical corpectomy fusion using titanium cages for reconstruction: analysis of outcome and long-term follow-up. Eur Spine J 2009;18:654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Grob D, Luca A. Surgery for cervical stenosis: anterior cervical decompression, corpectomy, and fusion. Eur Spine J 2010;19:1801–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sakaura H, Hosono N, Mukai Y, et al. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine (Phila Pa 1976) 2003;28:2447–51. [DOI] [PubMed] [Google Scholar]
- [68].Edwards CC, Heller JG, Murakami H. Corpectomy versus laminoplasty for multilevel cervical myelopathy: an independent matched-cohort analysis. Spine 2002;27:1168–75. [DOI] [PubMed] [Google Scholar]
- [69].Apfelbaum RI, Kriskovich MD, Haller JR. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine 2000;25:2906–12. [DOI] [PubMed] [Google Scholar]


