Supplemental Digital Content is available in the text
Keywords: hepatectomy, morbidity, risk model
Abstract
To construct a robust morbidity risk-prediction model based on a Japanese nationwide web-based database of patients who underwent liver surgery.
Although liver resection has become safer, patient mortality and morbidity still occur. This study investigated postoperative morbidity risks in patients who underwent hepatectomy in Japan at institutions registered in the National Clinical Database.
This analysis involved 14,970 patients who underwent hepatectomy of more than 1 section, except for left lateral sectionectomy, during 2011 and 2012 at 1192 hospitals in Japan. Patients were randomized into 2 subsets, with 80% of patients analyzed for model development and the remaining 20% for model validation.
Rates of 90-day inhospital mortality and overall morbidity were 3.7% and 25.7%, respectively. Rates of surgical site infection and bile leakage were 9.0% and 8.0%, respectively, but these morbidities showed little association with mortality. Rates of nonsurgical complications, including postoperative transfusion over 5 units, unexpected intubation, renal failure, cardiac events, septic shock, and postoperative pneumonia, ranged from 0.2% to 2.6%. These complications were highly associated with mortality, suggesting they were life-threatening. Risk models for morbidity yielded high C-indices for transfusion of over 5 units (0.758), unplanned intubation (0.755), renal failure (0.80), cardiac events (0.779), septic shock (0.783), pneumonia (0.768), and bile leakage (0.676).
Preoperative parameters/comorbidities can accurately predict life-threatening complications after hepatectomy. These models allow early identification of patients at risk of mortality and may be useful in deciding on surgical interventions and in improving surgical quality.
1. Introduction
Liver resection remains the standard therapeutic option for patients with a variety of liver tumors, including hepatocellular carcinoma (HCC), metastatic liver tumors, and tumors originating from the bile duct. Over the past 2 decades, liver resection has become safer. Reports based on Nationwide Inpatient Samples (NIS) have shown that overall mortality rates declined from 10.4% in 1988 to 1989 to 5.3% in 1999 to 2000,[1] and to 3.8% in 2010 to 2012.[2] These reductions are a result of advances in anesthesia and surgical techniques that reduce intraoperative blood loss.[3–6] Nevertheless, overall posthepatectomy morbidity rates remain high, ranging from 32% to 44.6% in the National Surgical Quality Improvement Project (NSQIP) and NIS datasets,[2] although studies at single institutions have reported lower rates.[7–9] Studies based on the National Clinical Database (NCD) of Japan showed that the 30-day mortality and 90-day inhospital mortality rates after hepatectomy of more than one segment (MOS) were higher than in patients undergoing esophagectomy, partial/total gastrectomy, right hemicolectomy, low anterior resection, and pancreaticoduodenectomy.[10–17]
Previous analyses, most involving patients treated at single institutions, have identified predictors or risk models for morbidities, including major morbidities,[18] morbidities classified as Clavien–Dindo grades III and IV,[19–21] and mortality.[22] To our knowledge, clear definitions of life-threatening morbidities and risk models for these morbidities have never been clarified using a robust nationwide database. This study focused on the risk of morbidities in patients registered in the NCD who underwent hepatectomy during the years 2011 and 2012.
2. Methods
2.1. Data collection
The NCD is a nationwide project, in cooperation with the board certification system for surgery in Japan. Submission of cases to the NCD is a prerequisite for all institutions that are members of the Japan Surgical Society and the Japanese Society of Gastroenterological Surgery (JSGS), and only registered cases can be used for the board certification.[17] The NCD collected data on over 4 million patients who underwent surgery at 4105 hospitals from January 1, 2011 to December 31, 2012. The NCD is a web-based data management system that continuously recruits individuals who approve the data collection, and members of various departments in charge of annual reviews, thereby assuring data traceability. Moreover, the project validates data consistency by inspecting institutions selected at random.
All variables, definitions, and inclusion criteria for the NCD are accessible to participating institutions on the NCD website (http://www.ncd.or.jp). Details about the database and the website have been reported.[17] The database administrators also provide e-learning systems to teach participants how to input consistent data. Morbidities recorded in the database included surgical site infection (SSI), blood transfusion of over 5 units, bile leakage, pneumonia, pulmonary embolism, sepsis, unplanned intubation, renal failure, central nervous system events, and cardiac events.
This analysis focused on hepatectomy procedures performed in 1192 hospitals in Japan from January 1, 2011 to December 31, 2012, including 957 hospitals in 2011 and 1047 hospitals in 2012. In particular, this study included 14,970 patients who underwent resection of more than 1 section, except for left lateral sectionectomy, as described.[15] Risk models of pivotal surgical and nonsurgical life-threatening complications were constructed based on their correlations with patient mortality. Types of hepatectomy were defined as described, with the liver divided into 4 sections and 8 segments.[23,24] Specific hepatectomy procedures were identified by variables indicating resected segments (S1–S8), which were also included in the development of the risk model. Hepatectomies that included secondary procedures, such as revascularization, and hepatectomy for gall bladder cancer or hilar bile duct cancer, were included in the development of the risk model.
2.2. Endpoints
The primary outcome measures were the identification of morbidities associated with hepatectomy and factors predicting these complications. Secondary endpoints included correlations between postoperative morbidities and 30-day mortality and 90-day inhospital mortality. Thirty-day mortality was defined as death within 30 days of surgery, regardless of the patient's geographical location, even if the patient had been discharged from the hospital. Ninety-day inhospital mortality was defined as death within the index hospitalization period, regardless of the length of hospital stay (up to 90 days), and also any death after discharge, up to 30 days after surgery.
2.3. Statistical analysis
Continuous variables were expressed as means and compared using Fisher exact test, unpaired Student t test, or the Mann–Whitney U test, as appropriate. Categorical variables were compared using the chi-square test. Statistical significance was defined as a P value <0.05. Correlations between each morbidity and 90-day inhospital mortality, and between each pair of morbidities were analyzed using the Pearson product-moment correlation.
Patients were randomized into 2 subsets, with 80% of patients (n = 12,002) analyzed for model development and the remaining 20% (n = 2968) for model validation. The NCD 2013 dataset, which included records of 7327 patients, was also used for the model validation.
Logistic regression models for the development dataset were constructed using step-wise selection of predictors having a P value <0.05. Goodness-of-fit tests were performed to determine how well the model could distinguish between patients with and without complications. Receiver-operating characteristic (ROC) curves for respective morbidities were created for the validation dataset. An ROC curve is a plot of a test's true positive rate (sensitivity) versus its false-positive rate (1 − specificity).
All statistical analyses were performed using IBM SPSS Statistics for Windows (Version 20.0; IBM Corp., Armonk, NY).
3. Results
3.1. Risk profile of the study population
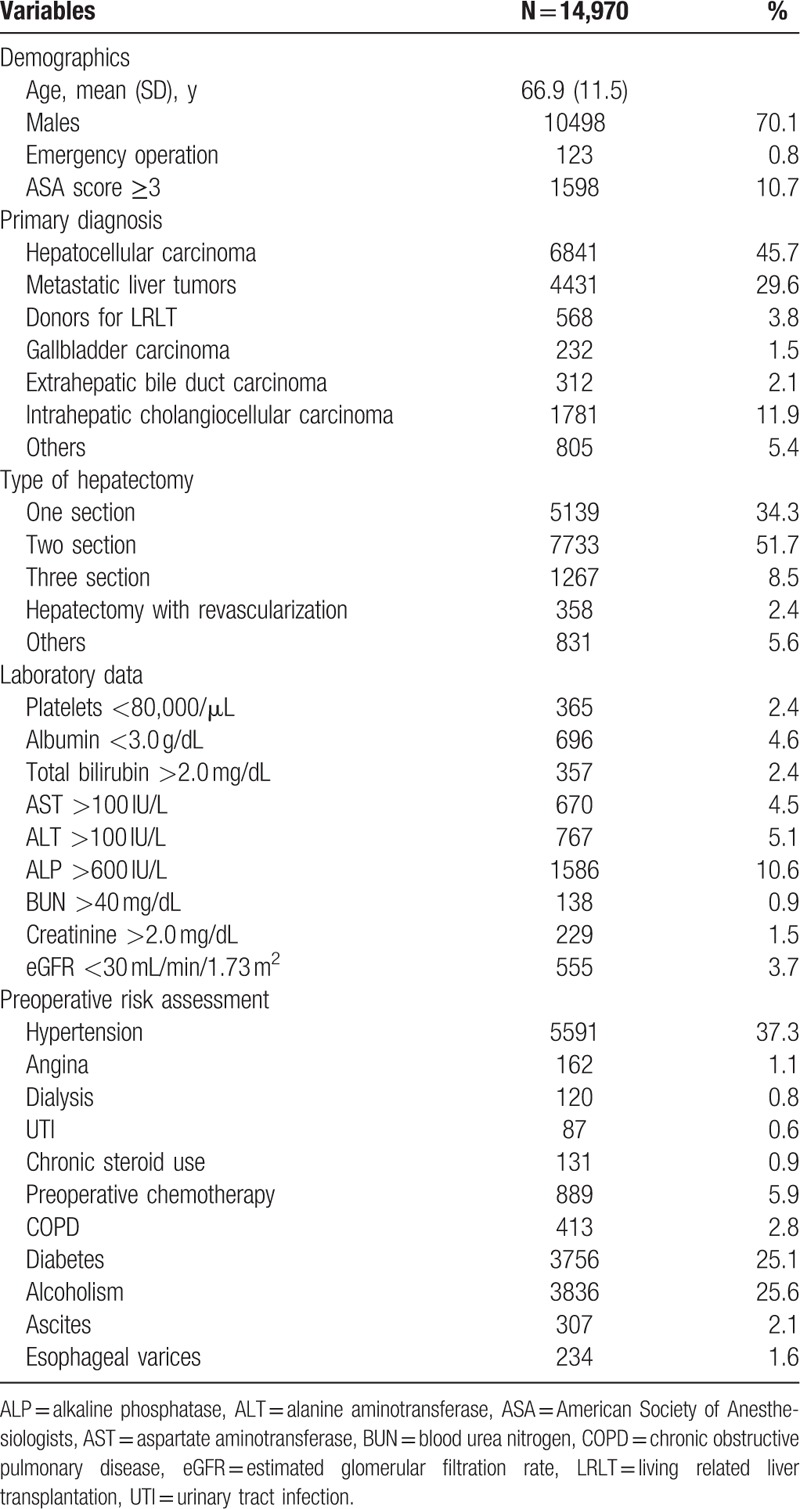
The average age of the 14,970 patients in the NCD who underwent MOS hepatectomy in 2011 and 2012 was 66.9 years, and 10,498 (70.1%) were men. An abbreviated risk profile of the study population is shown in Table 1. Of these patients, 45.7% were diagnosed with primary HCC, 29.6% with metastatic liver tumors, 11.9% with intrahepatic cholangiocellular carcinoma, and 1.5% with gallbladder carcinoma. Types of hepatectomy included removal of 1, 2, and 3 sections form 34.3%, 51.7%, and 8.5% of patients, respectively. Of the 14,970 patients, 0.8% required emergency hepatectomy and 10.7% had an American Society of Anesthesiologists’ (ASA's) physical status of grade 3 or higher. Pre-existing comorbidities included hypertension in 37.3% of patients, a history of preoperative chemotherapy in 5.9%, chronic obstructive pulmonary disease in 2.8%, diabetes mellitus in 25.1%, heavy alcohol use in 25.6%, and ascites in 2.1%.
Table 1.
Baseline demographic and clinical characteristics of patients undergoing hepatectomy.
3.2. Morbidities and outcomes after hepatectomy
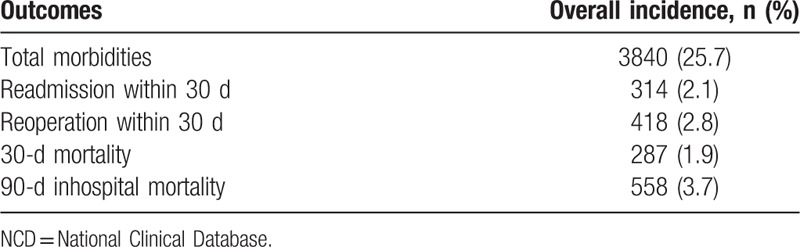
The overall morbidity rate in the NCD hepatectomy population was 25.7%, involving 3840 patients. The 30-day mortality rate was 1.9%, and the 90-day inhospital mortality rate was 3.7% (Table 2).
Table 2.
Outcomes in the NCD hepatectomy population (N = 14,970).
Surgical complications included SSI in 9.0% of patients, bile leakage in 8.0%, and transfusion of over 5 units of blood in 4.1%. Nonsurgical complications included unexpected intubation in 2.3% of patients, renal failure in 2.6%, heart disease in 0.9%, septic shock in 1.2%, and postoperative pneumonia in 2.4%. Each type of the complications, except for cardiac events, had a great impact on length of hospital stay (Supplemental Table 1).
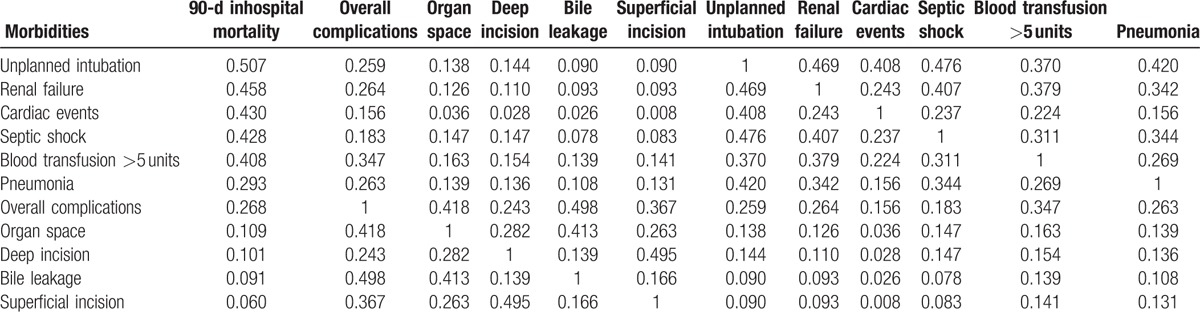
Table 3 shows the relationships between patient morbidity rates and 30-day mortality and 90-day inhospital mortality rates. Transfusion of over 5 units of blood, pneumonia, septic shock, unplanned intubation, renal failure, and cardiac events were found to correlate with both 30-day mortality and 90-day inhospital mortality rates. Sorting of these morbidities by their relationship to 90-day inhospital mortality (Table 4) identified 2 groups of morbidities, those highly and weakly correlated with mortality. Interestingly, morbidities weakly correlated with mortality, such as SSI and bile leakage, showed good correlations with each other. Transfusion of over 5 units of blood correlated with mortality and overall complication rates. The risk models created in this study included these life-threatening morbidities, and also bike leakage, which is specific to hepatectomy.
Table 3.
Relationship between morbidities and different types of mortality in the NCD hepatectomy population (N = 14,970).
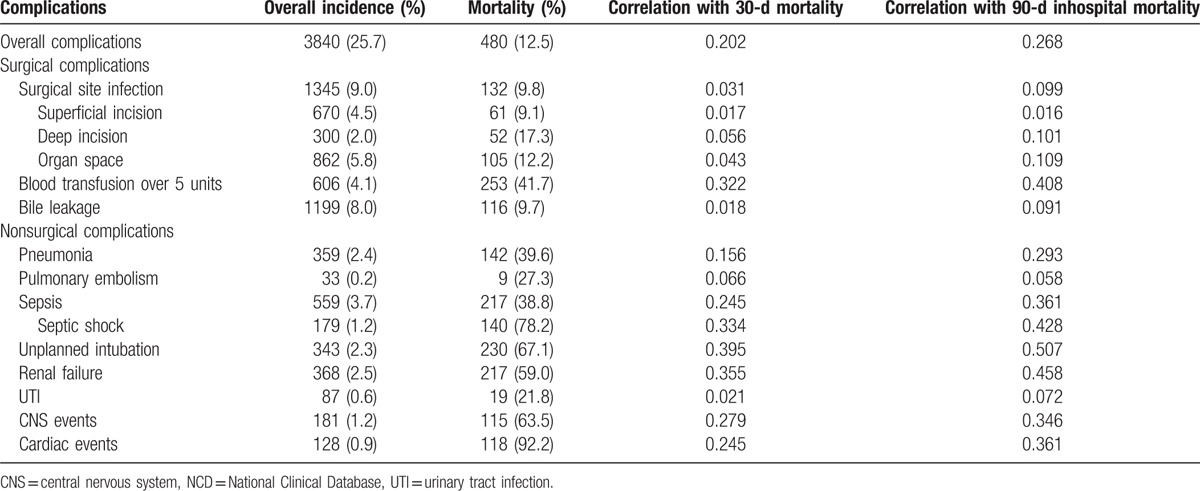
Table 4.
Correlation coefficients of morbidities in patients who underwent hepatectomy.
3.3. Risk models for morbidities
Risk models for postoperative morbidities after hepatectomy were constructed based on preoperative clinical parameters and types of liver resection. The final logistic regression models with odds ratios (ORs) are summarized in Table 5. The 95% confidence intervals (CIs) and statistical significance of each model and β-coefficient intercept (β0) are presented in Supplemental Table 2a, 2b, 2c. The scoring system for the morbidity risk models according to the logistic regression equation was as follows: predicted morbidity = e (β0 + ΣβiXi)/1 + e (β0 + ΣβiXi), where βi is the coefficient of the variable Xi in the logistic regression equation provided in Supplemental Table 2a, 2b, 2c for each morbidity. The risk of requiring transfusion of more than 5 units of blood could be predicted based on 21 factors, including segment 1 hepatectomy, surgery for gallbladder malignancy, and body mass index (BMI). Similarly, the risks of bile leakage and septic shock after liver resection could be predicted based on 17 and 14 factors, respectively. Other risk factors are shown in Table 5.
Table 5.
Risk model for morbidities in patients undergoing hepatectomy.

3.4. Model performance
To evaluate model performance, the C-index (measure of model discrimination), defined as the area under the ROC curves, was determined for each factor (Fig. 1). The C-index for the transfusion of over 5 units of blood was 0.703 (95% CI 0.655–0.750, P < 0.001). The C-indices for other complications of hepatectomy were 0.635 (95% CI 0.559–0.669, P < 0.001) for bile leakage, 0.718 (95% CI 0.664–0.773, P < 0.001) for unplanned intubation, 0.756 (95% CI 0.705–0.807, P < 0.001) for renal failure, 0.676 (95% CI 0.575–0.778, P = 0.002) for cardiac events, 0.784 (95% CI 0.723–0.844, P < 0.001) for sepsis, and 0.749 (95% CI 0.692–0.805, P < 0.001) for pneumonia. The supplemental figure shows the validation of these models and the ROC curves for model performance in the NCD 2013 dataset. These findings suggest good performance of these models.
Figure 1.
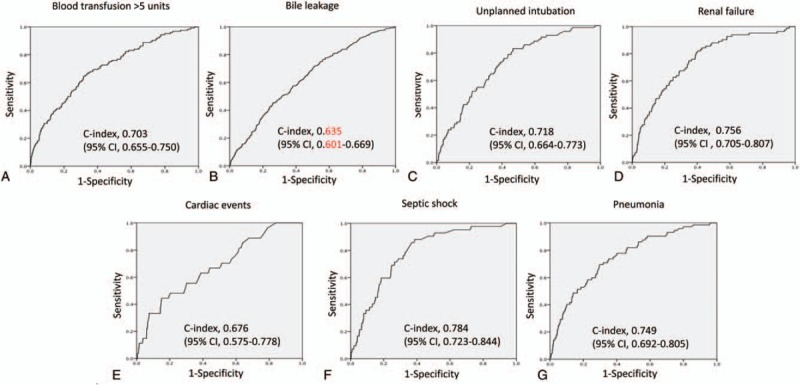
Receiver-operating characteristics (ROC) curves of model performance, as shown by C-indices and their 95% confidence intervals (CIs), for each postoperative complication: (A) transfusion of over 5 units of blood; (B) bile leakage; (C) unplanned intubation; (D) renal failure; (E) cardiac events; (F) septic shock; and (G) pneumonia.
4. Discussion
The study was undertaken to evaluate life-threatening morbidities highly associated with mortality after hepatectomy and to create risk models for each morbidity. A robust database of 14,970 patients in the NCD who underwent hepatectomy over a 2-year period (2011 and 2012) was used as a source for development (80%) and validation (20%) of the models. The 6 life-threatening morbidities were found to be unplanned intubation, renal failure, cardiac events, septic shock, pneumonia, and transfusion of over 5 units of blood, with some of these comorbidities highly correlated with each other. In contrast, all types of SSI and bile leakage appeared to be minor morbidities, with little relationship with patient mortality. Nevertheless, any type of morbidities, regardless of its severity, prolonged length of hospital stay. The risk models based on preoperative comorbidities, laboratory data, and procedure-specific variables were found to accurately predict each morbidities, as determined by the validation datasets. This was also confirmed when the datasets from 2013 were used for validation. To our knowledge, this is the first study to assess a risk model of postoperative complications based on preoperative comorbidities among patients in the Japanese NCD who underwent hepatectomy.
Many risk models have been constructed to predict outcomes after hepatectomy. Most of these models included an analysis of mortality and/or sets of major morbidities.[18–22] None, however, assessed the risks of specific morbidities, perhaps because the number of patients who underwent hepatectomy at a single center was insufficient and/or the variables assayed were not adequate. The American College of Surgeons (ACS)-NSQIP database has been accumulating a large number of patients who underwent various surgical procedures to predict mortality and morbidity from each procedure, with this database shown to be both accurate and useful.[25,26] Standard ACS-NSQIP variables were found to be less accurate for predicting outcomes in patients who underwent hepatobiliary than gastrointestinal tract surgery, suggesting that risk models include variables for specific procedures.[27,28] The inclusion of patients who underwent secondary procedures after hepatectomy was found to improve the prediction of mortality, and also major morbidities.[29] The NCD includes the standard variables in the ACS-NSQIP, and also variables associated with secondary procedures after hepatectomy, including biliary reconstruction, revascularization, and lymphadenectomy, and also variables associated with the resected liver segments.[15] These additional variables increased the accuracy of predicting mortality after hepatectomy.[15,17] The robust data entry system of the NCD, which included procedure-specific variables, resulted in risk models for life-threatening morbidities (unplanned intubation, renal failure, cardiac events, septic shock, pneumonia, and transfusion of over 5 units of blood) being accurate and having good discriminatory ability, generating areas under the ROC curves of 0.755 to 0.800.
The most common preoperative variables among the models, being included in more than 3 of the 6 models, included age, activities of daily living (ADL), ASA grade, hypertension, chronic obstructive pulmonary disease (COPD), preoperative pneumonia, chronic steroid use, systemic sepsis, low albumin levels, hyperbilirubinemia, and elevated aspartase transaminase levels. These risk models also found that secondary procedures and tumor location were significant variables. In contrast to models predicting these life-threatening morbidities, the risk model for bile leakage, although having a lower predictive power (C-index 0.676), selected characteristic variables, including open wound, peripheral vascular disease, C-reactive protein (CRP) concentration >1 mg/dL, and various types of complicated hepatectomy.
Assessment of life-threatening morbidities showed that cardiac events were less frequent (0.9%) than other major morbidities (1.2–4.1%). However, the mortality rate among patients with cardiac events was quite high (92.2%). Variables with OR >3 included ASA grade 5, BMI >35 kg/m2, previous cardiac surgery, recent cerebrovascular disease, COPD, preoperative dialysis, and bleeding disorder. Although septic shock was an uncommon morbidity (1.2%), its mortality rate was quite high (78.2%). Preoperative ADL, respiratory distress, COPD, preoperative systemic sepsis, and white blood cell (WBC) count <3500/μL were also strongly predictive (OR >2), as were secondary surgical procedures including revascularization, biliary reconstruction, and hepatectomy S4a + S5, which were often indicated for gall bladder cancer.
Renal failure occurred in 2.6% of patients, with a relatively high mortality rate (59%). Risk models of acute renal failure according to Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) criteria (absolute increase from baseline of serum creatinine ≥0.3 mg/dL within 48 hours after surgery) in patients who underwent hepatectomy found that alanine aminotransferase (ALT) concentration, pre-existing cardiovascular disease, chronic renal failure, and diabetes were the strongest predictors of acute renal failure.[30] In our database, the strongest variables (OR >3) included preoperative pneumonia, chronic steroid use, systemic sepsis, and creatinine >1.2 mg/dL. Other significant variables (OR 2–3) included ADL before 30 days, BMI >30 kg/m2, hepatectomy S4a and S5, and hepatectomy including S8. This study showed that variables other than preoperative renal insufficiency were significant predictors of renal failure after hepatectomy in Japanese patients.
Unplanned intubation occurred in 2.3% of patients, with these patients having a high mortality rate (67.1%). Significant predictors of unplanned intubation (OR >1.8) included BMI >35 kg/m2, ADL before 30 days, and COPD. Similar to a previous study,[35] results from the NCD showed that pulmonary complications were significantly more frequent in patients with high BMI, irrespective of the extent of resection.
Severe preoperative pneumonia, systemic sepsis, and renal insufficiency were associated with postoperative pneumonia, septic shock, and renal failure, respectively. The latter conditions were associated with each other, leading to mortality, possibly through multiple organ failure. According to the Clavien–Dindo classification,[31] these grade IVa life-threatening morbidities would advance to grade IVb and then to grade V. These patients required control of infection at the source and intensive care before undergoing surgery. Information about preoperative risks of specific morbidities would help in the prevention and treatment of these morbidities.[30]
Although several scoring systems, including the Physiological and Operative Severity Score for the enumeration of Mortality and Morbidity (POSSUM)[32] and the Estimation of Physiological Ability and Surgical Stress (E-PASS),[33] have been found to predict the risks associated with hepatectomy, these predictive models include intraoperative, and also preoperative factors. The 50-50 criteria, as assessed on postoperative day 5, may accurately predict postoperative liver failure and death after hepatectomy.[34] Predicting the risks for each patient undergoing an invasive procedure and preparing measures that can be applied intraoperatively or postoperatively to these patients may improve the quality of surgery. The risk models described in this study showed good discrimination in predicting the occurrence of life-threatening morbidities after hepatectomy.
Although this study evaluated over 14,000 patients who underwent hepatectomy, it had several limitations. First, this analysis included only patients who underwent MOS hepatectomy, but did not evaluate patients who underwent other types of hepatectomy, including partial hepatectomy, lateral sectionectomy, subsegmentectomy, and S4a + S5 resection. Mortality rates have been reported lower for patients undergoing these procedures than for those who underwent MOS hepatectomy.[15] At the start of the NCD in 2011, detailed input of these items was limited only to patients undergoing MOS hepatectomy, excluding the lateral segment. This study therefore did not evaluate morbidities in patients who underwent other types of hepatectomy. Beginning in 2015, the variables recorded for MOS hepatectomy were also recorded for patients who underwent other types of hepatectomy. Future studies may therefore analyze factors associated with mortality in patients who underwent non-MOS hepatectomy.
A second limitation was associated with the characteristics of the NCD system, which did not record the dates of occurrence and outcomes of each morbidity. The lack of these data may hamper sequential analysis of morbidities leading to mortality. We are also planning to include these variables in a future model.
In conclusion, this study identified life-threatening morbidities associated with mortality after hepatectomy. A robust morbidity risk-prediction model was constructed based on a Japanese nationwide web-based database of patients who underwent liver surgery. Using preoperative comorbidities and type of resection, this model could accurately predict the incidence of complications. The NCD has initiated feedback implementation, calculating the risk of mortality and analyzing performance reports at each participating hospital, using the risk models described here,[17] and risk models for morbidities may soon be utilized. This model may therefore help guide postoperative procedures. Real improvements in surgical quality should be monitored on a nationwide scale and be validated in future studies.
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank all data managers and hospitals participating in this NCD project for their great efforts in entering the data, and also thank the members and working members of the JSGS database committee.
Footnotes
Abbreviations: ACS = American College of Surgeons, ADL = activities of daily living, ASA = American Society of Anesthesiologists, BMI = body mass index, CIs = confidence intervals, COPD = chronic obstructive pulmonary disease, E-PASS = Estimation of Physiological Ability and Surgical Stress, HCC = hepatocellular carcinoma, MOS = more than one segment, NCD = National Clinical Database, ORs = odds ratios, POSSUM = Physiological and Operative Severity Score for the enumeration of Mortality and Morbidity, ROC = receiver-operating characteristic, SSI = surgical site infection.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Dimick JB, Wainess RM, Cowan JA, et al. National trends in the use and outcomes of hepatic resection. J Am Coll Surg 2004;199:31–8. [DOI] [PubMed] [Google Scholar]
- [2].He J, Amini N, Spolverato G, et al. National trends with a laparoscopic liver resection: results from a population-based analysis. HPB (Oxford) 2015;17:919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Smyrniotis V, Kostopanagiotou G, Theodoraki K, et al. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg 2004;187:398–402. [DOI] [PubMed] [Google Scholar]
- [4].Cunningham JD, Fong Y, Shriver C, et al. One hundred consecutive hepatic resections. Blood loss, transfusion, and operative technique. Arch Surg 1994;129:1050–6. [DOI] [PubMed] [Google Scholar]
- [5].Man K, Fan ST, Ng IO, et al. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg 1997;226:704–11. [discussion 711–713]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Clavien PA, Yadav S, Sindram D, et al. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg 2000;232:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Taketomi A, Kitagawa D, Itoh S, et al. Trends in morbidity and mortality after hepatic resection for hepatocellular carcinoma: an institute's experience with 625 patients. J Am Coll Surg 2007;204:580–7. [DOI] [PubMed] [Google Scholar]
- [8].Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg 2003;138:1198–206. [DOI] [PubMed] [Google Scholar]
- [9].Kamiyama T, Nakanishi K, Yokoo H, et al. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg 2010;211:443–9. [DOI] [PubMed] [Google Scholar]
- [10].Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014;260:259–66. [DOI] [PubMed] [Google Scholar]
- [11].Watanabe M, Miyata H, Gotoh M, et al. Total gastrectomy risk model: data from 20,011 Japanese patients in a nationwide internet-based database. Ann Surg 2014;260:1034–9. [DOI] [PubMed] [Google Scholar]
- [12].Kurita N, Miyata H, Gotoh M, et al. Risk model for distal gastrectomy when treating gastric cancer on the basis of data from 33,917 Japanese patients collected using a nationwide web-based data entry system. Ann Surg 2015;262:295–303. [DOI] [PubMed] [Google Scholar]
- [13].Kobayashi H, Miyata H, Gotoh M, et al. Risk model for right hemicolectomy based on 19,070 Japanese patients in the National Clinical Database. J Gastroenterol 2014;49:1047–55. [DOI] [PubMed] [Google Scholar]
- [14].Matsubara N, Miyata H, Gotoh M, et al. Mortality after common rectal surgery in Japan: a study on low anterior resection from a newly established nationwide large-scale clinical database. Dis Colon Rectum 2014;57:1075–81. [DOI] [PubMed] [Google Scholar]
- [15].Kenjo A, Miyata H, Gotoh M, et al. Risk stratification of 7,732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J Am Coll Surg 2014;218:412–22. [DOI] [PubMed] [Google Scholar]
- [16].Kimura W, Miyata H, Gotoh M, et al. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg 2014;259:773–80. [DOI] [PubMed] [Google Scholar]
- [17].Gotoh M, Miyata H, Hashimoto H, et al. National Clinical Database feedback implementation for quality improvement of cancer treatment in Japan: from good to great through transparency. Surg Today 2016;46:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schroeder RA, Marroquin CE, Bute BP, et al. Predictive indices of morbidity and mortality after liver resection. Ann Surg 2006;243:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 2004;240:698–708. discussion 708-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Abbass MA, Slezak JM, DiFronzo LA. Predictors of early postoperative outcomes in 375 consecutive hepatectomies: a single-institution experience. Am Surg 2013;79:961–7. [PubMed] [Google Scholar]
- [21].Farges O, Vibert E, Cosse C, et al. Surgeons’ intuition” versus “prognostic models”: predicting the risk of liver resections. Ann Surg 2014;260:923–8. discussion 928-930. [DOI] [PubMed] [Google Scholar]
- [22].Breitenstein S, DeOliveira ML, Raptis DA, et al. Novel and simple preoperative score predicting complications after liver resection in noncirrhotic patients. Ann Surg 2010;252:726–34. [DOI] [PubMed] [Google Scholar]
- [23].Couinaud C. Contribution of anatomical research to liver surgery. Fr Med 1956;19:5–12. [PubMed] [Google Scholar]
- [24].Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005;12:351–5. [DOI] [PubMed] [Google Scholar]
- [25].Cohen ME, Dimick JB, Bilimoria KY, et al. Risk adjustment in the American College of Surgeons National Surgical Quality Improvement Program: a comparison of logistic versus hierarchical modeling. J Am Coll Surg 2009;209:687–93. [DOI] [PubMed] [Google Scholar]
- [26].Cohen ME, Bilimoria KY, Ko CY, et al. Development of an American College of Surgeons National Surgery Quality Improvement Program: morbidity and mortality risk calculator for colorectal surgery. J Am Coll Surg 2009;208:1009–16. [DOI] [PubMed] [Google Scholar]
- [27].Aloia TA, Fahy BN, Fischer CP, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxford) 2009;11:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pitt HA, Kilbane M, Strasberg SM, et al. ACS-NSQIP has the potential to create an HPB-NSQIP option. HPB (Oxford) 2009;11:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Paruch JL, Merkow RP, Bentrem DJ, et al. Impact of hepatectomy surgical complexity on outcomes and hospital quality rankings. Ann Surg Oncol 2014;21:1773–80. [DOI] [PubMed] [Google Scholar]
- [30].Slankamenac K, Breitenstein S, Held U, et al. Development and validation of a prediction score for postoperative acute renal failure following liver resection. Ann Surg 2009;250:720–8. [DOI] [PubMed] [Google Scholar]
- [31].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lam CM, Fan ST, Yuen AW, et al. Validation of POSSUM scoring systems for audit of major hepatectomy. Br J Surg 2004;91:450–4. [DOI] [PubMed] [Google Scholar]
- [33].Haga Y, Ikejiri K, Takeuchi H, et al. Value of general surgical risk models for predicting postoperative liver failure and mortality following liver surgery. J Surg Oncol 2012;106:898–904. [DOI] [PubMed] [Google Scholar]
- [34].Balzan S, Belghiti J, Farges O, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824–8. discussion 828-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Balzan S, Nagarajan G, Farges O, et al. Safety of liver resections in obese and overweight patients. World J Surg 2010;34:2960–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


