Abstract
Vitamin D has been linked to various cardiovascular risk factors including indices of large-vessel disease. However, it remains unclear whether vitamin D is also associated with microvascular damage. In a community-dwelling population, we studied associations between vitamin D serum levels and retinal microvascular damage defined as retinopathy signs, narrower arterioles, and wider venules.
From the population-based Rotterdam Study, we included 5675 participants (age ≥45 years) with vitamin D data and gradable retinal photographs. Serum levels of vitamin D were measured using an antibody-based assay. Retinal exudates, microaneurysms, cotton wool spots, and dot/blot hemorrhages were graded on fundus photographs by experienced graders in the whole sample; retinal vascular calibers, that is, arteriolar and venular diameters, were semiautomatically measured in a subsample (n = 2973). We examined the cross-sectional association between vitamin D and retinal microvascular damage using logistic and linear regression models, adjusting for age, sex, and cardiovascular risk factors.
We found that persons with lower vitamin D levels were more likely to have retinopathy (adjusted odds ratio per standard deviation (SD) decrease of vitamin D = 1.30; 95% confidence interval (CI): = 1.12–1.49). Furthermore, lower vitamin D levels were associated with wider venular calibers (adjusted mean difference per SD decrease in vitamin D = 1.35; 95% CI = 0.64–2.06). This association was strongest among men (P for interaction = 0.023).
Lower levels of vitamin D are associated with retinal microvascular damage, suggesting that the link with cardiovascular risk may partly run through changes in the microvasculature.
Keywords: microcirculation, retina, vascular diseases, vitamin D
1. Introduction
Over the past decades, vitamin D deficiency has emerged as a potentially modifiable risk factor for cardiovascular disease (CVD).[1] The exact mechanisms for how vitamin D relates to CVD are uncertain, but existing data show a strong link between vitamin D and cardiovascular risk factors, which themselves contribute to the development of CVD. In particular, population-based studies have primarily focused on the relation of vitamin D with various indices of large-vessel disease such as atherosclerosis,[2] arterial stiffness,[3] and arterial stenosis.[4] Together with traditional cardiovascular risk factors, large-vessel disease explains about 60% of the variance of incident CVD.[5] Yet, a growing body of evidence shows that microvascular disease is also an important contributor to the development of CVD.[6] Retinal imaging provides a great opportunity to study the microvasculature in vivo, and retinal microvascular damage—assessed as retinopathy signs, arteriolar narrowing, and venular widening—has been widely used as markers of microvascular disease.[7] As such, studies have related these markers to incident hypertension,[8] stroke,[9] and coronary heart disease,[10] supporting the notion of a microvascular component in CVD.[11] In view of these observations, we hypothesize that vitamin D could be linked to CVD through the presence of a microvascular component. Studies investigating the link between vitamin D and indices of microvascular disease have shown that vitamin D deficiency was associated with poor coronary microcirculation,[12] endothelial dysfunction,[13] nephropathy,[14] and with markers of cerebral small-vessel disease.[15] Although these studies suggest a microvascular component in the link of vitamin D with CVD, no study has investigated the direct relation of vitamin D with markers of microvascular damage in humans. In this study, we investigated associations between vitamin D and direct visualization of microvascular damage using retinal imaging in a community-dwelling population.
2. Methods
2.1. Setting and study population
This study was performed as part of the Rotterdam Study (RS), a prospective population-based cohort study.[16] All inhabitants of the Ommoord district in the city of Rotterdam, the Netherlands, aged ≥55 years were invited to the study in 1990 (RS-I, n = 7932) and 2000 (RS-II, n = 3011). In 2006, a further extension of the cohort was initiated and participants aged 45 years or older were invited (RS-III, n = 3932). Vitamin D was measured in RS-II and RS-III. Signs of retinopathy were graded on fundus photographs in both cohorts, and retinal vascular calibers were measured only in RS-III. In total, data on vitamin D were available in 5918 persons. Of these 5918 persons, 243 persons had no (gradable) fundus photographs centered on the macula, or did not undergo ophthalmic examinations. Thus, data on both vitamin D and retinopathy were available in 5675 persons. In RS-III, data on vitamin D were available in 3445 persons. Of these, 472 had no (gradable) fundus photographs centered on the optic disc, or did not undergo ophthalmic examinations, resulting in 2973 persons with complete data on vitamin D and retinal vascular calibers. Baseline home interviews and examinations were performed in each cohort. The RS has been approved by the medical ethics committee according to the Population Study Act: Rotterdam Study, executed by the Ministry of Health, Welfare, and Sports of the Netherlands. A written informed consent was obtained from all participants.
2.2. Assessment of 25-hydroxyvitamin D
Plasma levels of 25-hydroxyvitamin D were measured once in non-fasting status using an electrochemiluminescense-based assay (Elecsys Vitamin D Total, Roche Diagnostics, Mannheim, Germany). This assay has a functional sensitivity of 10 nmol/L with 18.5% coefficient of variation for intra-assay analyses. The repeatability is given by the within-run precision of ≤6.5% and the reproducibility by the intermediate precision of ≤11.5%.[1] Vitamin D deficiency was considered as a level lower than 50 nmol/L.
2.3. Assessment of retinopathy signs
Participants underwent a full eye examination of both eyes including fundus photography centered on the macula (35° field, Topcon TRV-50VT, Tokyo Optical Co., Tokyo, Japan) and fundus photography centered on the optic disc (20° visual field, Topcon TRC-50VT, Tokyo Optical Co., Tokyo, Japan) after pharmacological mydriasis on both eyes. Fundus photographs were checked for quality and the presence of age-related maculopathy by 2 experienced graders. These graders, each having 20 years of experience, divided their work and graded all fundus photographs particularly focusing on fundus signs of age-related maculopathy. Consensus sessions and between-grader comparisons were performed regularly, and weighted κ coefficients ranged from 0.58 to 0.80 for various fundus lesions. Retinopathy was defined as the presence of one or more dot/blot hemorrhages, microaneurysms, hard exudates, cotton wool spots, or evidence of laser treatment for retinopathy in 1 eye (Figure 1A). Retinopathy was also considered to be present in participants with central retinal artery or vein occlusion.
Figure 1.
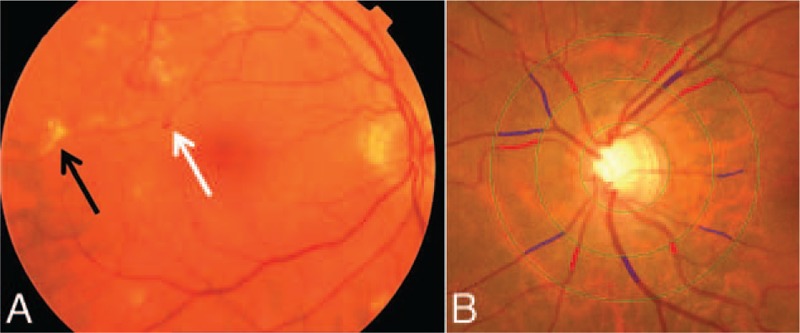
Fundus photographs showing (A) signs of retinopathy and (B) measurements of retinal vascular calibers. In (A), white arrow = small hemorrhages; black arrow = hard exudates. In (B), red lines = arteriolar calibers; blue lines = venular calibers.
2.4. Assessment of retinal vascular calibers
Retinal vascular calibers were measured in RS-III on fundus photographs centered on the optic disc (Figure 1B). For each participant, the image of 1 eye with the best quality was analyzed with a semiautomated system (IVAN, University of Wisconsin-Madison, Madison, WI), and 1 summary value was calculated for the arteriolar calibers (in μm) and 1 for the venular calibers (in μm).[17] As eyes may have different magnification due to refractive changes, we adjusted vessel measurements for possible magnification variations with Littmann formula to approximate absolute measures.[18] We verified in a random subsample of 100 participants that individual measurements in the left and right eye were similar. Measurements were performed by 1 rater, masked for participant characteristics. Pearson correlation coefficients for interrater and intrarater agreement (n = 100) were 0.85 and 0.86 for arteriolar calibers, and 0.87 and 0.87 for venular calibers, respectively.
2.5. Assessment of other measurements
Blood pressure was measured twice in sitting position at the right brachial artery with a random-zero sphygmomanometer. We used the average of 2 readings for analysis. We defined hypertension as a systolic blood pressure of 140 mm Hg or more, a diastolic blood pressure of 90 mm Hg or more, use of antihypertensive medication, or any combination of these 3 factors. Body mass index was computed as weight divided by height squared. Non-fasting serum total and high-density lipoprotein cholesterol concentrations were determined by an automated enzymatic procedure.[19] Diabetes mellitus was considered to be present if participants reported use of antidiabetic medication or when fasting serum glucose level was ≥7.0 mmol/L. Serum levels of C-reactive protein were determined by the Rate Near Infrared Particle Immunoassay method (Immage® high-sensitive CRP, Beckman Coulter Inc., Brea, CA). Atherosclerotic plaques were assessed by ultrasound at the carotid artery bifurcation, common carotid artery, and internal carotid artery on both sides. Presence of plaques were defined as focal thickening of the vessel wall of at least 2 mm relative to adjacent segments with or without calcified components at any site. Information on smoking (never, former, or current), antihypertensive and lipid-lowering medication use, and vitamin D supplement use was obtained during the home interview by a computerized questionnaire. Dietary intake data were collected using a semiquantitative 389-item food-frequency questionnaire. Vitamin D intake from foods was calculated using the Dutch Food Composition Table of 2006. Prevalent CVD was assessed as a history of myocardial infarction, coronary artery bypass graft, percutaneous coronary intervention, coronary revascularization, or stroke. An extensive description on definitions of cardiovascular outcomes has been described previously.[20,21] Kidney function was assessed by calculating an estimated glomerular filtration rate for serum creatinine and cystatin combined, according to the Chronic Kidney Disease Epidemiology Collaboration formula.[22] An estimated glomerular filtration rate <60 mL/min/1.73 m2 was considered as having kidney disease.
2.6. Statistical analyses
We standardized vitamin D values by creating z-scores (individual value minus population mean, divided by the standard deviation (SD)). In addition, we categorized participants into quartiles on the basis of vitamin D levels. We assessed associations of vitamin D with retinopathy using logistic regression models and with retinal vascular calibers using linear regression models. In model 1, we adjusted for age, sex, season when the blood was drawn, the other vascular caliber (if applicable), and subcohort (if applicable). In model 2, we additionally adjusted for the following cardiovascular risk factors: systolic blood pressure, diastolic blood pressure, use of antihypertensive and lipid-lowering medication, body mass index, total cholesterol, high-density lipoprotein cholesterol, C-reactive protein, and smoking. As the use of vitamin D supplements could influence these associations, we repeated our analyses after adjusting for use of any vitamin supplements, and again after excluding these persons. We explored effect modification by stratifying for sex, history of CVD, hypertension, diabetes mellitus, and kidney disease. We also created interaction terms with corresponding P-values in the statistical models. Missing values for covariates, if present, occurred in <3% of the cases, and were dealt with using multiple imputations with all covariates of interest as predictors. We explored the possibility of collinearity, given the Pearson correlation coefficient between arteriolar and venular diameter (r = 0.53), by calculating the variance inflation factor, but no collinearity was identified (variance inflation factor <1.2). Statistical tests were performed at the 0.05 level of significance (two-tailed) using SPSS 21.0 for Windows (IBM Corp., Armonk, NY).
3. Results
The characteristics of the study population are reported in Table 1. Of the total 5675 participants, 56% were women and the average age was 60.1 years (SD = 8.1). The average vitamin D level was 60.8 nmol/L (range = 8–175 nmol/L), and 40% of the participants had vitamin D deficiency. Persons with retinopathy had an average vitamin D level of 52.3 nmol/L (SD = 27.7), and persons without retinopathy 61.2 nmol/L (SD = 28.1).
Table 1.
Characteristics of the study population.
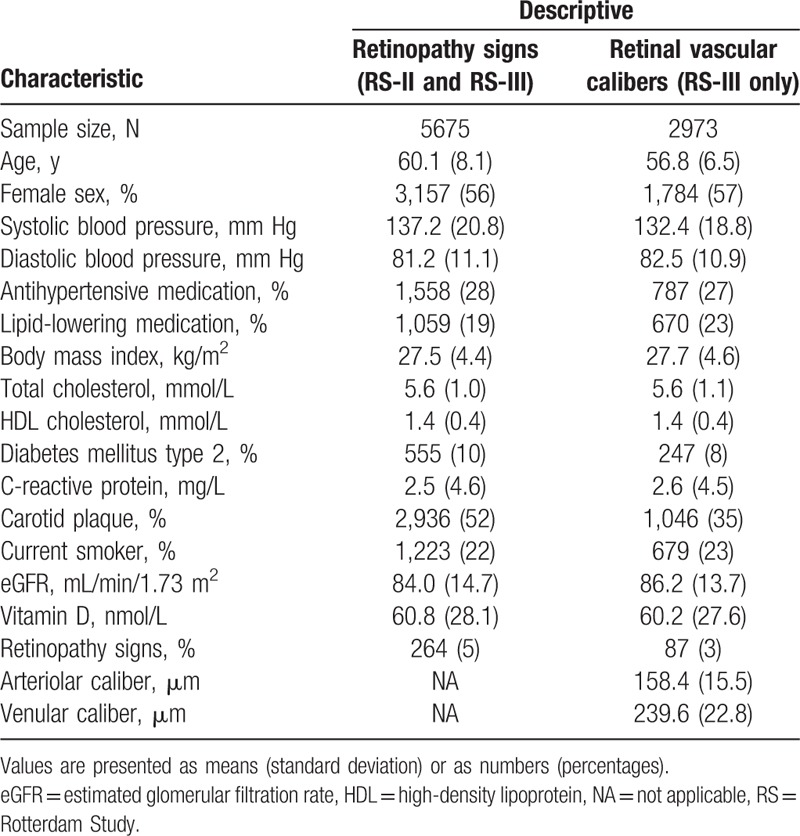
Table 2 shows the associations between vitamin D and the presence of retinopathy. Lower levels of vitamin D were significantly associated with the presence of retinopathy: odds ratio (OR) per SD decrease of vitamin D was 1.42 (95% confidence interval (CI) = 1.21–1.66). This association attenuated after adjusting for cardiovascular risk factors, but remained statistically significant (OR = 1.28 (1.09–1.50)). Adjusted OR for the presence of retinopathy was 1.62 (1.05–2.49) for persons in the first quartile of vitamin D compared with persons in the fourth quartile.
Table 2.
Associations between vitamin D and retinopathy.
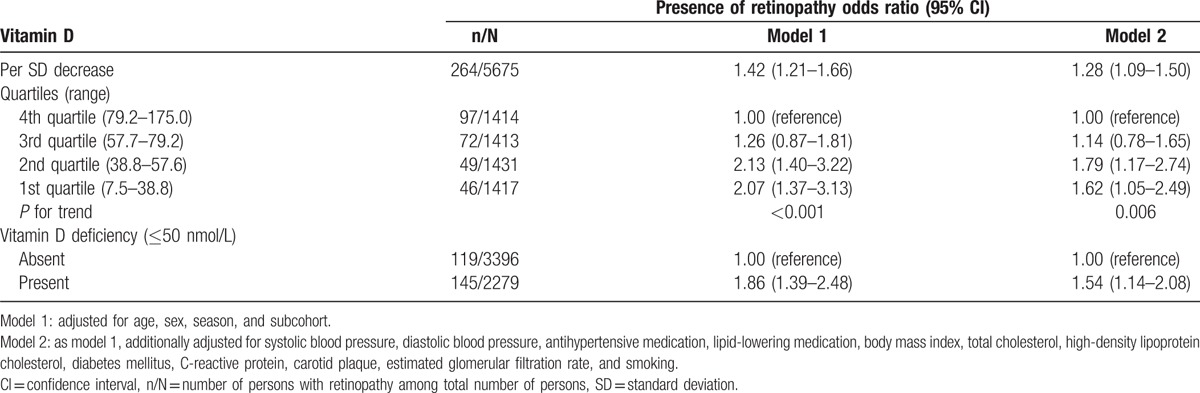
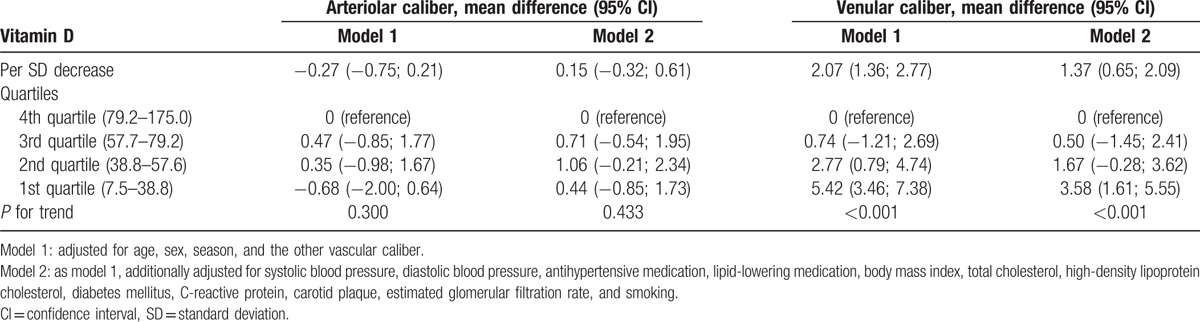
Table 3 shows associations of vitamin D with retinal microvascular calibers. We found that lower levels of vitamin D were significantly associated with wider venular calibers, irrespective of cardiovascular risk factors: adjusted mean difference per SD decrease of vitamin D was 1.37 (0.65–2.09). On the other hand, lower vitamin D levels were weakly associated with narrower arteriolar calibers: adjusted mean difference was 0.15 (−0.32; 0.61).
Table 3.
Associations between vitamin D and retinal vascular calibers.
Furthermore, the associations between vitamin D and microvascular damage remained similar after adjusting for vitamin supplement use, or excluding supplement users (n = 1343). Also, in RS-III, adjusting for vitamin D from food intake attenuated the associations, but it remained statistically significant: adjusted OR for the presence of retinopathy was 1.58 (1.18–2.13), and adjusted mean difference for venular calibers was 0.99 (0.14–1.83).
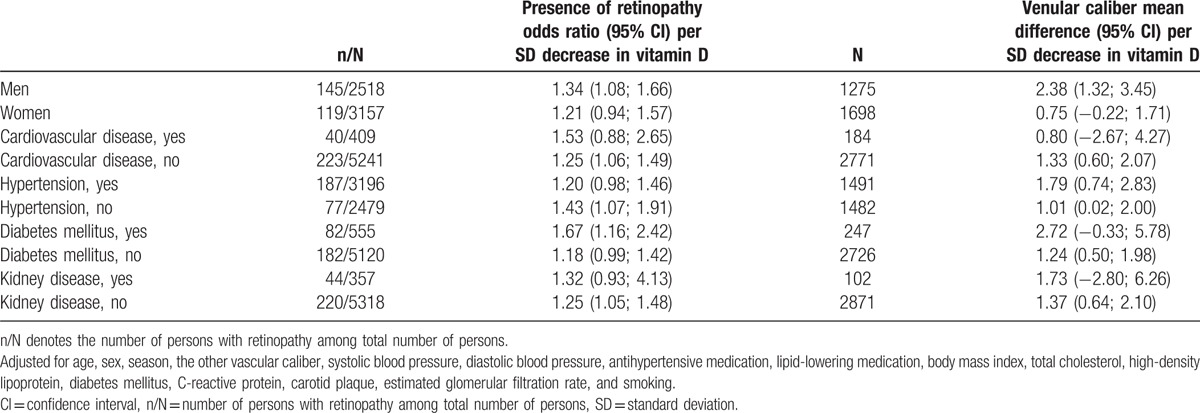
In stratified analyses (Table 4), we found that sex modified the association of vitamin D with venules with a significant p-value for formal interaction term (P = 0.023).
Table 4.
Stratified analyses for associations between serum vitamin D levels and retinal microvascular damage.
4. Discussion
In this population-based study, we found that lower vitamin D serum levels were associated with the presence of microvascular damage, independent of cardiovascular risk factors.
Vitamin D has been widely recognized to play important in the development of CVD, and investigators have repeatedly shown that patients deficient in vitamin D are more likely to develop coronary heart disease,[23] stroke,[24] and cardiovascular mortality.[25] Despite robust evidence of vitamin D to be a risk factor for CVD, exact mechanisms through which vitamin D leads to the development of CVD remain unclear. Thus far, studies have suggested that vitamin D affects cardiovascular health through its association with cardiovascular risk factors such as diabetes mellitus,[26] an unfavorable lipid profile,[27] inflammation,[28] and indices of large-vessel disease.[29] Apart from these factors, it has been suggested that vitamin D may act on cardiovascular health through changes in the microvasculature. Given the increasing importance of microvascular disease in the development of CVD,[30] it is possible that the link between vitamin D and CVD may be explained by a microvascular component. Indeed, recent studies have shown that vitamin D is associated with nephropathy and with structural magnetic resonance imaging (MRI) markers of microvascular disease in the brain, that is, white matter lesions and lacunar infarcts.[15] Extending these previous findings, the main novelty of our study is that we show a link between vitamin D and direct visualization of microvascular damage, as reflected by qualitative and quantitative retinal parameters. Several explanations can be proposed for the association of vitamin D with microvascular damage. First, vitamin D may alter the structure and arrangement of microvasculature by endothelium activation. Both in large-vessel disease and in microvascular disease, endothelium is the key component that initiates pathological vascular processes. As such, vitamin D receptors (i.e., DNA-binding transcription factors) expressed on endothelial cells modulate endothelial cell function by binding to these receptors. Subsequently, the activated endothelium promotes endothelial cell proliferation and migration by stimulating the production of nitric oxide production, and reducing the production of reactive oxygen species.[31] It also inhibits innate inflammatory process by modulating specific signaling pathways and reduces vascular tone via the production of endothelium-derived contracting factors.[32] Against this background, it is conceivable that these antioxidative and vasodilatory processes may not be initiated in case of low vitamin D levels, and therefore damage to the blood vessels may occur. It is noteworthy to mention that in recent years, apart from arterioles, the role of venules in CVD has gained attention, and converging evidence shows wider venules to be an important marker for CVD. Also in our study, vitamin D was particularly related to venules and not arterioles, which further points toward the importance of venules in CVD. Given that both lower vitamin D levels and wider venules are related to ischemia, it is likely that vitamin D and venules are connected in the pathways of ischemia.[33] How exactly these 2 factors are connected should be investigated in further research.
Other potential mechanisms through which vitamin D could lead to microvascular damage include inflammation, lipid metabolism, and renin–angiotensin–aldosterone system, which are all processes involved in the pathogenesis of arteriosclerosis.[29] In our study, adjusting for markers of these processes (e.g., C-reactive protein, cholesterol, and blood pressure) showed that the associations between vitamin D and retinal microvascular damage greatly attenuated, pointing further toward some effects through these processes. However, the associations remained statistically significant, indicating that other processes likely also play a role or that measurement error in covariates led to insufficient adjustment.
Several limitations need to be discussed. First, the cross-sectional design of our analyses limits our ability to infer a temporal link between vitamin D and retinal microvascular damage. Another limitation is that retinal fundus photographs were taken at a single time-point, and thus, we were unable to measure dynamic measures synchronized on the cardiac cycle. This may have caused random misclassification, leading to an underestimation of our associations. Third, we were not able to measure important confounding factors such as (lifetime) sun-exposure and food intake. These factors could confound the effect of vitamin D on microvascular damage. Finally, participants in the RS are mainly middle-class white persons, which limits the generalizability of our findings. Strengths of our study are the population-based setting, large study size, and the extensive information on covariates.
In conclusion, lower vitamin D serum levels are associated with the presence of retinal microvascular damage, suggesting that the link with cardiovascular risk factor may partly run through changes in the microvasculature.
Acknowledgments
The authors thank all collaborating general practitioners and pharmacists in Ommoord for their contribution to the Rotterdam Study, all in the Netherlands.
Footnotes
Abbreviations: CI = confidence interval, CVD = cardiovascular disease, RS = Rotterdam Study, SD = standard deviation.
Funding/support: The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare, and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. None of the funding organizations or sponsors was involved in study design, in collection, analysis, and interpretation of data, in writing of the report, or in the decision to submit the article for publication.
The authors have no conflicts of interest to disclose.
References
- [1].Holick MF. Vitamin D deficiency. N Engl J Med 2007;3573:266–81. [DOI] [PubMed] [Google Scholar]
- [2].de Boer IH, Kestenbaum B, Shoben AB, et al. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol 2009;208:1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dong Y, Stallmann-Jorgensen IS, Pollock NK, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab 2010;95:4584–91. [DOI] [PubMed] [Google Scholar]
- [4].Lim S, Shin H, Kim MJ, et al. Vitamin D inadequacy is associated with significant coronary artery stenosis in a community-based elderly cohort: the Korean Longitudinal Study on Health and Aging. J Clin Endocrinol Metab 2012;97:169–78. [DOI] [PubMed] [Google Scholar]
- [5].Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol 2014;2:634–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation 2010;121:2317–25. [DOI] [PubMed] [Google Scholar]
- [7].Liew G, Wang JJ, Mitchell P, et al. Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging 2008;1:156–61. [DOI] [PubMed] [Google Scholar]
- [8].Ikram MK, Witteman JC, Vingerling JR, et al. Retinal vessel diameters and risk of hypertension: the Rotterdam Study. Hypertension 2006;47:189–94. [DOI] [PubMed] [Google Scholar]
- [9].Ikram MK, de Jong FJ, Bos MJ, et al. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology 2006;66:1339–43. [DOI] [PubMed] [Google Scholar]
- [10].Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA 2002;287:1153–9. [DOI] [PubMed] [Google Scholar]
- [11].Grunwald JE, Ying GS, Maguire M, et al. Association between retinopathy and cardiovascular disease in patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort [CRIC] Study). Am J Cardiol 2012;110:246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Capitanio S, Sambuceti G, Giusti M, et al. 1,25-Dihydroxy vitamin D and coronary microvascular function. Eur J Nucl Med Mol Imaging 2013;40:280–9. [DOI] [PubMed] [Google Scholar]
- [13].Al Mheid I, Patel R, Murrow J, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol 2011;58:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Usluogullari CA, Balkan F, Caner S, et al. The relationship between microvascular complications and vitamin D deficiency in type 2 diabetes mellitus. BMC Endocr Disord 2015;15:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chung PW, Park KY, Kim JM, et al. 25-hydroxyvitamin D status is associated with chronic cerebral small vessel disease. Stroke 2015;46:248–51. [DOI] [PubMed] [Google Scholar]
- [16].Hofman A, Brusselle GG, Darwish Murad S, et al. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol 2015;30:661–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Knudtson MD, Lee KE, Hubbard LD, et al. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 2003;27:143–9. [DOI] [PubMed] [Google Scholar]
- [18].Littmann H. [Determining the true size of an object on the fundus of the living eye] Zur Bestimmung der wahren Grosse eines Objektes auf dem Hintergrund eines lebenden Auges. Klin Monbl Augenheilkd 1988;192:66–7. [DOI] [PubMed] [Google Scholar]
- [19].van Gent CM, van der Voort HA, de Bruyn AM, et al. Cholesterol determinations. A comparative study of methods with special reference to enzymatic procedures. Clin Chim Acta 1977;75:243–51. [DOI] [PubMed] [Google Scholar]
- [20].Leening MJ, Kavousi M, Heeringa J, et al. Methods of data collection and definitions of cardiac outcomes in the Rotterdam Study. Eur J Epidemiol 2012;27:173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bos MJ, Koudstaal PJ, Hofman A, et al. Modifiable etiological factors and the burden of stroke from the Rotterdam study: a population-based cohort study. PLoS Med 2014;11:e1001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Giovannucci E, Liu Y, Hollis BW, et al. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 2008;168:1174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Poole KE, Loveridge N, Barker PJ, et al. Reduced vitamin D in acute stroke. Stroke 2006;37:243–5. [DOI] [PubMed] [Google Scholar]
- [25].Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 2007;167:1730–7. [DOI] [PubMed] [Google Scholar]
- [26].Pittas AG, Lau J, Hu FB, et al. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ponda MP, Huang X, Odeh MA, et al. Vitamin D may not improve lipid levels: a serial clinical laboratory data study. Circulation 2012;126:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jablonski KL, Chonchol M, Pierce GL, et al. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 2011;57:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kassi E, Adamopoulos C, Basdra EK, et al. Role of vitamin D in atherosclerosis. Circulation 2013;128:2517–31. [DOI] [PubMed] [Google Scholar]
- [30].Grunwald JE, Pistilli M, Ying GS, et al. Retinopathy and the risk of cardiovascular disease in patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort study). Am J Cardiol 2015;116:1527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Molinari C, Rizzi M, Squarzanti DF, et al. 1alpha,25-Dihydroxycholecalciferol (Vitamin D3) induces NO-dependent endothelial cell proliferation and migration in a three-dimensional matrix. Cell Physiol Biochem 2013;31:815–22. [DOI] [PubMed] [Google Scholar]
- [32].Wong MS, Man RY, Vanhoutte PM. Calcium-independent phospholipase A(2) plays a key role in the endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 2010;298:H1260–1266. [DOI] [PubMed] [Google Scholar]
- [33].Ikram MK, De Jong FJ, Van Dijk EJ, et al. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain 2006;129:182–8. [DOI] [PubMed] [Google Scholar]


