Abstract
The aim of this study is to compare the effects of propofol and sevoflurane anesthesia on perioperative immune response in patients undergoing laparoscopic radical hysterectomy for cervical cancer.
Sixty patients with cervical cancer scheduled for elective laparoscopic radical hysterectomy under general anesthesia were randomized into 2 groups. TIVA group received propofol induction and maintenance and SEVO group received sevoflurane induction and maintenance. Blood samples were collected at 30 min before induction (T0); the end of the operation (T1); and 24 h (T2), 48 h (T3), and 72 h (T4) after operation. The T lymphocyte subsets (including CD3+ cells, CD4+ cells, and CD8+ cells) and CD4+/CD8+ ratio, natural killer (NK) cells, and B lymphocytes were analyzed by flow cytometry.
After surgery, all immunological indicators except CD8+ cells were significantly decreased in both groups compared to basal levels in T0, and the counts of CD3+ cells, CD4+ cells, NK cells, and the CD4+/CD8+ ratios were significantly lower in the SEVO groups than that in the TIVA group. However, the numbers of B cells were comparable at all the time points between 2 groups.
Laparoscopic radical hysterectomy for cervical cancer is associated with postoperative lymphopenia. In terms of protecting circulating lymphocytes, propofol is superior to sevoflurane.
Keywords: cervical cancer, immunity, laparoscopic radical hysterectomy, lymphocyte, propofol, sevoflurane
1. Introduction
Cervical cancer is the third most commonly diagnosed cancer and the fourth leading cause of cancer death in females worldwide. In contrast to the decreasing incidence trends in developed countries, a substantial increase in cervical cancer incidence was seen in China.[1,2]
For cervical cancer, radical surgery is one of the mainstays of treatment. However, surgery and anesthesia induced perioperative immunosuppression has been implicated in the development of postoperative complications, such as delayed wound healing, systemic inflammatory response and other septic events. Furthermore, impaired immune system may allow malignant cell to overcome host immunosurveillance so that a window is created for cancer metastasis and recurrence during perioperative period.[3–5]
Laparoscopic surgery is associated with lower surgical morbidity in terms of less intraoperative blood loss, shorter hospital stay, earlier resumption of daily activities, and increased quality of life.[6,7] And after a long-term disputes and practice, laparoscopic radical hysterectomy for cervical cancer has been accepted by most researchers.[8] Reports have suggested that laparoscopic surgery has greater ability for preservation of lymphocytes number and function than conventional open surgery.[9,10] Against this background, the effects of different anesthesia techniques and anesthetics on perioperative immune response become more prominent.
Propofol and sevoflurane are most widely used anesthetics for general anesthesia. It has been reported that compared with sevoflurane, propofol could better attenuate the surgical stress-induced adverse immune response, have more protective effects for circulating lymphocytes and provide better short-term consequence in patients receiving cancer or cardiac surgery.[11–13] Besides, Enlund et al[14] showed a higher overall 1-year survival rate in patients after radical colon and breast cancer surgery under general anesthesia with propofol than patients given sevoflurane. Nevertheless, there is no study has evaluated the effects of propofol and sevoflurane on perioperative immune response in patients undergoing laparoscopic radical hysterectomy for cervical cancer.
In this study, we compared the effects of propofol and sevoflurane anesthesia on peripheral lymphocyte counts, including CD3+, CD4+, CD8+, B, natural killer (NK) cells, and CD4+/CD8+ ratio in patients with cervical cancer who were scheduled for elective laparoscopic radical hysterectomy. We hypothesized that propofol would provide more protection for the circulating lymphocytes than sevoflurane during perioperative period.
2. Methods
The study was approved by the Ethics Committee of the Xuzhou Central Hospital. Written informed consent was obtained from all participants before the trial. Female patients classified as American Society of Anesthesiologists (ASA) physical status I to II and ages 30 to 65 years who had cervical cancer requiring radical surgery were recruited. All the patients were scheduled for elective laparoscopic radical hysterectomy under general anesthesia. None had a history of endocrine, immune, or circulatory system diseases. Other exclusion criteria included recent or concurrent chemotherapy, a requirement for perioperative blood transfusion, perioperative treatment with immunomodulatory agents and any contraindication to drugs used in this study. Patients who developed major surgical complications were also excluded from our study.
Sixty patients were enrolled and were randomly allocated to 2 groups using a computer-generated randomization list. None of the patients received any premedication. After the patients arrived in operating room, the radial artery was cannulated for invasive blood pressure monitoring. The electrocardiography (ECG), peripheral capillary oxygen saturation, end-tidal carbon dioxide (PETCO2), and bispectral index (BIS) were also continuously monitored during the operation. In the propofol induction and maintenance (TIVA) group, anesthesia was induced with midazolam 2 mg, propofol 2.0 to 2.5 mg/kg (Diprivan, AstraZeneca, Zug, Switzerland), fentanyl 2 to 3 μg/kg, and maintained with propofol 4 to 8 mg/kg per h. The sevoflurane induction and maintenance (SEVO) group was induced with midazolam 2 mg, inhalation of 8% sevoflurane (Sevofrane, Maruishi, Osaka, Japan) with fresh gas flow 5 L/min, fentanyl 2 to 3 μg/kg, and maintained with inhalation of 2% to 3% sevoflurane. Rocuronium 0.6 mg/kg was given to all patients to facilitate tracheal intubation. The lungs were ventilated with oxygen in air (50–60%). Mechanical ventilation was administered to maintain a PETCO2 concentration of 35 to 40 mm Hg. After induction, continuous infusion of remifentanil 0.1 to 0.2 μg/kg/min and cisatracurium 0.2 μg/kg/min were administered in all patients. The depth of anesthesia was monitored by BIS monitor and the concentration of sevoflurane or infusion rate of propofol was adjusted to keep the BIS between 40 and 60. Thirty minutes before the end of surgery, fentanyl 1 to 2 μg/kg was administered as an intravenous bolus in every patient. All patients received patient-controlled intravenous analgesia for postoperative pain therapy.
Two milliliters of peripheral venous blood was collected into anticoagulant test tubes (ethylenediaminetetraacetic acid tube) at 5 time points: 30 min before induction of anesthesia (T0); the end of the operation (T1); and 24 h (T2), 48 h (T3), and 72 h (T4) after operation. Vacutainer tubes were transported to the hematology laboratory immediately.
Lymphocyte subsets were analyzed on a FACScalibur Flow Cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ). A single-platform, lyse-no-wash procedure was performed with Trucount tubes (BD, Franklin, NJ) with the following 2- or 4-color monoclonal antibody combinations supplied in the MultiTEST IMK kit (BD): CD3FITC/CD8PE/CD45PerCP/CD4APC or CD3FITC/CD16 + 56PE/CD45PerCP/CD19APC. The stained blood sample was lysed with a diluted lysing solution, and special care was taken not to expose the stained sample to light. CD3+ T cells, CD4+ T helper cells, and CD8+ T cytotoxic cells were identified according to published protocols.[15] B cells were identified by CD19 expression, and NK cells were identified by the CD3− CD16+ and/or CD56+ phenotype.
During the perioperative period, the surgical details of every patient (i.e., operation time, blood loss, the volume of crystalloid or colloid received, urine volume, and intraoperative complications) and the postoperative characteristics (i.e., duration of catheterization, hospital stay period, and postoperative complications) were recorded.
We believe that the CD4+/CD8+ ratio 24 h after surgery is a more useful indicator for assessing immune system function. From published study,[15] the mean CD4+/CD8+ ratio before induction of anesthesia was estimated at about 1.5 (with standard deviation [SD] approximately 0.56). We considered that a difference of 0.5 would be clinically important. We judged that 27 patients in each group would be required to detect this difference with a power of 0.90 at a significance level of 0.05 (2-sided). To allow for 10% loss during the study period, recruitment of a total of 60 patients was intended. The results of this study were evaluated using the GraphPad Prism 5.0 (GraphPad Software Inc, San Diego, CA). Continuous variables were described as mean ± SD and differences between groups were analyzed by using unpaired t test for normally distributed data. Categorical variables were described as number (%) and analyzed by Fisher exact test. The differences of lymphocyte subsets counts across different time point in the same group were analyzed by 1-way analysis of variance (ANOVA) followed by post hoc Tukey HSD test. The differences of lymphocyte subset counts between groups according to the time points were analyzed by 2-way ANOVA followed by Bonferroni correction. P value < 0.05 was considered to be statistically significant.
3. Results
3.1. Patient recruitment
Patient recruitment took place from March 1, 2014 to August 1, 2014. A total of 70 patients with cervical cancer scheduled to undergo laparoscopic radical hysterectomy were assessed for eligibility, with 60 patients enrolled and allocated randomly (Fig. 1). Two of these patients were excluded during surgery (1 patient was due to receiving blood transfusion during surgery and another one was due to the conversion to abdominal radical hysterectomy). Data of patients screened but not finally enrolled were not collected. Thus, 29 patients in the TIVA group and 29 patients in the SEVO group were finally evaluated.
Figure 1.
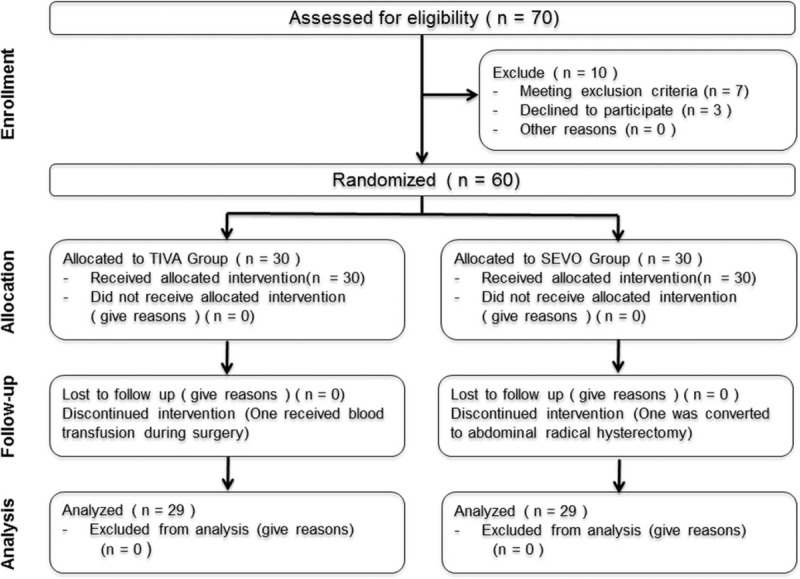
Patient flow diagram (according to the CONSORT chart). SEVO = sevoflurane induction and maintenance, TIVA = propofol induction and maintenance.
3.2. Demographics and surgical details
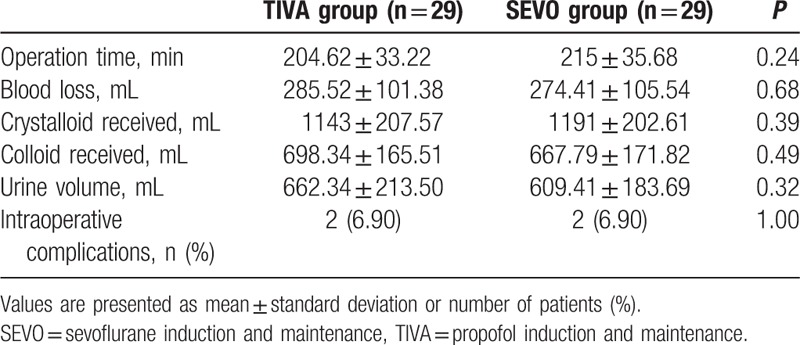
Patient characteristics are presented in Table 1. The 2 groups were comparable in terms of age, height, weight, ASA status, the International Federation of Gynecology and Obstetrics stage of tumor, and the histological types of tumor. The intraoperative parameters were not different, including the operation time, blood loss, crystalloid and colloid infused volume, urine volume, and intraoperative complications (e.g., bladder and ureteral injury) (Table 2).
Table 1.
Demographic characteristics.
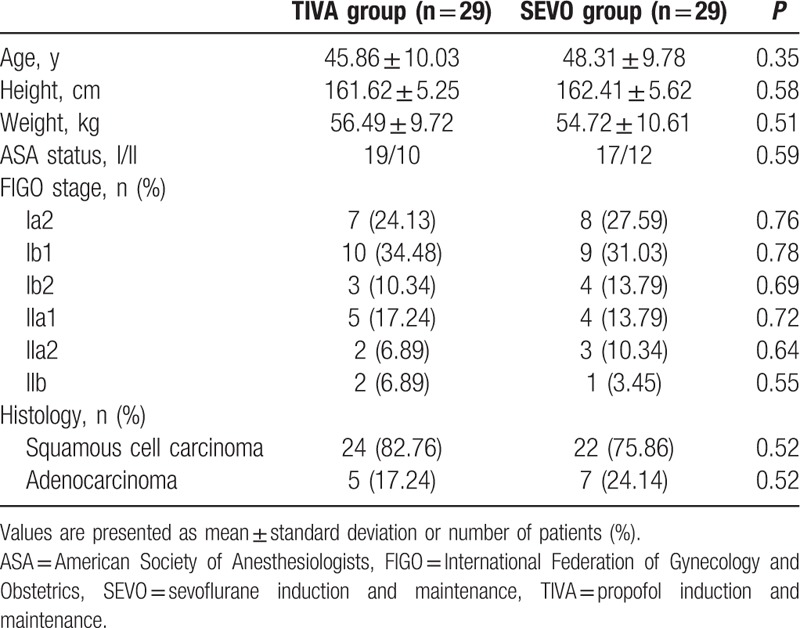
Table 2.
Surgical details.
3.3. Lymphocyte subset counts
As shown in Table 3, there were no significant differences concerning the numbers of circulating lymphocyte subsets and the CD4+/CD8+ ratio between groups before anesthesia induction.
Table 3.
Perioperative circulating lymphocyte counts.
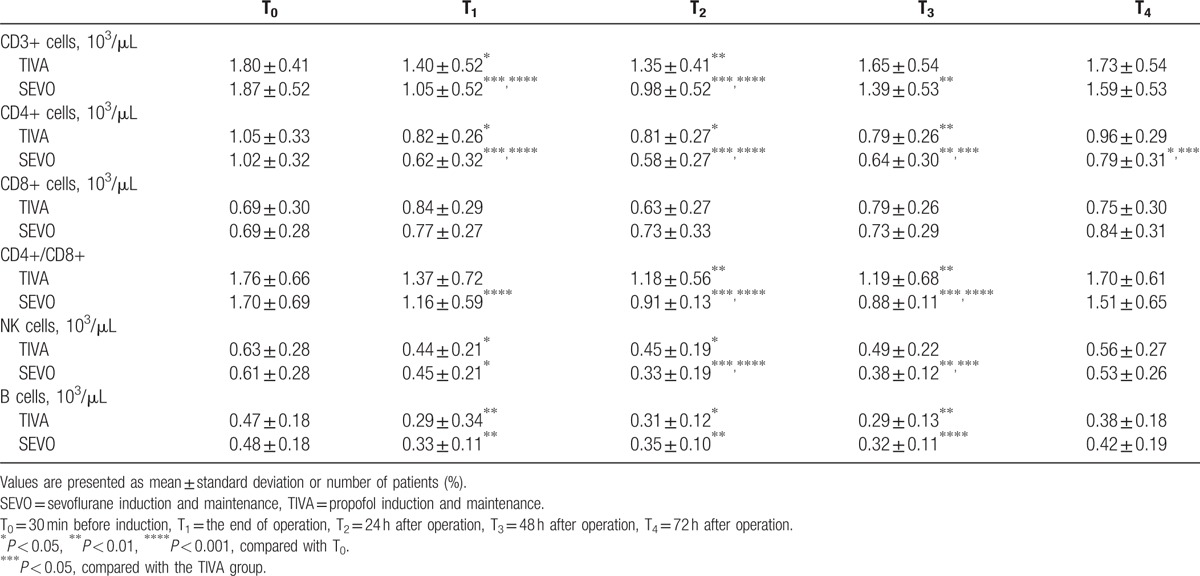
The number of CD3+ cells was significantly decreased after surgery at T1–T2 in TIVA group and T1–T3 in SEVO group compared with the baseline value at T0. And at T1–T2 time points, the CD3+ cells reduced more in SEVO group than in TIVA group. The CD4+ cells were also reduced significantly in both groups after surgery, but recovered to the normal level only in TIVA group at T4. The CD4+ lymphocyte counts were lower in SEVO group than that of TIVA group by 72 h after surgery (T4). There were no obvious changes of CD8+ cell counts were detected during this study period in both groups. The CD4+/CD8+ ratio was significantly lower in TIVA group at T2–T3 and in SEVO group at T1–T3. They all recovered gradually to the physiologic level 72 h after surgery. We also found that the ratio of CD4+/CD8+ was lower at T2–T3 in SEVO group than in TIVA group.
The NK cell counts showed a marked decrease at T1–T2 in TIVA group and at T1–T3 in SEVO group. The SEVO group also showed a statistically lower number of NK cells than TIVA group at 24 h (T2) and 48 h (T3) after surgery.
Compared with the preoperative value, the number of B lymphocytes at T1–T3 was significantly lower in both groups, but there were no statistically significant differences between groups.
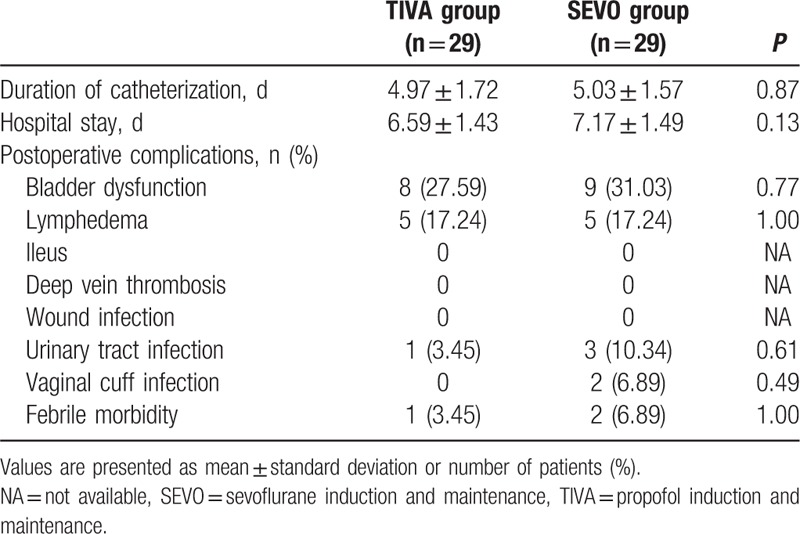
3.4. Postoperative characteristics
The postoperative data are shown in Table 4. The duration of catheterization and the hospital stay period were comparable between 2 groups. Similarly, no statistical differences were found between groups regarding bladder dysfunction and lymphedema. There is no patient who experienced severe complications, such as ileus and deep vein thrombosis. Infection was observed in 4 patients of SEVO group, while only in 1 patient of TIVA group. However, the difference was not statistically significant.
Table 4.
Postoperative characteristics.
4. Discussion
The different effects of inhalational anesthetics and propofol on the perioperative lymphocyte counts and function in patients undergoing cancer surgery have been studied for a long time. For cervical cancer, several studies have reported that the perioperative lymphocyte counts are important prognostic factors for evaluating postoperative complication and predicting relapse.[16–19] Furthermore, Wu et al suggested that pre- and post-treatment lymphopenia might be associated with deceased survival in patients with cervical cancer.[20]
CD4+ and CD8+ T cells are important effector cells of cell-mediated immunity (CMI). The CD4+/CD8+ ratio is considered to have a positive association with the function of CMI.[21] NK cells, as a firstline of defense, play a key role in destroying tumor cells and micrometastasis[22,23]; and NK cell levels have prognostic significance in a range of neoplasms.[24–28] Our results showed that the counts of CD3+, CD4+ T cells, NK cells, and the CD4+/CD8+ ratio were decreased after surgery and significant lower in SEVO group (sevoflurane induction and maintenance). Besides, the indicators in SEVO group recovered later than that in TIVA group (propofol induction and maintenance). B cells are the major cells involved in the creation of antibodies that circulate in blood plasma and lymph, known as humoral immunity. Here, we found that the number of B lymphocytes was significantly lower than preoperative levels, but there was no statistically significant difference between groups. These data suggested that propofol is less associated with the impairment of cellular immunity function rather than humoral immunity in such patients with cervical cancer.
The mechanism by which propofol provides favorable effects on the immune system than sevoflurane remains elusive. However, several studies have suggested that immune changes occurring perioperatively are primarily as a result of surgical trauma and subsequent neuroendocrine responses.[3] Activation of the hypothalamic–pituitary–adrenal (HPA) axis is the key response to stress and plays a central role in mediating the effect of surgery on the immune system.[29,30] The activation of HPA axis finally induces the release of glucocorticoids such as cortisol which is known to suppress CMI.[31,32] Besides, activation of the sympathetic nervous system during surgery also has a profound effect on the immune system since the immune organs or lymphoid organs are innervated by sympathetic nerve fibers.[33] The subsequent release of catecholamines from the nerve terminals has predominantly immunosuppressive effects.[34] Several studies indicated that inhalational anesthetics were associated with higher serum concentration of catecholamines and cortisol than propofol.[35–37] Moreover, Marana et al[38] showed that the plasma levels of norepinephrine, epinephrine adrenocorticotropic hormone, and cortisol were significantly lower in patients receiving TIVA anesthesia than patients receiving sevoflurane anesthesia in gynecological laparoscopy, suggesting a better inhibitory effect of propofol on HPA axis and sympathetic nervous system. These evidences may provide explanation, at least partially, for our present results.
Despite the indirect effects of propofol and sevoflurane on immunomodulation, they can also directly affect the lymphocyte biological characteristics. It has been reported that propofol could preserve NK activity and enhance cytotoxic T lymphocyte activity.[39,40] Besides, propofol would not alter the oxidative state of peripheral T cells and might attenuate oxidative injury of lymphocytes induced by sevoflurane.[41,42] In addition, studies have shown that sevoflurane could induce apoptosis in peripheral lymphocyte in dose-dependent and time-dependent manners in vitro via increased mitochondrial membrane permeability and caspase-3 activation.[43,44] Clinically, propofol has been shown to preferably promote the helper T cells to differentiate into Th1 cells, which maintains the Th1/Th2 ratio balance and inhibits surgical stress.[11,45] Jia et al[12] found that propofol was superior to sevoflurane in protecting the lymphocyte from apoptosis induced by caspase-3 or apoptosis-inducing factor so that provide a protective effect for circulating lymphocytes in patients undergoing off-pump coronary artery bypass graft surgery. These in vivo and in vitro mechanisms contribute to the immunoprotective effect of propofol on surgical stress that occurs in perioperative period.
In all forms of surgery, oncological and otherwise, perioperative immunosuppression can result in immediate consequences for patients including delayed wound healing and other septic events.[3] Here, we recorded postoperative characteristics including duration of catheterization, hospital stay period, and postoperative complications and found that no statistical difference was found regarding total incidence of postoperative complication. However, there were 4 patients experienced infection-related postoperative complications in SEVO group; while only 1 was observed in TIVA group. Due to the small sample size of our study, we cannot exclude the possibility that patients receiving sevoflurane anesthesia may develop more infection-related complications than that receiving propofol anesthesia after laparoscopic radical hysterectomy for cervical cancer. Another limitation is that we must not disregard the possibility that perioperative immunosuppression could be associated with long-term sequelae such as tumor recurrence, metastasis, and mortality[4,5,46]; however, we did not evaluate actual long-term clinical outcomes of the patients. Therefore, further studies regarding the long-term effects of propofol and sevoflurane on patients with cervical cancer are warranted to provide us with a comprehensive evaluation.
5. Conclusion
The present study finds that laparoscopic radical hysterectomy for cervical cancer is associated with postoperative lymphopenia. In terms of protecting circulating lymphocytes, propofol was superior to sevoflurane. Although further studies are needed, the present study provides helpful suggestions for selecting suitable anesthesia techniques and anesthetics to minimize immunosuppression during perioperative period and reduce potential short-term and long-term adverse consequence to patients with cervical cancer.
Acknowledgments
The authors thank all nursing staff in operating rooms and wards for their efforts and performance during this study.
Footnotes
Abbreviations: ANOVA = analysis of variance, ASA = American Society of Anesthesiologists, BIS = bispectral index, CMI = cell-mediated immunity, ECG = electrocardiography, HPA = hypothalamic–pituitary–adrenal, NK = natural killer, PETCO2 = end-tidal carbon dioxide.
SL and XG have contributed equally to the work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [3].Hogan BV, Peter MB, Shenoy HG, et al. Surgery induced immunosuppression. Surgeon 2011;9:38–43. [DOI] [PubMed] [Google Scholar]
- [4].Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth 2010;105:106–15. [DOI] [PubMed] [Google Scholar]
- [5].Tavare AN, Perry NJ, Benzonana LL, et al. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer 2012;130:1237–50. [DOI] [PubMed] [Google Scholar]
- [6].Diaz-Feijoo B, Gil-Moreno A, Perez-Benavente MA, et al. Sentinel lymph node identification and radical hysterectomy with lymphadenectomy in early stage cervical cancer: laparoscopy versus laparotomy. J Minim Invasive Gynecol 2008;15:531–7. [DOI] [PubMed] [Google Scholar]
- [7].Salicru S, Gil-Moreno A, Montero A, et al. Laparoscopic radical hysterectomy with pelvic lymphadenectomy in early invasive cervical cancer. J Minim Invasive Gynecol 2011;18:555–68. [DOI] [PubMed] [Google Scholar]
- [8].Maestri D, Reis RJ, Bacha OM, et al. A comparison of radical vaginal hysterectomy combined with extraperitoneal or laparoscopic pelvic lymphadenectomy in the treatment of cervical cancer. Int J Gynecol Cancer 2012;22:1238–43. [DOI] [PubMed] [Google Scholar]
- [9].Decker D, Lindemann C, Springer W, et al. Endoscopic vs conventional hernia repair from an immunologic point of view. Surg Endosc 1999;13:335–9. [DOI] [PubMed] [Google Scholar]
- [10].Ishikawa M, Nishioka M, Hanaki N, et al. Perioperative immune responses in cancer patients undergoing digestive surgeries. World J Surg Oncol 2009;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ji FH, Wang YL, Yang JP. Effects of propofol anesthesia and sevoflurane anesthesia on the differentiation of human T-helper cells during surgery. Chin Med J 2011;124:525–9. [PubMed] [Google Scholar]
- [12].Jia L, Dong R, Zhang F, et al. Propofol provides more effective protection for circulating lymphocytes than sevoflurane in patients undergoing off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2015;29:1172–9. [DOI] [PubMed] [Google Scholar]
- [13].Zhang T, Fan Y, Liu K, et al. Effects of different general anaesthetic techniques on immune responses in patients undergoing surgery for tongue cancer. Anaesth Intensive Care 2014;42:220–7. [DOI] [PubMed] [Google Scholar]
- [14].Enlund M, Berglund A, Andreasson K, et al. The choice of anaesthetic—sevoflurane or propofol—and outcome from cancer surgery: a retrospective analysis. Upsala J Med Sci 2014;119:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chng WJ, Tan GB, Kuperan P. Establishment of adult peripheral blood lymphocyte subset reference range for an Asian population by single-platform flow cytometry: influence of age, sex, and race and comparison with other published studies. Clin Diag Lab Immunol 2004;11:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee YY, Choi CH, Sung CO, et al. Clinical significance of changes in peripheral lymphocyte count after surgery in early cervical cancer. Gynecol Oncol 2012;127:107–13. [DOI] [PubMed] [Google Scholar]
- [17].Lomivorotov VV, Efremov SM, Boboshko VA, et al. Preoperative total lymphocyte count in peripheral blood as a predictor of poor outcome in adult cardiac surgery. J Cardiothorac Vasc Anesth 2011;25:975–80. [DOI] [PubMed] [Google Scholar]
- [18].Nedergaard BS, Ladekarl M, Thomsen HF, et al. Low density of CD3+, CD4+ and CD8+ cells is associated with increased risk of relapse in squamous cell cervical cancer. Br J Cancer 2007;97:1135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shah W, Yan X, Jing L, et al. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4(+)FOXP3(+) regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol 2011;8:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wu ES, Oduyebo T, Cobb LP, et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol 2016;140:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hori Y, Ibuki T, Hosokawa T, et al. The effects of neurosurgical stress on peripheral lymphocyte subpopulations. J Clin Anesth 2003;15:1–8. [DOI] [PubMed] [Google Scholar]
- [22].Yang Q, Goding SR, Hokland ME, et al. Antitumor activity of NK cells. Immunol Res 2006;36:13–25. [DOI] [PubMed] [Google Scholar]
- [23].Zamai L, Ponti C, Mirandola P, et al. NK cells and cancer. J Immunol (Baltimore, Md: 1950) 2007;178:4011–6. [DOI] [PubMed] [Google Scholar]
- [24].Coca S, Perez-Piqueras J, Martinez D, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 1997;79:2320–8. [DOI] [PubMed] [Google Scholar]
- [25].Fries R, Platz H, Wagner R, et al. Carcinoma of the oral cavity: on the prognostic significance of the primary tumour site (by levels and areas). J Maxillofac Surg 1979;7:15–31. [DOI] [PubMed] [Google Scholar]
- [26].Hsia JY, Chen JT, Chen CY, et al. Prognostic significance of intratumoral natural killer cells in primary resected esophageal squamous cell carcinoma. Chang Gung Med J 2005;28:335–40. [PubMed] [Google Scholar]
- [27].Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 2000;88:577–83. [PubMed] [Google Scholar]
- [28].Villegas FR, Coca S, Villarrubia VG, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer (Amsterdam, Netherlands) 2002;35:23–8. [DOI] [PubMed] [Google Scholar]
- [29].Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 1995;332:1351–62. [DOI] [PubMed] [Google Scholar]
- [30].Kennedy BC, Hall GM. Neuroendocrine and inflammatory aspects of surgery: do they affect outcome? Acta Anaesthesiol Belg 1999;50:205–9. [PubMed] [Google Scholar]
- [31].Larsson EL. Cyclosporin A and dexamethasone suppress T cell responses by selectively acting at distinct sites of the triggering process. J Immunol (Baltimore, Md: 1950) 1980;124:2828–33. [PubMed] [Google Scholar]
- [32].Arya SK, Wong-Staal F, Gallo RC. Dexamethasone-mediated inhibition of human T cell growth factor and gamma-interferon messenger RNA. J Immunol (Baltimore, Md: 1950) 1984;133:273–6. [PubMed] [Google Scholar]
- [33].Panuncio AL, De La Pena S, Gualco G, et al. Adrenergic innervation in reactive human lymph nodes. J Anat 1999;194(pt 1):143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Elenkov IJ, Wilder RL, Chrousos GP, et al. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 2000;52:595–638. [PubMed] [Google Scholar]
- [35].Adams HA, Schmitz CS, Baltes-Gotz B. Endocrine stress reaction, hemodynamics and recovery in total intravenous and inhalation anesthesia. Propofol versus isoflurane. Der Anaesthesist 1994;43:730–7. [DOI] [PubMed] [Google Scholar]
- [36].Hertel VG, Olthoff D, Vetter B, et al. Comparison of various methods of anesthesia by plasma catecholamine determination. Anaesthesiol Reanim 1995;20:116–25. [PubMed] [Google Scholar]
- [37].Pirttikangas CO, Salo M, Mansikka M, et al. The influence of anaesthetic technique upon the immune response to hysterectomy. A comparison of propofol infusion and isoflurane. Anaesthesia 1995;50:1056–61. [DOI] [PubMed] [Google Scholar]
- [38].Marana E, Colicci S, Meo F, et al. Neuroendocrine stress response in gynecological laparoscopy: TIVA with propofol versus sevoflurane anesthesia. J Clin Anesth 2010;22:250–5. [DOI] [PubMed] [Google Scholar]
- [39].Kushida A, Inada T, Shingu K. Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol Immunotoxicol 2007;29:477–86. [DOI] [PubMed] [Google Scholar]
- [40].Melamed R, Bar-Yosef S, Shakhar G, et al. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg 2003;97:1331–9. [DOI] [PubMed] [Google Scholar]
- [41].Delogu G, Antonucci A, Moretti S, et al. Oxidative stress and mitochondrial glutathione in human lymphocytes exposed to clinically relevant anesthetic drug concentrations. J Clin Anesth 2004;16:189–94. [DOI] [PubMed] [Google Scholar]
- [42].Zhou Y, Li E, Li Y, et al. Attenuating sevoflurane-induced cellular injury of human peripheral lymphocytes by propofol in a concentration-dependent manner. Arch Pharm Res 2011;34:1535–43. [DOI] [PubMed] [Google Scholar]
- [43].Matsuoka H, Kurosawa S, Horinouchi T, et al. Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology 2001;95:1467–72. [DOI] [PubMed] [Google Scholar]
- [44].Loop T, Dovi-Akue D, Frick M, et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology 2005;102:1147–57. [DOI] [PubMed] [Google Scholar]
- [45].Inada T, Yamanouchi Y, Jomura S, et al. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia 2004;59:954–9. [DOI] [PubMed] [Google Scholar]
- [46].Homburger JA, Meiler SE. Anesthesia drugs, immunity, and long-term outcome. Curr Opin Anaesthesiol 2006;19:423–8. [DOI] [PubMed] [Google Scholar]


