Supplemental Digital Content is available in the text
Keywords: medullary thyroid carcinoma, papillary thyroid carcinoma, ultrasonography
Abstract
This study was designed to retrospectively compare the sonographic features of medullary thyroid carcinoma (MTC) and the features of papillary thyroid carcinoma (PTC).
A total of 97 patients with 127 MTCs between January 2000 and January 2016 and 107 consecutive patients with 132 PTCs were included in this study. Two radiologists retrospectively determined the sonographic features and compared the findings of MTCs and PTCs.
Compared with the patients with PTCs, the patients with MTCs were older (46.9 years vs 42.9 years, P = 0.016) and the male proportion was higher (53.6% vs 33.6%, P = 0.005). Most of the MTCs had an irregular shape (72.4%), a length/width ratio <1 (75.6%), an unclear boundary (63.8%), no peripheral halo ring (93.7%), hypoechogenicity (96.9%), heterogeneous echotexture (76.4%), no cystic change (78.7%), calcification (63.8%), and hypervascularity (72.4%). There was no significant difference in the boundary, peripheral halo ring, echogenicity, and calcification between the MTCs and PTCs. However, compared with the PTCs, a larger size (2.2 vs 1.2 cm, P <0.001), a regular shape (27.6% vs 7.6%, P <0.001), a length/width ratio <1 (75.6% vs 51.5%, P<0.001), heterogeneous echotexture (76.4% vs 54.5%, P <0.001), cystic change (21.3 vs 8.3%, P = 0.005), and hypervascularity (72.4% vs 47.7%, P <0.001) were more frequent in the MTCs.
The sonographic features with a higher likelihood of malignancy are common in MTCs, including a shape taller than the width, irregular infiltrative margins, an absent halo, hypoechogenicity, the presence of microcalcifications, and increased intranodular vascularity. However, MTCs tend to possess these suspicious sonographic features less often than PTCs, with the exception of hypervascularity, which was more frequent in MTCs.
1. Introduction
Medullary thyroid carcinoma (MTC) originates from thyroid C cells and is a rare malignancy, accounting for approximately 5% to 8% of thyroid malignancies.[1,2] MTC could be diagnosed by fine needle aspiration, but the detection rate by fine needle aspiration is only 56%.[3] The routine measurement of plasma calcitonin levels has not been widely adopted.[4] The growth of MTC is relatively slow, but at the time of initial diagnosis, 35% of patients with MTCs had lymphatic metastatic spread.[5] Therefore, it is important to diagnose MTC prior to surgery.
Thyroid ultrasonography has been widely used and is the pivotal imaging tool to differentiate benign from malignant thyroid nodules. Specific sonographic features with a higher likelihood of malignancy have been widely reported.[6–8] However, in the majority of studies, these sonographic features are used to differentiate benign nodules from papillary thyroid carcinoma (PTC), and only some studies with small sample sizes have focused on the sonographic features for possible MTCs.[9–17] Only one study with 12 MTCs compared the vascularity of MTCs and PTCs and found that intranodular vascularity was more frequent in MTCs.[14] However, because of the small sample size, the difference was not significant. In this retrospective study, we compared the sonographic features of MTCs and PTCs in a relatively large sample size to improve the diagnostic value of ultrasonography for MTCs.
2. Patients and methods
2.1. Patients
The Peking Union Medical College Hospital ethics committee approved and supported this retrospective study, and the need for written informed consent from the participants was waived. The records data and sonograms of all patients were deidentified and analyzed anonymously (see Table, Supplemental Content, which provides the data of all cases). The records of 125 consecutive patients who underwent surgery for primary MTC confirmed by pathological examination in this institution between January 2000 and January 2016 were retrospectively reviewed. Preoperative sonographic findings were available for 97 patients in this institution. The final study group consisted of 97 patients with 127 MTCs; there was a single tumor in 72 patients, 2 tumors in 20 patients, and 3 tumors in 5 patients.
As a control group, 107 consecutive patients with 132 PTCs that were diagnosed after surgery between October 2015 and January 2016 in this institution were included in this retrospective study.
2.2. Imaging and image analysis
Preoperative thyroid ultrasonography was performed as a baseline study in each patient using a Philips HDI, HDI5000 or iU22 (Philips Medical Systems, Bothell, WA) and GE logic 7 or logic 9 (GE Healthcare, Wauwatosa, WI) equipped with a 5- to 12-MHz linear array transducer. All equipments had been used less than 5 years and had good status and variability. Transverse, longitudinal, and oblique plane sonograms of the thyroid and the cervical lymph nodes were obtained. The preoperative sonograms were retrospectively reviewed by two experienced radiologists (XL and YX) by consensus. The 2 radiologists had 9 and 15 years of thyroid ultrasonography experience and were blinded to the pathologic information.
The size, shape, ratio of length/width, boundary, peripheral halo ring, echogenicity, echotexture, cystic change, calcification, and vascularity of each thyroid nodule were recorded. The shape was determined as regular (round or oval) or irregular (including lobulated). The ratio of length/width was determined as <1 or ≥1. The boundary was determined as clear or unclear. The peripheral halo ring was determined as with or without peripheral halo ring. The echogenicity was determined as hypoechogenicity, isoechogenicity, or hyperechogenicity. The echotexture was determined as homogeneous or heterogeneous. The cystic change was determined as solid, predominantly solid (<50% cystic) or predominantly cystic (≥50% cystic). The calcification was determined as no calcification, microcalcification, or macrocalcification. The vascularity was determined as hypervascularity (more than adjacent tissue), normal vascularity (similar to adjacent tissue), or avascularity (no blood flow).[18]
2.3. Statistical analysis
The clinical features (age, gender, bilaterality, multifocality, lymph node metastasis, distant metastasis, and accompanying disease) and sonographic findings (size, shape, ratio of length/width, boundary, peripheral halo ring, echogenicity, echotexture, calcification, cystic change, and vascularity) were compared between the MTCs and PTCs.
All statistical analyses were performed using the SPSS 11.5 software package (SPSS, Chicago, IL). Categorical variables were summarized as percentages, and continuous variables were summarized as the means ± standard deviation, median, and range. The χ2 test or Fisher exact test was used, as appropriate. Statistical analysis for a difference in age and size was performed with the Mann–Whitney test. Statistical significance was determined when a P value was less than 0.05.
3. Results
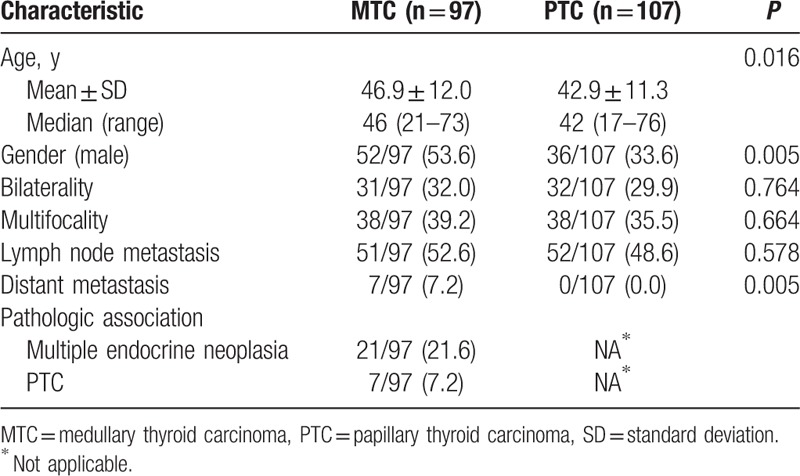
The final study group consisted of 97 patients with 127 MTCs and the control group consisted of 107 consecutive patients with 132 PTCs. A summary of the clinical features of all patients is presented in Table 1. The patients with MTCs were older than the patients with PTCs (46.9 years vs 42.9 years, P = 0.016). The male proportion of patients with MTCs was higher than that of patients with PTCs (53.6% vs 33.6%, P = 0.005). Seven patients with MTCs and no patients with PTCs had distant metastases (P = 0.005). However, the bilaterality, multifocality, and cervical lymph node metastases were not significantly different between the patients with MTCs and the patients with PTCs. In addition, 21 patients with MTCs had multiple endocrine neoplasia, and 7 patients with MTCs also had PTCs.
Table 1.
Comparison of clinical features between medullary thyroid carcinomas and papillary thyroid carcinomas.
A summary of the sonographic findings of all patients is presented in Table 2. The majority of the MTCs had an irregular shape (72.4%), a length/width ratio <1 (75.6%), an unclear boundary (63.8%), no peripheral halo ring (93.7%), hypoechogenicity (96.9%), heterogeneous echotexture (76.4%), no cystic change (78.7%), calcification (63.8%), and hypervascularity (72.4%) (Figs. 1 and 2). There was no significant difference in the boundary, peripheral halo ring, echogenicity, and calcification between the MTCs and PTCs. However, compared with the PTCs, a larger size (2.2 cm vs 1.2 cm, P<0.001), a regular shape (27.6% vs 7.6%, P < 0.001), a length/width ratio <1 (75.6% vs 51.5%, P < 0.001), heterogeneous echotexture (76.4% vs 54.5%, P < 0.001), cystic change (21.3 vs 8.3%, P = 0.005), and hypervascularity (72.4% vs 47.7%, P < 0.001) were more frequent in the MTCs.
Table 2.
Comparison of sonographic features between medullary thyroid carcinomas and papillary thyroid carcinomas.
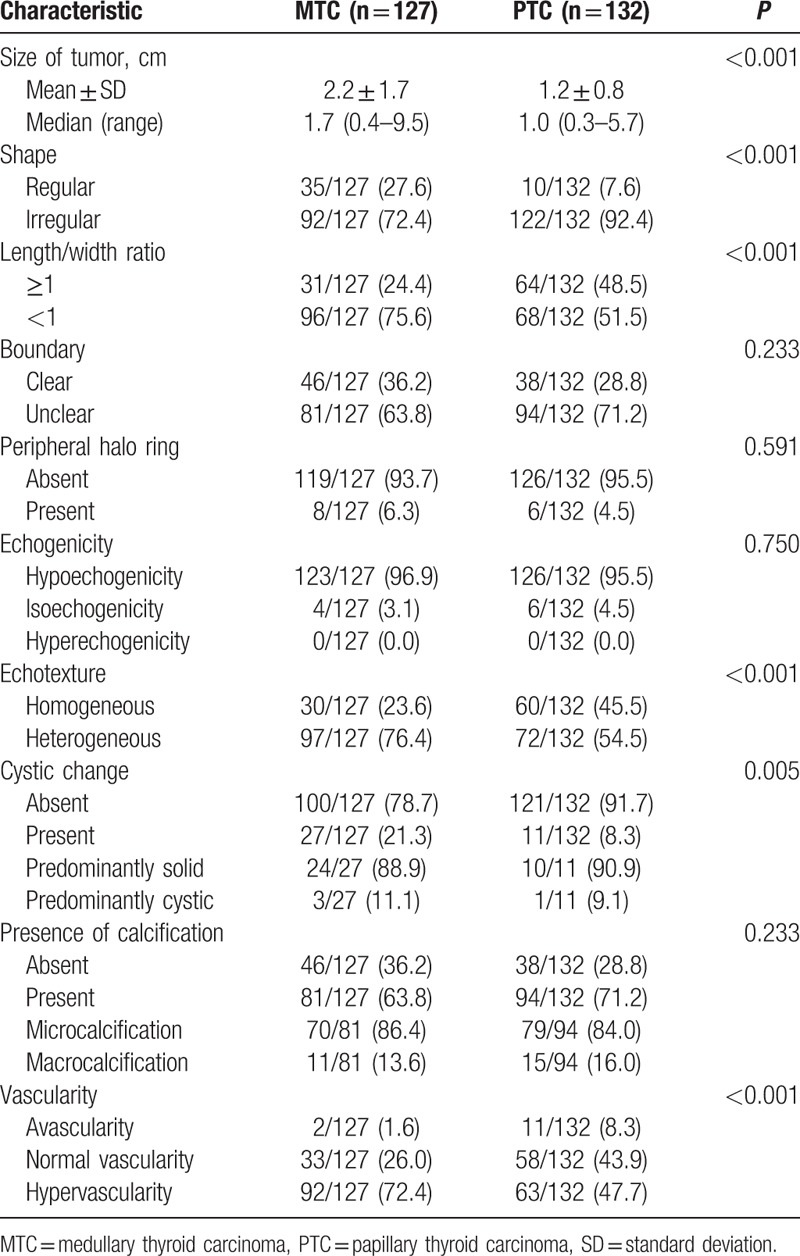
Figure 1.
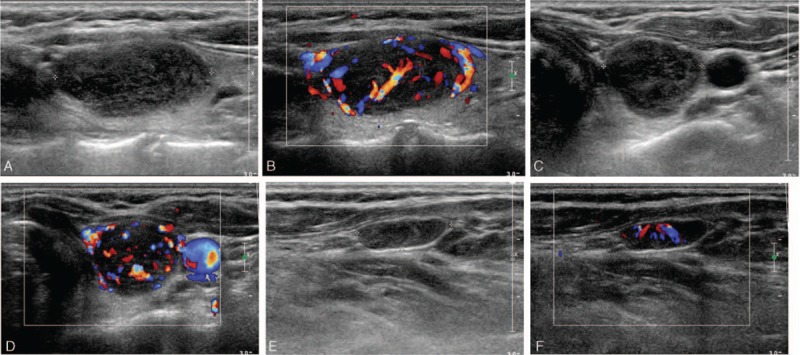
A 48-year-old male patient with medullary thyroid carcinoma and cervical lymph node metastasis. A, The longitudinal gray scale sonogram of the tumor shows a regular shape, a length/width ratio of <1, a clear boundary, no peripheral halo ring, hypoechogenicity, heterogeneous echotexture, no cystic change, and no calcification. B, The longitudinal color sonogram of the tumor shows hypervascularity. C, The transverse gray scale sonogram of the tumor shows a regular shape, a length/width ratio of <1, a clear boundary, no peripheral halo ring, hypoechogenicity, heterogeneous echotexture, no cystic change, and no calcification. D, The transverse color sonogram of the tumor shows hypervascularity. E, The longitudinal gray scale sonogram of the cervical lymph node shows loss of the fatty hilus. F, The longitudinal color sonogram of the cervical lymph node shows hypervascularity.
Figure 2.
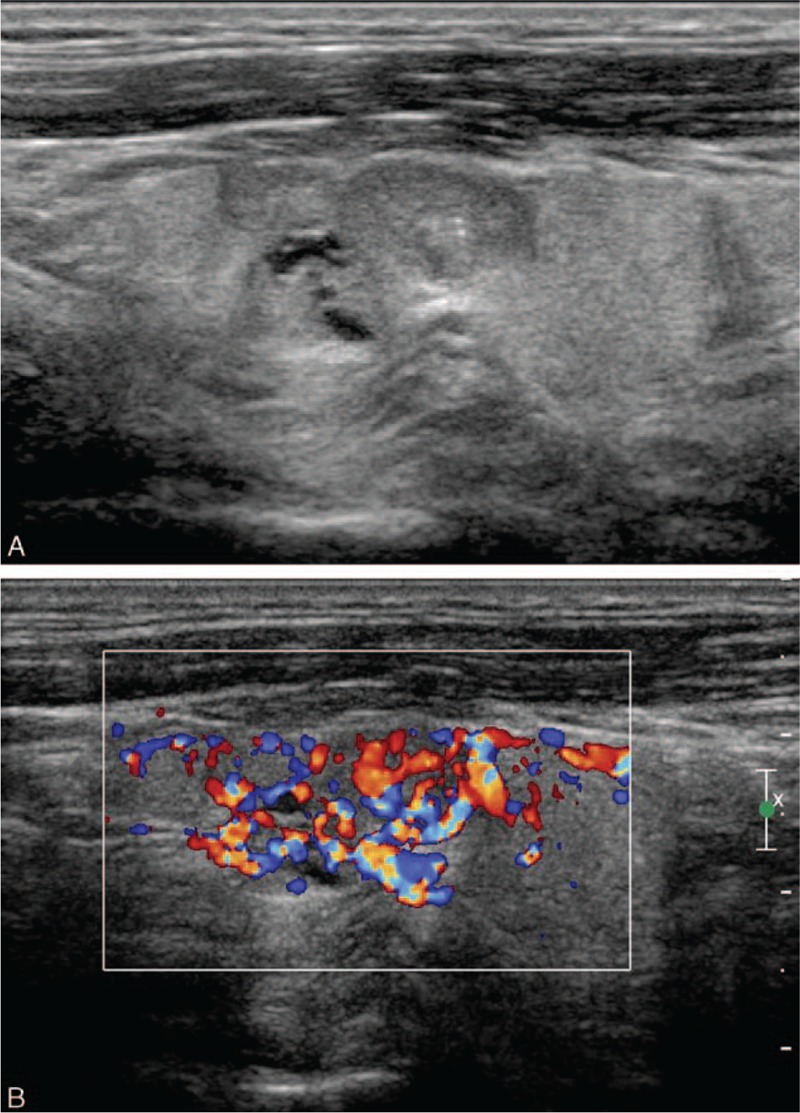
A 44-year-old male patient with medullary thyroid carcinoma. A, The longitudinal gray scale sonogram of the tumor shows an irregular (lobulated) shape, a length/width ratio of <1, an unclear boundary, no peripheral halo ring, hypoechogenicity, homogeneous echotexture, cystic change, and microcalcification. B, The longitudinal color sonogram of the tumor shows hypervascularity.
4. Discussion
In this study, the patients with MTCs were older than the patients with PTCs (46.9 years vs 42.9 years), and the MTCs had a larger size (2.2 cm vs 1.2 cm). One feasible reason for this discrepancy might be that the diagnosis of MTC represents a diagnostic challenge in clinical practice, and many MTCs are still incidentally discovered after thyroid excision.[2] Although the cervical lymph node metastases were not significantly different between the patients with MTCs and the patients with PTCs, more patients with MTCs had distant metastases at the time of initial diagnosis, confirming that MTCs were more aggressive than PTCs.
Several studies have evaluated the sonographic features of MTCs, and some studies also included comparisons with PTCs.[9–17] However, because of the rarity of MTC, the sample sizes in those studies were small. We evaluated the sonographic features of MTCs in this study with a relatively large sample size and found that an irregular shape, a length/width ratio <1, an unclear boundary, no peripheral halo ring, hypoechogenicity, heterogeneous echotexture, no cystic change, calcification, and hypervascularity were the most common sonographic features of MTCs.
Various sonographic features including a shape taller than the width, irregular infiltrative margins, an absent halo, hypoechogenicity, the presence of microcalcifications, and increased intranodular vascularity were associated with a higher likelihood of PTC.[6–8] The results of this study confirmed that these specific sonographic features were also associated with a higher likelihood of MTC.
In this study, an irregular shape and a shape taller than the width were significantly less infrequent in MTCs than in PTCs, and irregular infiltrative margins were less infrequent in MTCs than in PTCs without a significant difference. Previous studies obtained similar results. Kim et al[11] reported that 57% of MTCs and 25% of PTCs exhibited an ovoid to round shape, 19% of MTCs and 42% of PTCs exhibited a shape taller than the width, and 19% of MTCs and 19% of PTCs exhibited smooth margins. Lee et al[12] reported that 67% of MTCs and 40% of PTCs exhibited an ovoid to round shape, 13% of MTCs and 36% of PTCs exhibited a shape taller than the width, and 41% of MTCs and 59% of PTCs exhibited non-circumscribed margins.
Some studies have evaluated the halo presence in MTCs and found that the presence of a halo was not common in MTCs and occurred in 3% to 28% MTCs.[10,13,14,16] Trimboli et al[14] reported that a hypoechoic halo was detected in 2 MTCs and in none of the PTCs. In this study, we found that peripheral halo rings were more frequent in MTCs (6.3%) than in PTCs (4.5%), although the difference was not significant.
A cystic change was significantly more common in MTCs (21.3%) than in PTCs (8.3%) with significant discrepancy. However, the majority of MTCs (88.9%) with a cystic change were predominantly solid, and only 3 MTCs were predominantly cystic. The sizes of the 3 predominantly cystic MTCs were 2 to 4 cm. Therefore, the size of the MTCs and cystic change has a not significant relationship. Lee et al reported similar results. In their study, 32.6% of MTCs and 3.6% of PTCs exhibited a cystic change.[12]
In agreement with the results of the previous studies, we found in this study that the presence of calcification was common in MTCs and microcalcifications were detected in majority of MTCs with calcifications (86.4%). These results confirmed that both MTCs and PTCs were associated with calcifications more than other thyroid malignancies.[11] Amyloid deposits were common in MTCs and might give rise to reactive fibrosis and calcified deposits.[19] These factors might be 1 feasible explanation for the more frequent heterogeneous echotexture in MTCs than in PTCs (76.4% vs 54.5%) in this study.
To the best of our knowledge, only limited literature has reported the vascular signal pattern of MTCs. Trimboli et al[14] reported that 25% of MTCs and 15% of PTCs exhibited an intranodular vascular signal, without significant difference. However, in the current reported series, hypervascularity was significantly more frequent in MTCs than in PTCs. This discrepancy might be explained by the small sample size in the previous study. Only 12 MTCs were included in that study. Therefore, a statistically significant difference was unable to be demonstrated. The CDFI display technology had improved obviously in the past 16 years. However, in this study, the vascularity was determined as hypervascularity (more than adjacent tissue), normal vascularity (similar to adjacent tissue), or avascularity (no blood flow). When the CDFI display technology improved, the sensitivity of detecting intranodular vascular signals and adjacent tissue vascular signals both improved. Therefore, the vascularity of nodules (hypervascularity, normal vascularity, or avascularity) was not affected by the improvement of CDFI display technology.
There are several limitations of this study. First, this was a retrospective study. Therefore, evaluating the sonographic findings in real time, which might provide more information, was impossible, and the interpretive results of sonograms might vary among different radiologists. However, 2 experienced radiologists interpreted all preoperative sonograms in this study by consensus. Second, this study did not contain information regarding the appearance of MTCs in soelastography, which is believed to be an important improvement on conventional sonography.[20]
In conclusion, the sonographic features with a higher likelihood of malignancy are common in MTCs, including a shape taller than the width, irregular infiltrative margins, an absent halo, hypoechogenicity, the presence of microcalcifications, and increased intranodular vascularity. However, MTCs tend to possess these suspicious sonographic features less often than PTCs, with the exception of hypervascularity, which was more frequent in MTCs.
Supplementary Material
Footnotes
Abbreviations: MTC = medullary thyroid carcinoma, PTC = papillary thyroid carcinoma.
XL is first author and ML is co-first author.
This work was supported by the Science and Technology Project of Ministry of Labor and Personnel of China (M426300).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Wells SA, Jr, Asa SL, Dralle H, et al. American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Trimboli P, Giovanella L, Valabrega S, et al. Ultrasound features of medullary thyroid carcinoma correlate with cancer aggressiveness: a retrospective multicenter study. J Exp Clin Cancer Res 2014;25:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Trimboli P, Treglia G, Guidobaldi L, et al. Detection rate of FNA cytology in medullary thyroid carcinoma: a meta-analysis. Clin Endocrinol (Oxf) 2015;82:280–5. [DOI] [PubMed] [Google Scholar]
- [4].Trimboli P, Giovanella L, Crescenzi A, et al. Medullary thyroid cancer diagnosis: an appraisal. Head Neck 2014;36:1216–23. [DOI] [PubMed] [Google Scholar]
- [5].Lairmore TC, Wells SA., Jr Medullary carcinoma of the thyroid: current diagnosis and management. Semin Surg Oncol 1991;7:92–9. [DOI] [PubMed] [Google Scholar]
- [6].American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- [7].Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology 2005;237:794–800. [DOI] [PubMed] [Google Scholar]
- [8].Moon WJ, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation-multicenter retrospective study. Radiology 2008;247:762–70. [DOI] [PubMed] [Google Scholar]
- [9].Gorman B, Charboneau JW, James EM, et al. Medullary thyroid carcinoma: role of high-resolution US. Radiology 1987;162:147–50. [DOI] [PubMed] [Google Scholar]
- [10].Saller B, Moeller L, Gorges R, et al. Role of conventional ultrasound and color Doppler sonography in the diagnosis of medullary thyroid carcinoma. Exp Clin Endocrinol Diabetes 2002;110:403–7. [DOI] [PubMed] [Google Scholar]
- [11].Kim SH, Kim BS, Jung SL, et al. Ultrasonographic findings of medullary thyroid carcinoma: a comparison with papillary thyroid carcinoma. Korean J Radiol 2009;10:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee S, Shin JH, Han BK, et al. Medullary thyroid carcinoma: comparison with papillary thyroid carcinoma and application of current sonographic criteria. Am J Roentgenol 2010;194:1090–4. [DOI] [PubMed] [Google Scholar]
- [13].Choi N, Moon WJ, Lee JH, et al. Ultrasonographic findings of medullary thyroid cancer: differences according to tumor size and correlation with fine needle aspiration results. Acta Radiol 2011;52:312–6. [DOI] [PubMed] [Google Scholar]
- [14].Trimboli P, Nasrollah N, Amendola S, et al. Should we use ultrasound features associated with papillary thyroid cancer in diagnosing medullary thyroid cancer? Endocr J 2012;59:503–8. [DOI] [PubMed] [Google Scholar]
- [15].Fukushima M, Ito Y, Hirokawa M, et al. Excellent prognosis of patients with nonhereditary medullary thyroid carcinoma with ultrasonographic findings of follicular tumor or benign nodule. World J Surg 2009;33:963–8. [DOI] [PubMed] [Google Scholar]
- [16].Cai S, Liu H, Li WB, et al. Ultrasonographic features of medullary thyroid carcinoma and their diagnostic values. Chin Med J (Engl) 2010;123:3074–8. [PubMed] [Google Scholar]
- [17].Zhou L, Chen B, Zhao M, et al. Sonographic features of medullary thyroid carcinomas according to tumor size: comparison with papillary thyroid carcinomas. J Ultrasound Med 2015;34:1003–9. [DOI] [PubMed] [Google Scholar]
- [18].Xia Y, Wang L, Jiang Y, et al. Sonographic appearance of primary thyroid lymphoma-preliminary experience. PLoS One 2014;9:e114080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Williams ED. Histogenesis of medullary carcinoma of the thyroid. J Clin Pathol 1966;19:114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Trimboli P, Guglielmi R, Monti S, et al. Ultrasound sensitivity for thyroid malignancy is increased by real-time elastography: a prospective multicenter study. J Clin Endocrinol Metab 2012;97:4524–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


