Abstract
The aim of this study was to explore the incidence and risk factors of postoperative neurological deterioration after posterior decompression with or without instrumented fusion for thoracic myelopathy, and hope to provide references in decision-making and surgical planning for both spinal surgeon and thoracic stenosis patients.
By retrieving the medical records from January 2001 to November 2015, 168 patients were retrospectively reviewed. According to the occurrence of postoperative neurological deterioration, patients were divided into 2 groups: neurological deterioration (ND) group and non-ND group. To investigate risk values for the occurrence of ND, 3 categorized factors were analyzed statistically: patient characteristics—preoperative data of age, sex, body mass index, bone mineral density, the duration of disease (from first symptoms to operation), the preoperative neurological function (Frankel grade), and diagnosis; surgical variables—surgery time, the amount of bleeding, mean arterial pressure, intervertebral fusion or not, and instrumentation or not; radiographic parameters—the spinal canal occupancy ratio, location of the lesion, thoracic kyphosis, and kyphosis correction.
Postoperative neurological deterioration was developed in 23 of 168 patients (13.7%), and were enrolled as ND group. There was no statistically significant difference between the 2 groups in age at operation, sex composition, body mass index, and bone mineral density. The preoperative diagnosis presented significant difference between the 2 groups, because ossification of posterior longitudinal ligament combined with ossification of the ligamentum flavum was more common in ND group, whereas ossification of the ligamentum flavum alone was more common in non-ND group. There was no difference between the 2 groups in mean surgery time, the incidence of intraoperative direct trauma, and the number of patients that received instrumentation. The mean bleeding was much more in ND group than that in non-ND group, and the mean arterial pressure was lower in ND group than that in non-ND group. Also, the mean spinal canal occupancy ratio was more severe in ND group than that in non-ND group. There were no statistically significant difference between the 2 groups in stenosis location and preoperative thoracic kyphosis. The mean kyphosis correction was more significant in ND group. When included in a multivariate logistic regression model, thoracic disc herniation + ossification of posterior longitudinal ligament, spinal canal occupancy ratio more than 70%, bleeding more than 800 mL, and mean arterial pressure less than 81 mm Hg were independently associated with the postoperative neurological deterioration.
In conclusion, ossification of posterior longitudinal ligament combined with ossification of the ligamentum flavum, spinal canal occupancy ratio more than 70%, intraoperative bleeding more than 800 mL, and mean arterial pressure less than 81 mm Hg are risk factors for the occurrence of postoperative neurologic deterioration. Improving surgical technique, shortening operation time, and paying more attention to hemostasis could provide opportunities to reduce the incidence of neurologic deterioration and to improve therapeutic outcomes.
Keywords: mean arterial pressure, neurological deterioration, ossification of posterior longitudinal ligament, ossification of the ligamentum flavum, thoracic disc herniation
1. Introduction
Thoracic myelopathy (TM) is a relatively rare spinal disorder secondary to several degenerative disease, including intervertebral disc herniation, ossification of posterior longitudinal ligament (OPLL), and ossification of the ligamentum flavum (OLF).[1–5] Symptomatic thoracic disc herniations (TDHs) are rare and are mostly found in the lower thoracic spine, with more than 75% occurring below T-8.[6,7] The prevalence of thoracic OPLL in the Japanese population was 1.9%; posterior decompression with instrumented fusion surgery for thoracic OPLL is considered a relatively safe and stable surgical procedure considering the mid to long-term outcomes.[8,9] Thoracic OLF is the most common cause for thoracic spinal stenosis, and is usually complicated by TDHs, OPLL, and degenerative spinal diseases such as cervical spondylosis and lumbar spinal stenosis. The most prominent clinical manifestations were progressive weakness and numbness of the lower extremities, sensory abnormalities in the saddle area, urination, and defecation function disturbance. The resultant superposition of several symptoms makes the clinical manifestations complex.[10,11]
Regardless of its cause, prompt surgical decompression plays a key role in improving the functional outcome of myelopathy, but the surgical intervention has high incidence of complications.[12–15] Postoperative neurological deterioration (ND) is a devastating complication in thoracic decompressive surgery. There are several theories regarding the neurologic deterioration, such as vascular compromise, hypotension, or ischemia-reperfusion injury, direct trauma, microthrombi, and altered perfusion due to internal recoil of spinal cord architecture after decompression, epidural hematoma, and stretching of the neural elements.[16–19] However, the exact mechanisms and risk factors of postoperative ND after thoracic decompressive surgery remain unclear. The purpose of this study is therefore to explore the incidence and risk factors of postoperative ND after posterior decompression with or without instrumented fusion for TM, and hope to provide references in decision-making and surgical planning for both spinal surgeon and thoracic stenosis patients.
2. Materials and methods
2.1. Subjects
This is a retrospective study, and it was approved by the Institutional Review Board of the Third Hospital of HeBei Medical University before data collection and analysis. The inclusion criteria were as follows: TM derived from TDH, thoracic OPLL, OLF, and hypertrophy of the ligamentum flavum; undertake posterior decompression with or without instrumented fusion; preoperative Frankel grade C, D, or E. The exclusion criteria were as follows: preoperative Frankel grade A or B; cervical spinal cord compression of disc herniation, OPLL, or OLF; spinal space-occupying lesion, such as neurinoma and meningioma; cerebral ischemic lesion or cerebral infarction.
By retrieving the medical records from January 2001 to November 2015 in our hospital, 168 patients who met both the inclusion and exclusion criteria were retrospectively reviewed: 91 female and 77 male, with mean age of 39.8 ± 6.5 years (range 30–53 years). There were 17 cases of TDH, 52 cases of OPLL, 85 cases of OLF, and 14 cases of both OPLL and OLF. Sixty-six cases undertook posterior decompression with instrumental fusion and 102 cases undertook decompression alone.
2.2. Clinical and radiological evaluation
The Frankel grading was evaluated preoperatively, postoperatively, and at 3-month follow-up. The Frankel grade for evaluating neurological function was as follows: A: complete paralysis; B: sensory function only below the injury level; C: incomplete motor function below injury level; D: fair to good motor function below injury level; E: normal function. All the patients enrolled in this study were Frankel C, D, or E preoperatively, and patients who experienced Frankel grade decrease to A or B postoperatively and retained the neurological status at 3-month follow-up were regarded as ND.
According to the occurrence of postoperative ND, patients were divided into 2 groups: ND group and non-ND group. To investigate risk values for the occurrence of ND, 3 categorized factors were analyzed statistically: patient characteristics—preoperative data of age, sex, body mass index (BMI), bone mineral density (BMD), the duration of disease (from first symptoms to operation), the preoperative neurological function (Frankel grade), and diagnosis; surgical variables—surgery time, the amount of bleeding, mean arterial pressure (MAP), and instrumentation or not; radiographic parameters—the spinal canal occupancy ratio (at the narrowest section), location of the lesion (upper thoracic: T1-4, middle thoracic: T5-8, lower thoracic: T9-12), thoracic kyphosis (the Cobb angle between upper endplate of T4 and lower endplate of T12), and kyphosis correction (preoperative value − postoperative value).
2.3. Statistical analysis
Data were analyzed using Statistical Product and Service Solutions software (version 13; SPSS, Chicago, IL). Continuous variables were measured as mean ± standard deviation (SD), and categorical variables were expressed as frequency or percentages. An independent t test was used to analyze the difference of continuous variables between the 2 groups. A chi-square analysis and Fisher exact test were used to examine the differences among categorical variables. Variables with P values smaller than 0.05 in the univariate analyses, and also a number of variables selected by experts, were entered into a multivariate logistic regression model. For each variable, we computed the odds ratio (OR) with its 95% confidence interval (CI).
3. Results
Postoperative ND was developed in 23 of 168 patients (13.7%), and was enrolled as ND group. Six patients decreased from preoperative Frankel C to Frankel A postoperatively, 5 patients decreased from preoperative Frankel C to Frankel B postoperatively, 2 patients decreased from preoperative Frankel D to Frankel A postoperatively, 8 patients decreased from preoperative Frankel D to Frankel B postoperatively, and 2 patients decreased from preoperative Frankel E to Frankel B postoperatively.
There was no statistically significant difference between the 2 groups in age at operation, sex composition, BMI, and BMD. The preoperative diagnosis presented significant difference between the 2 groups, because OPLL + OLF was more common in ND group, whereas OLF was more common in non-ND group (Table 1).
Table 1.
Comparison of patient characteristics between ND and non-ND group.
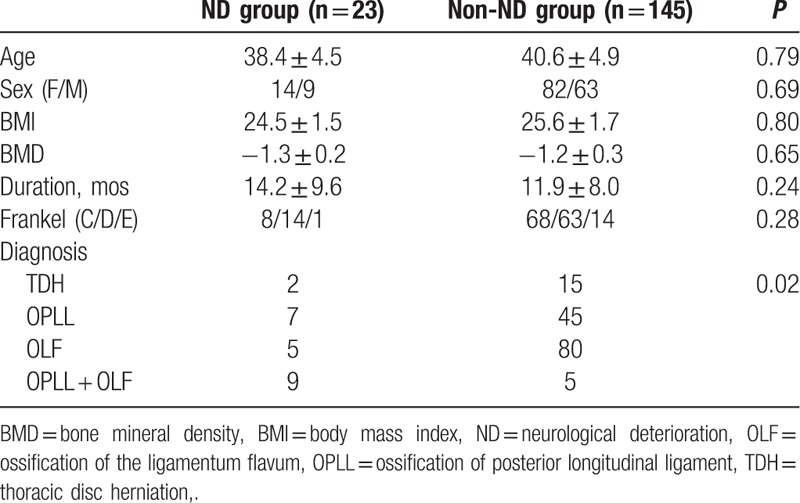
There was no statistically significant difference between the 2 groups in mean surgery time. The mean bleeding was much more in ND group than that in non-ND group, with statistically significant difference. The MAP was lower in ND group than that in non-ND group, with statistically significant difference. There was no statistically significant difference between the 2 groups in the incidence of intraoperative direct trauma and the number of patients that received instrumentation (Table 2).
Table 2.
Comparison of surgical variables between ND and non-ND group.
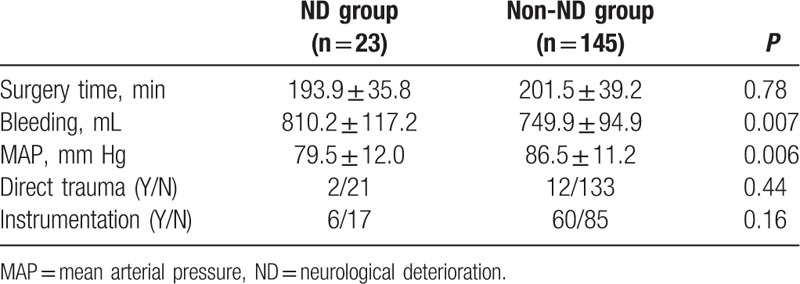
The mean spinal canal occupancy ratio was more severe in ND group than that in non-ND group, with statistically significant difference. There were no statistically significant difference between the 2 groups in stenosis location and preoperative thoracic kyphosis. The mean kyphosis correction was more significant in ND group than that in non-ND group (Table 3).
Table 3.
Comparison of radiographic parameters between ND and non-ND group.
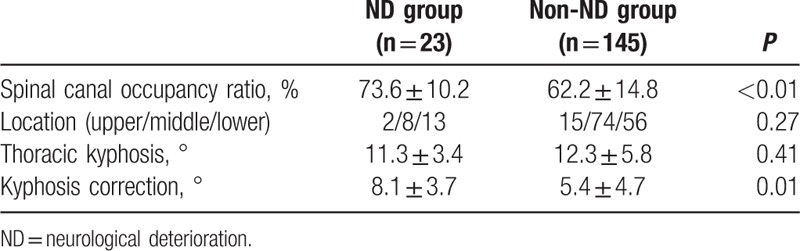
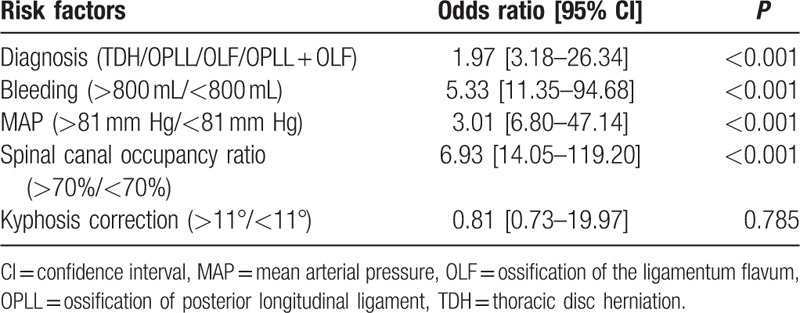
The following variables were entered into the multivariate model: age, sex, BMI, BMD, duration, preoperative Frankel grade, diagnosis, surgery time, bleeding, MAP, direct trauma, instrumentation, spinal canal occupancy ratio, stenosis location, thoracic kyphosis, and kyphosis correction. Multivariate logistic regression model revealed that OPLL + OLF, spinal canal occupancy ratio more than 70%, bleeding more than 800 mL, and MAP less than 81 mm Hg were independently associated with the postoperative ND (Table 4).
Table 4.
Risk factors for postoperative neurological deterioration, identified by multivariate analysis.
4. Discussion
In the current study, 13.7% of the patients (23/168) experienced postoperative ND, and we found that OPLL + OLF and spinal canal occupancy ratio more than 70% were significantly and independently associated with the occurrence of ND, and can be assessed before surgery. Intraoperative bleeding more than 800 mL and MAP less than 81 mm Hg were also associated with ND. These results were not confounded by other variables potentially affecting postoperative ND.
Both OPLL and OLF are individual conditions leading to TM; OPLL most commonly occurs in the upper thoracic area, whereas OLF most frequently occurs in the lower thoracic region.[20] TM caused by OPLL and OLF at the same vertebral level always leads to serious spinal cord lesions due to simultaneous ventral and dorsal compression, then pinch the spinal cord anteriorly and posteriorly, which is relatively rare, but has been reported in many literatures. Surgical intervention has generally been accepted to provide minimal neurological improvement, and be accompanied by the real possibility of postoperative ND. There are 2 possible explanations for the risk. First, the prognosis of decompression for thoracic stenosis is closely related to the preoperative severity of myelopathy, the spinal cord is always compressed severely due to the coexistence of anterior OPLL and posterior OLF. Some of the patients with spinal canal occupancy ratio more than 70% always present high signal in magnetic resonance imaging (MRI) (Fig. 1), indicating that the spinal cord is in the condition of ischemia, and postoperative ND may occur due to the ischemia reperfusion.[21] As Zhang et al[22] noted that patients with preoperative signal change ratio (SCR) ≥1.54 can experience poor postoperative recovery, Li et al[23] demonstrated that intramedullary signal change on T2WI and preoperative severity of myelopathy were confirmed and significantly correlated with the surgical outcome. Second, for patients suffering from both ventral and dorsal compression to the dural mater, the spinal cord is vulnerable, and mild traction or only slight vibration may cause severe paralysis at the procedure of extirpation of the OPLL through 1-stage posterior approach. (Fig. 2) Therefore, preoperative communication to make sure the patient is well-informed about the risk of postoperative ND is a necessity, especially for patients with OPLL + OLF and spinal canal occupancy ratio more than 70%. To decrease the incidence of postoperative ND, comprehensive clinical assessment is required to avoid delays in diagnosis and treatment, and early surgery for OPLL and OLF in the thoracic spine is strongly recommended.[24,25]
Figure 1.
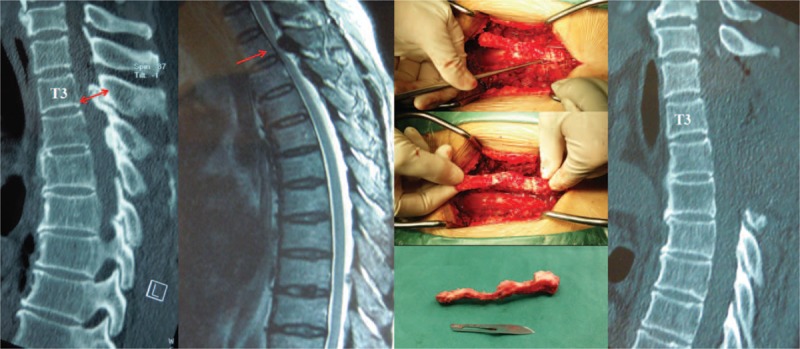
The patient presented thoracic OLF of T3 and T4 in CT scan with spinal canal occupancy ratio of 72%, and high signal in MRI. We performed laminectomy and resection of the OLF completely, which was confirmed by postoperative CT scan. Unfortunately, she experienced postoperative neurological deterioration from preoperative Frankel C to Frankel A. CT = computed tomography, MRI = magnetic resonance imaging, OLF = ossification of the ligamentum flavum.
Figure 2.
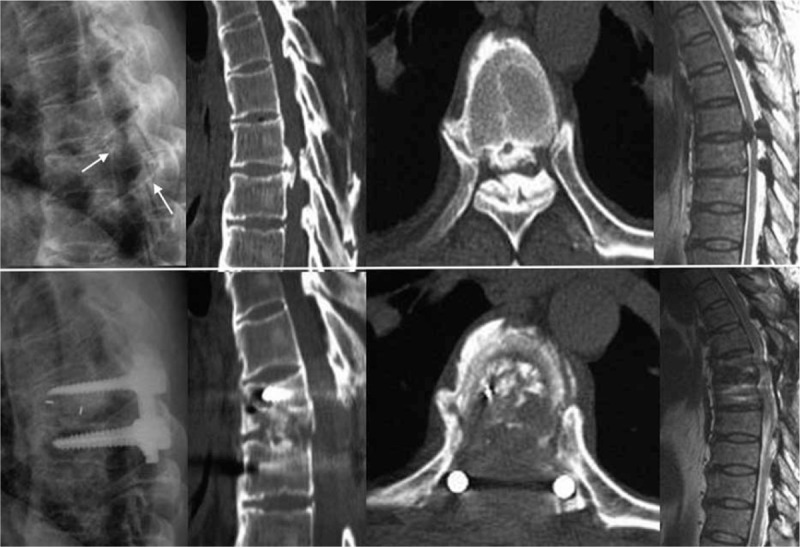
The patient suffered from both ventral and dorsal compression to the dural mater, and the spinal cord was vulnerable. We performed posterior decompression with transforaminal interbody fusion for thoracic myelopathy caused by combined ossification of the posterior longitudinal ligament and ligamentum flavum. Intraoperative mild traction or only slight vibration was prevented, but he experienced postoperative neurological deterioration from preoperative Frankel D to Frankel B.
Severe thoracic stenosis leads to increased pressure in adjacent neural structures, which in turn require an increase in MAP to maintain adequate spinal cord perfusion. Anatomically, the spinal cord is nourished by arterial supply, which follows a general path from vessels to parenchyma of white and gray matter throughout the spinal cord. In the thoracic spine, the segmental vessels come from the aorta or the subclavian artery and continue on as intercostal arteries. Colman et al[26] noted that the blood supply to the spinal cord might be interrupted iatrogenically during surgical intervention, including aortic aneurysm repair, tumor resection, and spinal deformity surgery. During descending aortic surgical repair, spinal cord damage due to ischemia is a dreadful complication that results in paraparesis/paraplegia in 6% to 40% of patients, and the Adamkiewicz artery should be carefully protected, as it lies on the left side at T7-L4 in about 80% of cases and usually supplies the nutrient arteries to the spinal cord at T9-L1.[27] When the spinal cord experiences an ischemic event, lactate release is the epiphenomenon of cellular impairment due to insufficient oxygen delivery, whereas lactate production occurs as neurons switch to anaerobic metabolism.[28] To avoid injury to the blood supply to the spinal cord, the microcirculation around the intervertebral foramen should be protected when ligating the vertebral segmental vessels.
A large amount of intraoperative bleeding results in insufficient venous return, then the decrease of MAP and spinal cord perfusion pressure is inevitable. Postoperative paralysis derived from spinal cord ischemia happens sometimes, the mechanism of ND may involve global hypoperfusion (aortic cross-clamping or systemic hypotension), or selective ischemia from ligation of dominant segmental vessels. One possible reason for the neurologic deterioration after thoracic decompression was thought to relate to inadequate cord perfusion pressures. Zuckerman et al reported a case series of 3 patients with severe TDH; they found that the mean intraoperative arterial pressures (MAPs) at the time of deterioration were noted to be approximately 65%, 92%, and 60% those of baseline values, respectively. As far as they are concerned, to prevent such complication, new guidelines of preoperative optimization of volume status and aggressive maintenance of MAPs more than 110% of preoperative values in the procedure of spinal cord decompression were recommended in the surgical management of TDH.[29] It is a contradiction on the proper intraoperative MAP, as low blood pressure leads to spinal cord ischemia, whereas high pressure may cause excessive bleeding, then decrease the venous return. Therefore, the effective measures to prevent cord ischemia are to improve surgical technique, shorten operation time, and pay more attention to hemostasis.
The intraoperative instability produced by wide laminectomy and the increased kyphotic spinal deformity after laminectomy are supposed to contribute to the potential for neurologic deterioration. Yamazaki et al[12] reported that 3 of 16 patients who underwent posterior decompression had postoperative ND, and 2 of the 3 patients recovered after the performance of revision surgery with posterior instrumentation. Zhang et al[30] reported a series of 11 patients with TM, and confirmed that a considerable degree of neurological recovery was observed after posterior decompression and kyphosis correction for patients whose spinal cords are severely impinged by OLF and OPLL at the same level. If the correction angle of kyphosis is less than 10°, it is possible that the spinal cord is not sufficiently detached, achieving a larger correction angle is crucial to elimination of paralysis.[16] In the current study, the kyphosis correction presented significant difference between the 2 groups in univariate analyses (Fig. 3). However, this univariate association did not persist after risk adjustment, as kyphosis correction was no longer independently associated with neurologic deterioration in the multivariable analysis. The possible reason may be the fact that the median value was selected as the classification point of kyphosis correction in the procedure of logistic regression analysis, which may result in systematic error. Moreover, the relatively small number of cases could also cause selective bias. Use of instrumentation could stabilize the thoracic spine and was able to prevent the progression or even to correct thoracic kyphosis, and thereby enhance spinal cord decompression. To minimize the risk of spinal instability or kyphotic deformity after thoracic decompression alone, we strongly recommend laminectomy combined with instrumentation for patients with thoracic stenosis, especially for patients who require wide decompression.
Figure 3.
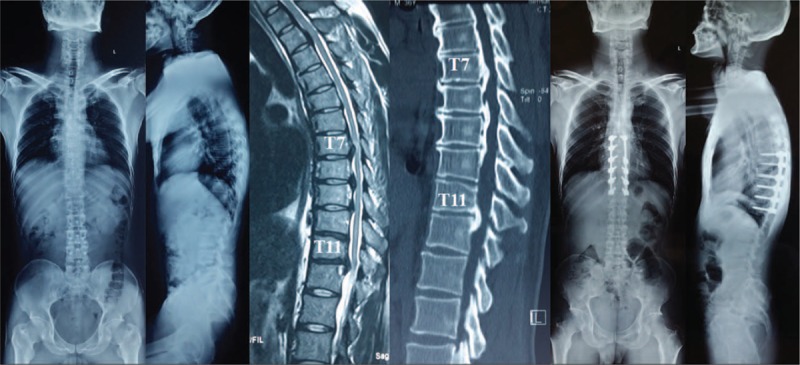
The patient demonstrated thoracic OPLL at T7-12, we adopted laminectomy combined with instrumentation of all decompressive segments (T7-12), the thoracic kyphosis decreased from preoperative 32° to 14° postoperatively. The patient do not experience neurological function deterioration postoperatively. OPLL = ossification of posterior longitudinal ligament.
There were several potential limitations in this study. First, only Chinese Han individuals were included in this study and ethnic variation was not covered. Second, the number of patients was relatively small, and the study may be under powered to detect the significance of some risk factors. Even with these issues in this study, we find that those with OPLL + OLF, spinal canal occupancy ratio more than 70%, intraoperative bleeding more than 800 mL, and MAP less than 81 mm Hg were risk factors for the occurrence of postoperative neurologic deterioration. Our data are of great value in decision-making and surgical planning for both spinal surgeon and thoracic stenosis patients.
Footnotes
Abbreviations: BMD = bone mineral density, BMI = body mass index, MAP = mean arterial pressure, ND = neurological deterioration, OLF = ossification of the ligamentum flavum, OPLL = ossification of posterior longitudinal ligament, TDH = thoracic disc herniation.
HW, LM, RX, DY, TW, and YW are equal contributors.
Funding disclosures: No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Ethical statement: The study was approved by Ethics Committee of The Third Hospital of HeBei Medical University, all patients provided written informed consent to participate in this study before the enrollment.
The authors report no conflicts of interest.
References
- [1].Hussain M, Ahmed Raja R, Makhdoom A. Ossification and hypertrophy of ligamentum flavum at thoracic spine. J Ayub Med Coll Abbottabad 2014;26:294–6. [PubMed] [Google Scholar]
- [2].Amato V, Giannachi L, Irace C, et al. Thoracic spinal stenosis and myelopathy: report of two rare cases and review of the literature. J Neurosurg Sci 2012;56:373–8. [PubMed] [Google Scholar]
- [3].Oppenlander ME, Clark JC, Kalyvas J, et al. Surgical management and clinical outcomes of multiple-level symptomatic herniated thoracic discs. J Neurosurg Spine 2013;19:774–83. [DOI] [PubMed] [Google Scholar]
- [4].Li M, Wang Z, Du J, et al. Thoracic myelopathy caused by ossification of the ligamentum flavum: a retrospective study in Chinese patients. J Spinal Disord Tech 2013;26:E35–40. [DOI] [PubMed] [Google Scholar]
- [5].Cornips EM, Janssen ML, Beuls EA. Thoracic disc herniation and acute myelopathy: clinical presentation, neuroimaging findings, surgical considerations, and outcome. J Neurosurg Spine 2011;14:520–8. [DOI] [PubMed] [Google Scholar]
- [6].Yamasaki R, Okuda S, Maeno T, et al. Surgical outcomes of posterior thoracic interbody fusion for thoracic disc herniations. Eur Spine J 2013;22:2496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Arnold PM, Johnson PL, Anderson KK. Surgical management of multiple thoracic disc herniations via a transfacet approach: a report of 15 cases. J Neurosurg Spine 2011;15:76–81. [DOI] [PubMed] [Google Scholar]
- [8].Mori K, Imai S, Kasahara T, et al. Prevalence, distribution, and morphology of thoracic ossification of the posterior longitudinal ligament in Japanese: results of CT-based cross-sectional study. Spine (Phila Pa 1976) 2014;39:394–9. [DOI] [PubMed] [Google Scholar]
- [9].Koda M, Furuya T, Okawa A, et al. Mid- to long-term outcomes of posterior decompression with instrumented fusion for thoracic ossification of the posterior longitudinal ligament. J Clin Neurosci 2016;27:87–90. [DOI] [PubMed] [Google Scholar]
- [10].Yin H, Shi H. Concurrent ossification of posterior longitudinal ligament and ossification of ligamentum flavum in the thoracic spine demonstrated by SPECT/CT imaging. Clin Nucl Med 2015;40:228–30. [DOI] [PubMed] [Google Scholar]
- [11].Feng FB, Sun CG, Chen ZQ. Progress on clinical characteristics and identification of location of thoracic ossification of the ligamentum flavum. Orthop Surg 2015;7:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yamazaki M, Okawa A, Fujiyoshi T, et al. Posterior decompression with instrumented fusion for thoracic myelopathy caused by ossification of the posterior longitudinal ligament. Eur Spine J 2010;19:691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang YQ, Liu XG, Jiang L, et al. Intraoperative ultrasonography in “cave-in” 360° circumferential decompression for thoracic spinal stenosis. Chin Med J 2011;124:3879–85. [PubMed] [Google Scholar]
- [14].Machino M, Yukawa Y, Ito K, et al. A new thoracic reconstruction technique “transforaminal thoracic interbody fusion”: a preliminary report of clinical outcomes. Spine (Phila Pa 1976) 2010;35:E1000–5. [DOI] [PubMed] [Google Scholar]
- [15].Sun X, Sun C, Liu X, et al. The frequency and treatment of dural tears and cerebrospinal fluid leakage in 266 patients with thoracic myelopathy caused by ossification of the ligamentum flavum. Spine (Phila Pa 1976) 2012;37:E702–7. [DOI] [PubMed] [Google Scholar]
- [16].Ito Z, Matsuyama Y, Ando M, et al. Postoperative paralysis from thoracic ossification of posterior longitudinal ligament (OPLL) surgery-risk factor of neurologic injury: nationwide multi-institution survey. Spine (Phila Pa 1976) 2016;41:E1159–63. [DOI] [PubMed] [Google Scholar]
- [17].Matsumoto M, Toyama Y, Chikuda H, et al. Outcomes of fusion surgery for ossification of the posterior longitudinal ligament of the thoracic spine: a multicenter retrospective survey: clinical article. J Neurosurg Spine 2011;15:380–5. [DOI] [PubMed] [Google Scholar]
- [18].Hou X, Sun C, Liu X, et al. Clinical features of thoracic spinal stenosis-associated myelopathy: a retrospective analysis of 427 cases. Clin Spine Surg 2016;29:86–9. [DOI] [PubMed] [Google Scholar]
- [19].Yu S, Wu D, Li F, et al. Surgical results and prognostic factors for thoracic myelopathy caused by ossification of ligamentum flavum: posterior surgery by laminectomy. Acta Neurochir 2013;155:1169–77. [DOI] [PubMed] [Google Scholar]
- [20].Yamazaki M, Okawa A, Koda M, et al. Transient paraparesis after laminectomy for thoracic myelopathy due to ossification of the posterior longitudinal ligament: a case report. Spine (Phila Pa 1976) 2005;30:E343–6. [DOI] [PubMed] [Google Scholar]
- [21].Hitchon PW, Abode-Iyamah K, Dahdaleh NS, et al. Risk factors and outcomes in thoracic stenosis with myelopathy: a single center experience. Clin Neurol Neurosurg 2016;147:84–9. [DOI] [PubMed] [Google Scholar]
- [22].Zhang J, Wang L, Li J, et al. Predictors of surgical outcome in thoracic ossification of the ligamentum flavum: focusing on the quantitative signal intensity. Sci Rep 2016;V6N:23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li Z, Ren D, Zhao Y, et al. Clinical characteristics and surgical outcome of thoracic myelopathy caused by ossification of the ligamentum flavum: a retrospective analysis of 85 cases. Spinal Cord 2016;54:188–96. [DOI] [PubMed] [Google Scholar]
- [24].Onishi E, Sano H, Matsushita M. Surgical treatment for thoracic myelopathy due to simultaneous ossification of the posterior longitudinal ligament and ligamentum flavum at the same level. Clin Spine Surg 2016;29:E389–95. [DOI] [PubMed] [Google Scholar]
- [25].Cinà CS, Laganà A, Bruin G, et al. Thoracoabdominal aortic aneurysm repair: a prospective cohort study of 121 cases. Ann Vasc Surg 2002;16:631–8. [DOI] [PubMed] [Google Scholar]
- [26].Colman MW, Hornicek FJ, Schwab JH. Spinal cord blood supply and its surgical implications. J Am Acad Orthop Surg 2015;23:581–91. [DOI] [PubMed] [Google Scholar]
- [27].Weigang E, Hartert M, Siegenthaler MP, et al. Perioperative management to improve neurological outcome in thoracic or thoracoabdominal aortic stent-grafting. Ann Thorac Surg 2006;82:1679–87. [DOI] [PubMed] [Google Scholar]
- [28].Giustiniano E, Ruggieri N. Is intrathecal lactate concentration monitoring helpful for postoperative paraplegia after descending aorta surgery? J Clin Anesth 2014;26:506–8. [DOI] [PubMed] [Google Scholar]
- [29].Zuckerman SL, Forbes JA, Mistry AM, et al. Electrophysiologic deterioration in surgery for thoracic disc herniation: impact of mean arterial pressures on surgical outcome. Eur Spine J 2014;23:2279–90. [DOI] [PubMed] [Google Scholar]
- [30].Zhang HQ, Chen LQ, Liu SH, et al. Posterior decompression with kyphosis correction for thoracic myelopathy due to ossification of the ligamentum flavum and ossification of the posterior longitudinal ligament at the same level. J Neurosurg Spine 2010;13:116–22. [DOI] [PubMed] [Google Scholar]


