Supplemental Digital Content is available in the text
Keywords: β-catenin, colorectal cancer, meta-analysis, prognosis
Abstract
Background:
The differential subcellular localizations of β-catenin (including membrane, cytoplasm, and nucleus) play different roles in the progression of colorectal cancer (CRC). However, the correlation between each subcellular localization of β-catenin and the prognosis of CRC patients remains undetermined.
Methods:
Systematic strategies were applied to search for eligible published studies in the PubMed, Embase, and Web of Science databases. The correlation between each subcellular localizations of β-catenin expression and patients’ clinicopathological features or prognosis was analyzed.
Results:
Finally, this meta-analysis, including 6238 cases from 34 studies, revealed that β-catenin overexpression in the nucleus (HR: 1.50[95% CI: 1.08–2.10]) or reduced expression of β-catenin in the membrane (HR: 1.33[95% CI: 1.15–1.54]) significantly correlated with lower 5-year overall survival (OS). Conversely, overexpression of β-catenin in the cytoplasm (HR: 1.00[95% CI: 0.85–1.18]) did not show significant association with 5-year OS.
Conclusion:
This study suggested that β-catenin overexpression in the nucleus or reduced expression in the membrane, but not its overexpression in cytoplasm, could serve as a valuable prognostic predictor for CRC. However, additional large and well-designed prospective studies are required to verify our results.
1. Introduction
Colorectal cancer (CRC) is the fourth most frequent human malignancies worldwide.[1] Although the mortality of colorectal cancer has been decreased by almost 35%through earlier screening and better treatment modalities,[2] CRC remains the second highest cause of cancer-related deaths. The 5-year survival rate for CRC exceeds 50%, but it is highly variable depending on the stage of the disease.[3] The molecular pathways involved in the tumorigenesis of CRC are complicated and heterogeneous. Fearon and Vogelstein[4] have reported a series of genetic alterations, including the activation of certain oncogenes and the inactivation of particular tumor suppressor genes, which are responsible for colorectal tumorigenesis.
β-catenin localizes in the membrane, cytoplasm, or nucleus and exerts different functions related to cell differentiation and proliferation. Membranous β-catenin was identified as a protein associated with E-cadherin in maintaining cell-to-cell interactions. Interestingly, the membranous expression of β-catenin exerts a restrictive effect on tumor cell movement and growth. Loss of β-catenin expression on the cell surface increases cell motility, growth, and transformation and thus promotes tumorigenesis.[5] Cytoplasmic β-catenin, which can translocate to the nucleus and activate the downstream target genes relevant to cell proliferation, migration, invasion, cell cycle progression and metastasis, serves as a downstream transcriptional transactivator through int/Wingless family (Wnt) transduction signaling.[6] Pre-existing intracellularβ-catenin directly connects scaffolding proteins Axin, adenomatous polyposis coli (APC), serine/threonine kinases, Casein kinase 1 alpha (CK1α), and glycogen synthase kinase 3β (GSK3β) to form a destruction complex without Wnt ligands.[7] However, if the APC is inactivated, Axinor β-catenin mutates; therefore, free β-catenin cannot be degraded and accumulates in the cytosol. Subsequently, β-catenin translocates to nucleus as a co-factor for T-cell factor (TCF) family of transcription factors to activate the downstream Wnttarget genes.[6] This aberrant Wnt/β-catenin-TCF signaling plays a key role in the development and progression of colorectal cancer. The Wnt/β-catenin pathway has been recognized to play a critical role in maintaining the stem cell features by targeting genes, such as Lgr5, Ascl2, and Sox9.[8–10] The hyperactivation of Wnt/β-catenin signaling enhances the invasive and metastatic potential of CRC cells.[11] Knockdown of β-catenin in CRC cells dampens cell proliferation and invasion.[12,13] Nuclear β-catenin expression detected by immunohistochemistry has been reported to be associated with high tumor burden and worse survival outcomes of CRC.[14–17] However, other studies did not present this association.[18,19] The variable and contradictory results were also observed regarding the correlation between the reduced membranous β-catenin expression and the prognosis in patients with CRC.[17,19–21] A previous analysis suggested that β-catenin overexpression in nucleus, rather than in cytoplasm, was associated with poor prognosis of CRC.[22] In this paper, we collected and added updated articles regarding β-catenin expression in CRC to reanalyze the prognostic value of β-cateninin cytoplasm and/or nucleus in CRC patients. More importantly, we also extracted relevant data to analyze the prognostic significance of reduced membranous β-catenin expression in patients with CRC. The pooled results suggested that β-catenin overexpression in nucleus or reduced β-catenin expression in the membrane was associated with worse prognosis of CRC.
2. Methods
2.1. Literature selection
We systematically searched the PubMed, Embase, and Web of Science databases to identifying pertinent articles published prior to November 2015. The terms used in the search were as follows with all possible combinations: “β-catenin, Beta-catenin, or CTNNB1,” “WNT/β-catenin signal pathway,” “prognostic, prognosis, or survival,” and “colorectal neoplasms, colorectal cancer, colorectal carcinoma, colorectal tumor.” The reference lists associated with all the studies were also inspected for additional available studies.
2.2. Inclusion and exclusion criteria
To obtain high-quality literatures to meet the high standards for this meta-analysis, studies must fulfill the following criteria: (1) patients included have definite pathological diagnosis of colorectal carcinoma; (2) β-catenin expression was evaluated by immunohistochemistry in the CRC tissue; (3) evaluation of the correlation between β-catenin expression and CRC pathological features and overall survival (OS) or disease-free survival (DFS) were done; and (4) studies included were published in English. In addition, the following articles were excluded: (1) articles published in a non-English language; (2) articles where the relevant data provided could not be extracted; (3) articles where no relevant data was provided for necessary analysis; (4) duplicated articles; (5) cut-off scoring of positive immunoreactivity of β-catenin expression in nucleus was higher than 30%; and (6) the quality of included study was too low (score < 4). The studies were evaluated and selected by 2 reviewers (XY and JS), and the disagreements were settled by a third reviewer (DL). Then, the eligible articles were included for further data processing.
2.3. Data extraction and quality evaluation
For each eligible study, the following information were extracted by 2 reviewers (SZ and XW): (1) first author's name and country and the publication year; (2) number of patients; (3) age and gender of patients; (4) characteristics of the disease; (5) accumulated percentage of the different subcellular locations of β-catenin expression; (6) antibody source and dilution rate; and (7) definition of β-catenin positive. The quality of the eligible studies was evaluated using the Newcastle–Ottawa scale (NOS), which was described previously.[22,23]
2.4. Statistical analysis
The STATA (version 12.0, Stata Corp. College Station, TX) was utilized for this meta-analysis. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to evaluate the association between the different subcellular localizations of β-catenin expression and the prognosis or clinicopathological parameters. We pooled the statistical variables directly if they were depicted in articles. Otherwise, Kaplan–Meier curves were read by Engauge Digitizer to obtain the necessary data. The chi-square based Q statistical test was used to evaluate the heterogeneity among the outcomes of enrolled studies.[24] In addition, I2 statistic represented the proportion of total variation caused by heterogeneity, and I2 >50% meant significant heterogeneity. According to the results of the Q statistical test, P > 0.10 indicated the outcomes of analysis among the results with low heterogeneity and a fixed-effects model was selected. In addition, the random-effects model was used for studies with P < 0.05. Egger's test and Begg's test were used to examine the potential risk of publication bias. Sensitivity analysis was performed following sequential omission of individual studies to evaluate the stability of the results.
3. Results
3.1. Studies description
A total of 445 eligible studies for inclusion based on the title was collected from different sources (Fig. 1). Then, 411 of them were excluded on the basis of inclusion and exclusion criteria. A total of 6216 cases, based on 29 studies that revealed a relationship between nuclear β-catenin expression and pathological features or DFS/5-year OS, were investigated and their major clinical characteristics are summarized in Table 1.[14,15,18–21,25–47] In addition, 9 studies showed a relationship between cytoplasmic β-catenin expression and pathological features or OS[17,19–21,25,33,34,37,43] (Table 2). Thirteen studies were selected for analysis of the prognostic value of reduced β-catenin expression in the membrane of CRC[17,19–21,26,31,34,35,38,43,48–50] (Table 3). There were also 3 studies included to examine the predictive role of nuclear β-catenin expression in the invasive front of tumor[17,41,51] (Table 4).
Figure 1.
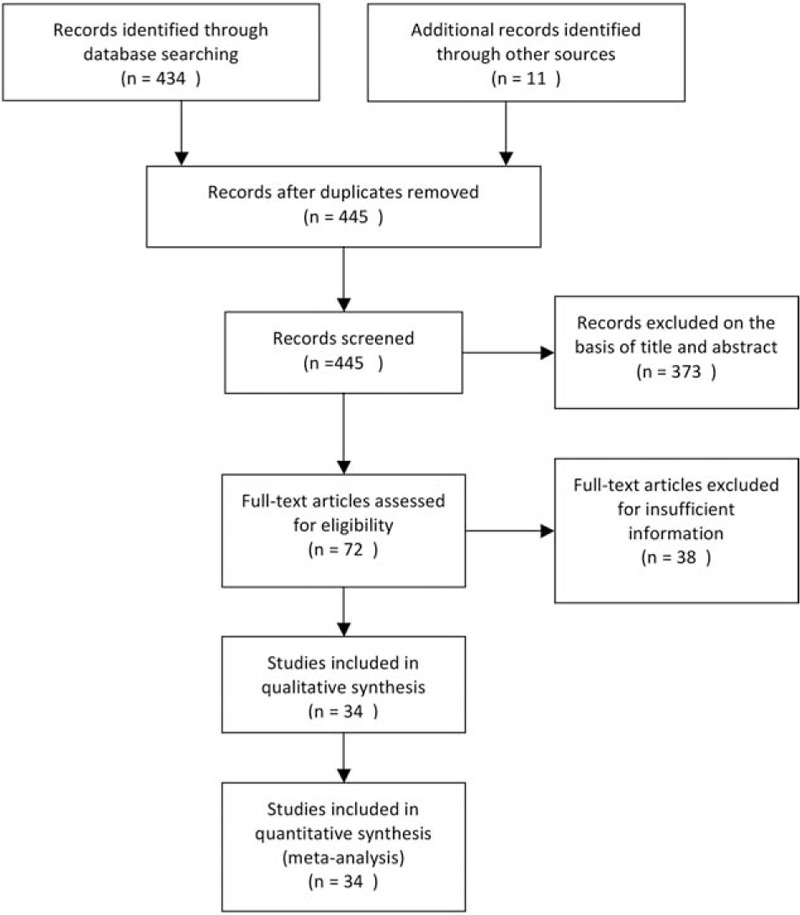
Flow diagram of study selection procedure.
Table 1.
Characteristics of the studies of the nuclear β-catenin expression.
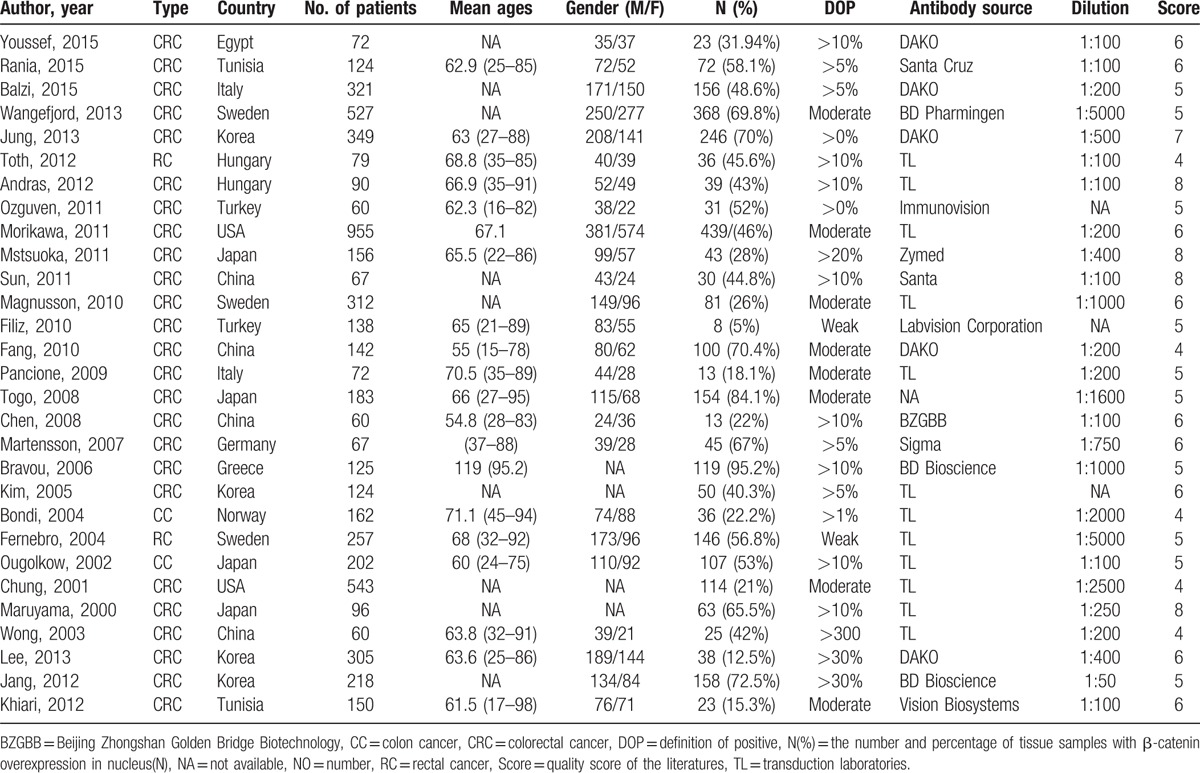
Table 2.
Characteristics of the studies of the β-catenin expression in cytoplasm.
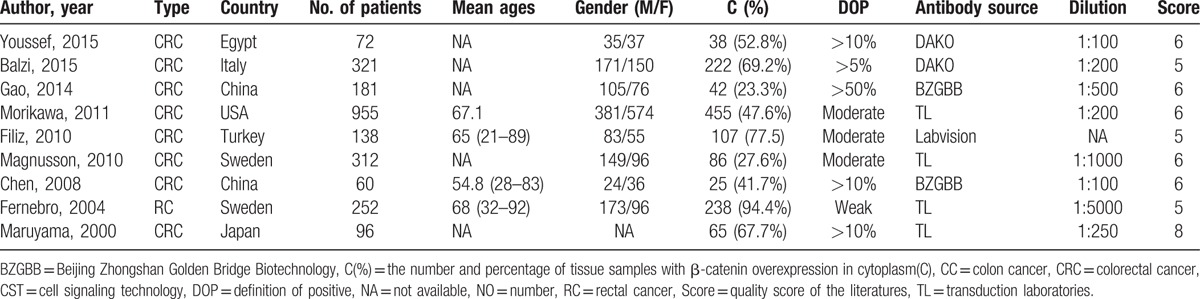
Table 3.
Characteristics of the studies of the reduced β-catenin expression in the membrane.
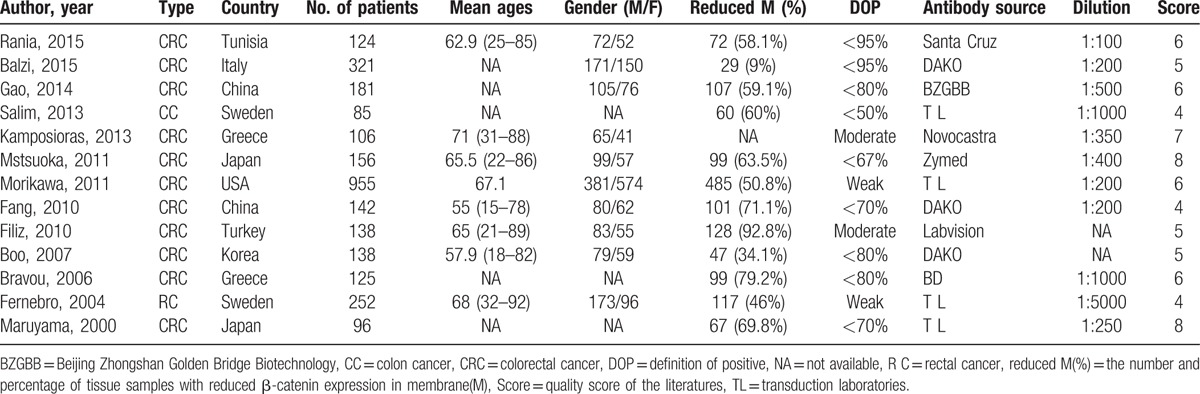
Table 4.
Characteristics of the studies of the nuclear β-catenin expression in the invasive front of cancer.
3.2. Quality of eligible studies
The Newcastle–Ottawa Scale (NOS) was conducted to assess the methodological quality of the studies. As described previously,[23] a score of 9 represented the highest quality and a score of 5 or more was considered as high quality. Twenty-seven studies included in our meta-analysis were of high quality with scores of 5 or more after quality assessment.
3.3. Prognostic value of β-catenin expression in colorectal cancer
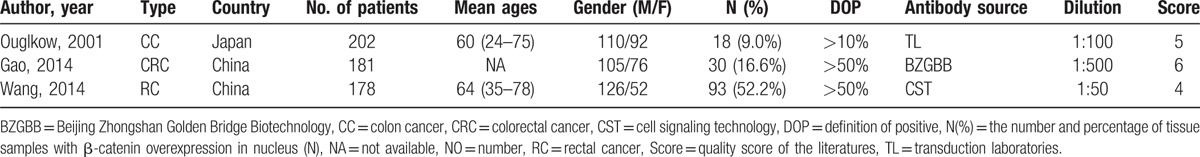
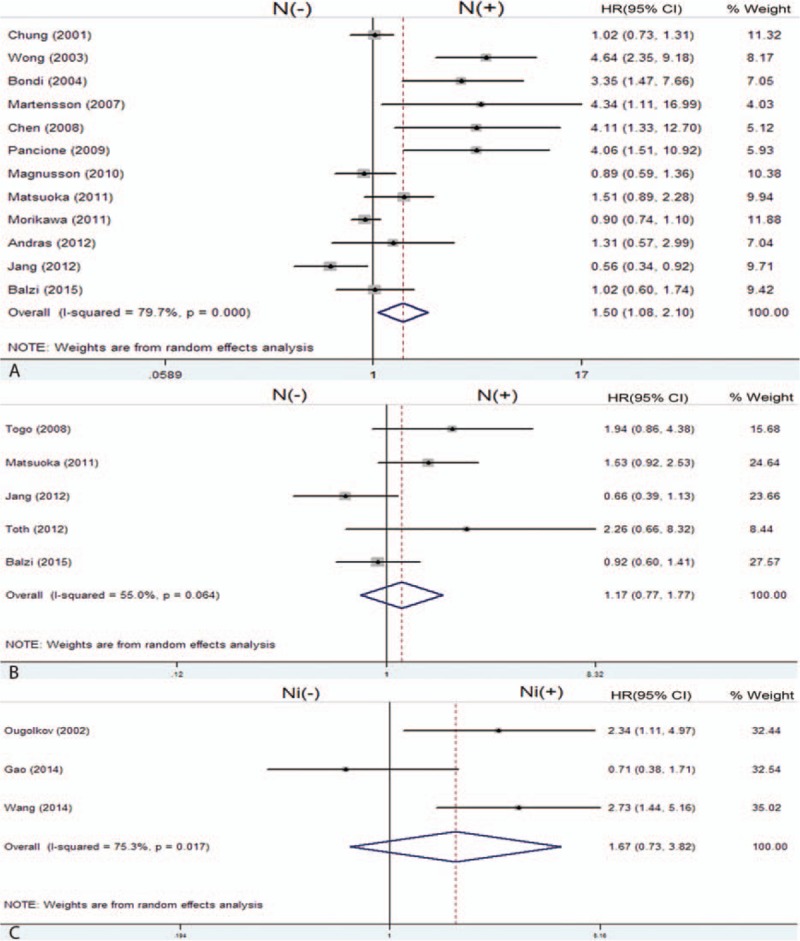
Twelve enrolled studies provided the HRs and 95%CI directly or indirectly about the correlation between nuclear β-catenin overexpression and 5-year OS. The pooled HR of the β-catenin overexpression in nucleus with OS was 1.50 (95% CI: 1.08–2.10; Z = 2.40; P = 0.016) (Fig. 2A), but heterogeneity did exist (I2 = 79.7% P = 0.000). The association of β-catenin overexpression in nucleus with DFS was analyzed based on 5 studies; the pooled HR was 1.17 (95% CI: 0.77–1.77; Z = 0.73; P = 0.463) (Fig. 2B). In addition, 3 studies assessed the association of nuclear β-catenin overexpression in the invasive front of tumor with OS; the pooled HR was 1.67 (95% CI: 0.73–3.82; Z = 1.22; P = 0.221) (Fig. 2C). Then, we evaluated the correlation between β-catenin overexpression in the cytoplasm and 5-year OS based on 5 studies, and the pooled HR was 1.00 (95% CI: 0.85–1.18; Z = 0.01; P = 0.991) (Fig. 3A). The pooled HR of the association of reduced membranous β-catenin expression and OS was 1.33 (95% CI: 1.15–1.54; Z = 3.81; P = 0.0001) based on 9 studies (Fig. 3B). The above results suggested that β-catenin overexpression in the nucleus was associated with lower OS, but not with DFS. In addition, reduced β-catenin expression in the membrane was correlated with a worse prognosis of CRC. However, β-catenin overexpressed in the cytoplasm or nuclear β-catenin overexpressed in the invasive front of tumor had no relationship with prognosis of CRC.
Figure 2.
Forest plot of hazard ratio for the association of β-catenin expression with survival. (N (−): β-catenin negative expression in the nucleus; N (+): β-catenin overexpression in the nucleus). (A) HRs with corresponding 95% CIs of the β-catenin expression in nucleus with 5-year OS; (B) HRs with corresponding 95% CIs of the β-catenin expression in nucleus with DFS; (C) HRs with corresponding 95% CIs of the nuclear β-catenin expression in the invasive front of tumor with 5-year OS. CI = confidence interval, DFS = disease-free survival, HR = hazard ratio, OS = overall survival.
Figure 3.
Forest plot of hazard ratio for the association of β-catenin expression in cytoplasm (A) and membrane (B) with 5-year OS. (C (−): β-catenin negative expression in the cytoplasm; C (+): β-catenin overexpression in the cytoplasm; M (−): reduced β-catenin expression in the membrane; M (+): β-catenin expression in the membrane). OS = overall survival.
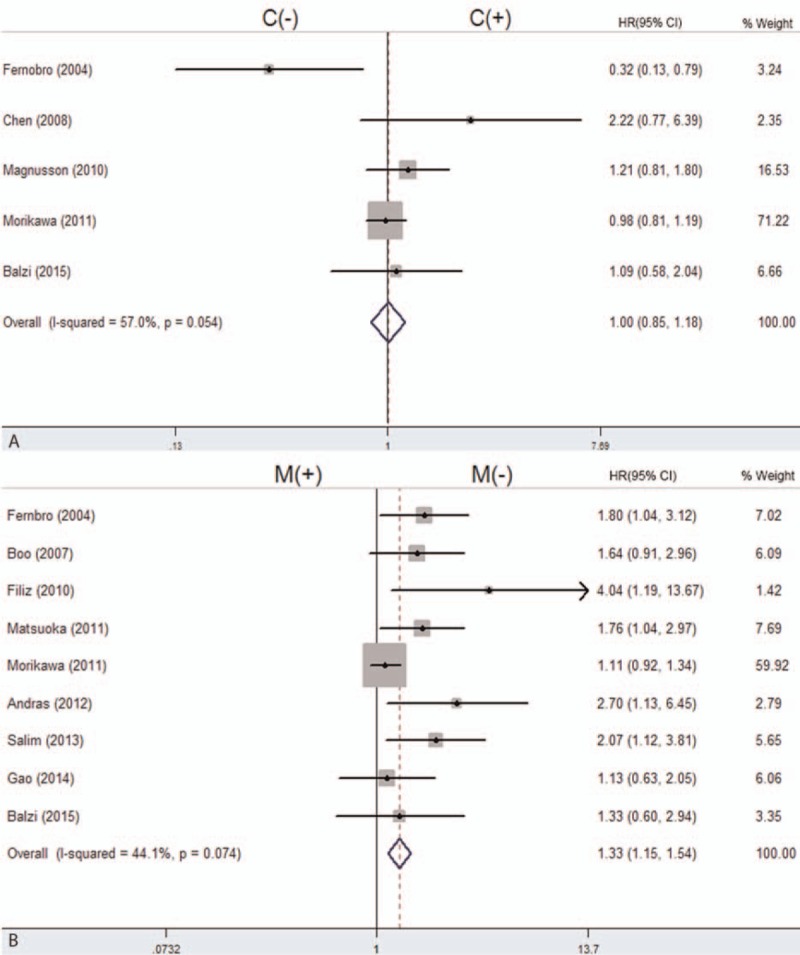
Subgroup analysis was performed by the publication year, study location, source of primary antibodies, and definition of β-catenin positive to explain the heterogeneity of the studies about the association of nuclear β-catenin with 5-year OS (Fig. 4). However, as indicated by subgroup analysis, a significant relationship between nuclear β-catenin overexpression and 5-year OS was shown only by an antibody sourced from the Transduction Laboratory (HR = 1.61; 95%CI: 1.04–2.47; I2 = 83.4%, P = 0.000). Other factors including study location and number of patients altered the significant prognostic impact of nuclear β-catenin expression. We could not identify the source of heterogeneity in this study by subgroup analysis; however, we inferred that the heterogeneity may have been caused by the different clinical features of patients or other factors we could not assess.
Figure 4.
Forest plot of hazard ratio for the association of β-catenin expression in the nucleus with OS by subgroup analysis by study location (A), primary antibody sources (B), and evaluation standards (C). (N (−): β-catenin negative expression in the nucleus; N (+): β-catenin overexpression in the nucleus). OS = overall survival.
3.4. Correlations between β-catenin expression and clinicopathological factors
Next, we evaluated the correlations of the different subcellular localizations of β-catenin expression with clinicopathological characteristics in patients with CRC. As shown in Table 5, 13 studies were included for evaluation of the correlation between β-catenin expression in the nucleus and tumor differentiation grade. The pooled OR was 0.72 (95% CI: 0.54–0.96, Z = 2.24, P = 0.002) without heterogeneity (I2 34.8% P = 0.104) (Fig. 5A), which meansβ-catenin expression in the nucleus inversely correlated with differentiation grade. Patients with T3 and T4 CRC had significant nuclear β-catenin expression compared to patients with T1 and T2 CRC (OR = 1.76, 95%CI: 1.16–2.68, Z = 2.68, P = 0.007) and without heterogeneity (I2 0.0% P = 0.771) (Fig. 5B). However, other clinicopathological parameters, such as lymph nodal status (OR = 0.91, 95%CI: 0.47–1.77), TNM stage (OR = 1.18, 95%CI: 0.87–1.62), lymphatic invasion (OR = 0.88, 95%CI: 0.62–1.27), venous invasion (OR = 1.19, 95%CI: 0.75–1.89), and tumor site (OR = 1.15, 95%CI: 0.79–1.69) had no relationship with β-catenin expression in the nucleus (Table 5). We also observed the significant association of β-catenin overexpression in the cytoplasm with tumor site, TNM stages, and differentiation grade (Table 5). The pooled ORs were 1.85 (95% CI: 1.42–2.44, Z = 4.49, P = 0.000), 1.28 (95% CI: 1.02–1.59, Z = 2.18, P = 0.029), and 0.59 (95% CI: 0.41–0.86, Z = 2.16, P = 0.030), respectively. All combined results were without heterogeneity (I20% P = 0.751), (I2 11.0% P = 0.345) and (I2 33.8% P = 0.196), respectively. Furthermore, reduced β-catenin expression in membrane was significantly associated with differentiation grade and tumor stage, the pooled OR were 2.28 (95% CI: 1.06–4.82, Z = 2.16, P = 0.030) and 1.82 (95% CI: 1.05–4.82, Z = 2.12, P = 0.034), respectively (Table 5), but they were not associated with lymph nodal status (OR = 1.16, 95%CI: 0.49–2.74, Z = 0.33, P = 0.739).
Table 5.
Meta-analysis of β-catenin in the nucleus = cytoplasm and membrane.
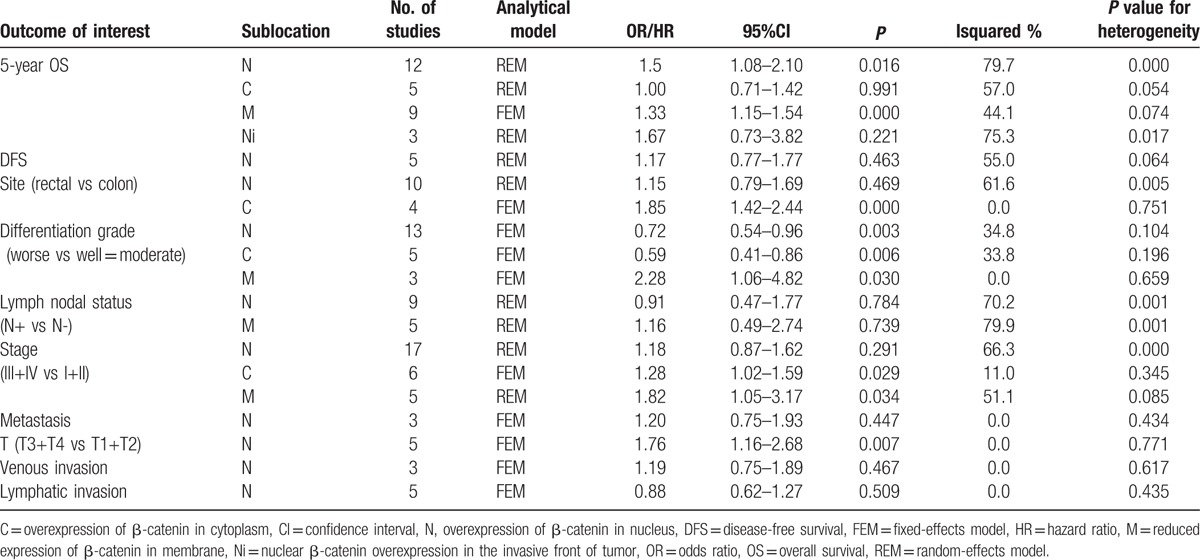
Figure 5.
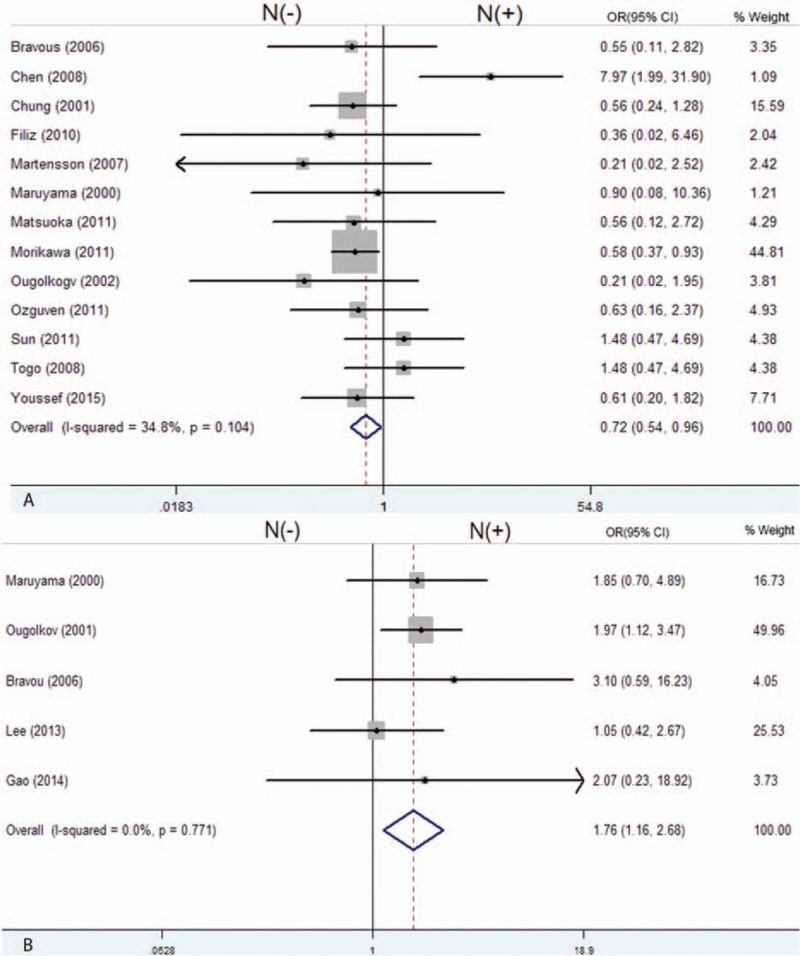
Forest plot of odd ratios for the association of nuclear β-catenin overexpression with differentiation grade (A) and depth of invasion (B). (N (−): β-catenin negative expression in the nucleus; N (+): β-catenin overexpression in the nucleus).
3.5. Publication bias
We assessed the publication bias by constructing a funnel plot (S1A Fig, S1B Fig, S2 Fig) as more than 10 studies were included for meta-analysis. Egger's test indicated that publication bias existed when we evaluated the impact of β-catenin in the nucleus with 5-year OS, although Begg's Test showed no significant publications bias (P = 0.064). However, with Egger's test, there is inadequate power of testing when the number of included studies is fewer than 20.[52] We performed sensitivity analysis and demonstrated that the pooled HRs were not significantly influenced by omitting any single study (S1C Fig).
4. Discussion
Colorectal carcinogenesis is a complicated multistage process including multiple genetic alterations. The aberrant Wnt/β-catenin pathway has been proven to be involved in progression of CRC. Approximately 60% to 80% of CRCs development is due to the aberrant activation of the Wnt/β-catenin signaling pathway.[53] Wnt signaling play a central role in both early colorectal tumorigenesis and later progression.[54] Activated Wnt/β-catenin signaling promotes EMT, migration, and invasion of CRC cells by targeting miR-150, BOP1, CKS2, and NFL3 genes, which induced a mesenchymal-like morphological change and experimental metastasis of CRC cells.[55,56] High Wnt/β-catenin signaling is also critical in the maintenance of the stem cell niche, which leads to tumor progression and metastasis.[10] β-catenin accumulation in the nucleus or cytoplasm was identified as a poor prognosis marker and nuclear β-catenin was implicated as a potential target for cancer therapy.[57,58] However, there were also contradictory results suggesting that β-catenin expression in the nucleus was associated with noninvasive tumors and a more favorable outcome.[59,60] Therefore, the prognostic significance of β-catenin expression in patients with CRC remains controversial and a systematic analysis is required to achieve a reliable conclusion. In this meta-analysis, we explored the prognostic significance of the different subcellular localizations of β-catenin expression for patients with CRC. The results indicated that nuclear expression of β-catenin or decreased expression of β-catenin in the membrane was associated with lower OS. However, no significant association was observed between β-catenin overexpression in the cytoplasm and 5-year OS, which was consistent with the previous results.[22] Unexpectedly, our results indicated that β-catenin overexpression in the nucleus and cytoplasm was negatively associated with differentiation grade, which needs further study to verify this conclusion.
Genetic mutations, such as mutation of APC or CTNNB1, are the main cause of accumulation of nuclear β-catenin.[61] Previous studies reported that the mutation rate of the CTNNB1 gene in CRC ranged from 10% to 50%.[62–64] Therefore, the β-cateninin the nucleus could be either mutant type or wild type; however, these 2 different typesof β-cateninin the nucleus are of functionally distinct. It is necessary to separate the wild-type and mutant-type β-catenin proteins by expression staining and analyze their prognostic value. Here, we failed to distinguish whether the nuclear β-catenin was mutant type or wild type due to lack of relevant information. We inferred that the variable outcomes of the relationship between nuclear β-catenin expression and prognosis in CRC may be caused by the analysis of the mutant-type and wild-type β-catenin. Furthermore, such analysis may also contribute to inter-study heterogeneity.
In addition, we could not ignore the limitations in this meta-analysis. First, heterogeneity that would affect the results of meta-analysis does exist. The subjective evaluation of β-catenin expression, different source and dilution of primary antibodies, and different characteristics of patients in each study contributed to significant heterogeneity. However, we failed to identify the source of heterogeneity by stratified analysis. To eliminate variations across studies, the random-effects model was performed accordingly. Second, we did not include non-English studies, which might introduce potential language bias. In addition, publication bias existed as only studies performed with positive results or significant outcomes were suitable for publication. Another potential source of bias might have come from the less reliable data that were extrapolated from survival curves. As the presence of inevitable limitations exists in this meta-analysis, additional large and well-designed prospective studies are should to be conducted.
Our study is the first to meta-analyze the association between the reduced membranous expression of β-catenin and prognosis in CRC patients. The pooled data suggested that reduced expression of β-catenin in the membrane is significantly associated with poor survival in patients with CRC. In addition, nuclear β-catenin overexpression, rather thancytoplasmic β-catenin overexpression, could serve as a biomarker of poor prognosis on CRC. New approaches to therapeutically target Wnt/β-catenin pathway needs to be explored, and it is necessary to distinguish the differential subcellular localizations of β-catenin to develop different therapeutic strategies.
Supplementary Material
Acknowledgments
The authors would like to thank Professor YanqinHuang (Cancer Institute, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China) for providing statistical consultation in this study.
Footnotes
Abbreviations: CRC = colorectal cancer, CI = confidence interval, HR = hazard ratio, OR = odds ratio, OS = overall survival, DFS = disease-free survival.
All analyses were based on previous published studies; thus, no ethical approval and patient consent were required
SZ, ZW, and JS contributed equally to this work.
Funding: This study was supported by project grants from the Zhejiang Provincial Natural Science Foundation of China (LY12H16014, LY13H160014 and LY14H160043), and the Science and Technology Department of Zhejiang Province (2012C24016).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Siegel R, Ward E, Brawley O, et al. Cancer statistics: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212–36. [DOI] [PubMed] [Google Scholar]
- [3].Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- [4].Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759–67. [DOI] [PubMed] [Google Scholar]
- [5].Gavert N, Conacci-Sorrell M, Gast D, et al. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol 2005;168:633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee JM, Yang J, Newell P, et al. Beta-catenin signaling in hepatocellular cancer: Implications in inflammation, fibrosis, and proliferation. Cancer Lett 2014;343:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu C, Li Y, Semenov M, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002;108:837–47. [DOI] [PubMed] [Google Scholar]
- [8].de Sousa EM, Vermeulen L, Richel D, et al. Targeting Wnt signaling in colon cancer stem cells. Clin Cancer Res 2011;17:647–53. [DOI] [PubMed] [Google Scholar]
- [9].Vermeulen L, De Sousa EMF, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 2010;12:468–76. [DOI] [PubMed] [Google Scholar]
- [10].Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell 2013;154:274–84. [DOI] [PubMed] [Google Scholar]
- [11].Haase G, Gavert N, Brabletz T, et al. The Wnt target gene L1 in colon cancer invasion and metastasis. Cancers 2016;8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ji S, Ye G, Zhang J, et al. miR-574-5p negatively regulates Qki6/7 to impact beta-catenin/Wnt signalling and the development of colorectal cancer. Gut 2013;62:716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li K, Zhou ZY, Ji PP, et al. Knockdown of beta-catenin by siRNA influences proliferation, apoptosis and invasion of the colon cancer cell line SW480. Oncol Lett 2016;11:3896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Martensson A, Oberg A, Jung A, et al. Beta-catenin expression in relation to genetic instability and prognosis in colorectal cancer. Oncol Rep 2007;17:447–52. [PubMed] [Google Scholar]
- [15].Pancione M, Forte N, Sabatino L, et al. Reduced beta-catenin and peroxisome proliferator-activated receptor-gamma expression levels are associated with colorectal cancer metastatic progression: correlation with tumor-associated macrophages, cyclooxygenase 2, and patient outcome. Hum Pathol 2009;40:714–25. [DOI] [PubMed] [Google Scholar]
- [16].Cheah PY, Choo PH, Yao J, et al. A survival-stratification model of human colorectal carcinomas with beta-catenin and p27kip1. Cancer 2002;95:2479–86. [DOI] [PubMed] [Google Scholar]
- [17].Gao ZH, Lu C, Wang MX, et al. Differential beta-catenin expression levels are associated with morphological features and prognosis of colorectal cancer. Oncol Lett 2014;8:2069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wangefjord S, Brandstedt J, Lindquist KE, et al. Associations of beta-catenin alterations and MSI screening status with expression of key cell cycle regulating proteins and survival from colorectal cancer. Diagn Pathol 2013;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Morikawa T, Kuchiba A, Yamauchi M, et al. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA 2011;305:1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fernebro E, Bendahl PO, Dictor M, et al. Immunohistochemical patterns in rectal cancer: application of tissue microarray with prognostic correlations. Int J Cancer 2004;111:921–8. [DOI] [PubMed] [Google Scholar]
- [21].Balzi M, Ringressi MN, Faraoni P, et al. B-cell lymphoma 2 and beta-catenin expression in colorectal cancer and their prognostic role following surgery. Mol Med Rep 2015;12:553–60. [DOI] [PubMed] [Google Scholar]
- [22].Chen Z, He X, Jia M, et al. Beta-catenin overexpression in the nucleus predicts progress disease and unfavourable survival in colorectal cancer: a meta-analysis. PloS One 2013;8:e63854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen J, Liu J, Jin R, et al. Cytoplasmic and/or nuclear expression of beta-catenin correlate with poor prognosis and unfavorable clinicopathological factors in hepatocellular carcinoma: a meta-analysis. PLoS One 2014;9:e111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ (Clin Res ed) 2007;335:914–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Youssef NS, Osman WM. Relationship between osteopontin and beta-catenin immunohistochemical expression and prognostic parameters of colorectal carcinoma. Int J Clin Exp Pathol 2015;8:1503–14. [PMC free article] [PubMed] [Google Scholar]
- [26].Abdelmaksoud-Damak R, Miladi-Abdennadher I, Triki M, et al. Expression and mutation pattern of beta-catenin and adenomatous polyposis coli in colorectal cancer patients. Arch Med Res 2015;46:54–62. [DOI] [PubMed] [Google Scholar]
- [27].Jung W, Hong KD, Jung WY, et al. SIRT1 expression is associated with good prognosis in colorectal cancer. Korean J Pathol 2013;47:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Toth L, Andras C, Molnar C, et al. Investigation of beta-catenin and E-cadherin expression in Dukes B2 stage colorectal cancer with tissue microarray method. Is it a marker of metastatic potential in rectal cancer? Pathol Oncol Res 2012;18:429–37. [DOI] [PubMed] [Google Scholar]
- [29].Andras C, Toth L, Molnar C, et al. Correlations between clinicopathological parameters and molecular signatures of primary tumors for patients with stage T3n0 colorectal adenocarcinomas: a single center retrospective study on 100 cases. Hepatogastroenterology 2012;59:1091–7. [DOI] [PubMed] [Google Scholar]
- [30].Ozguven BY, Karacetin D, Kabukcuoglu F, et al. Immunohistochemical study of E-cadherin and beta-catenin expression in colorectal carcinomas. Polish J Pathol 2011;62:19–24. [PubMed] [Google Scholar]
- [31].Matsuoka T, Mitomi H, Fukui N, et al. Cluster analysis of claudin-1 and -4, E-cadherin, and beta-catenin expression in colorectal cancers. J Surg Oncol 2011;103:674–86. [DOI] [PubMed] [Google Scholar]
- [32].Sun L, Hu H, Peng L, et al. P-cadherin promotes liver metastasis and is associated with poor prognosis in colon cancer. Am J Pathol 2011;179:380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Magnusson C, Mezhybovska M, Lorinc E, et al. Low expression of CysLT1R and high expression of CysLT2R mediate good prognosis in colorectal cancer. Eur J Cancer (Oxford, England: 1990) 2010;46:826–35. [DOI] [PubMed] [Google Scholar]
- [34].Filiz AI, Senol Z, Sucullu I, et al. The survival effect of E-cadherin and catenins in colorectal carcinomas. Colorectal Dis 2010;12:1223–30. [DOI] [PubMed] [Google Scholar]
- [35].Fang QX, Lu LZ, Yang B, et al. L1, beta-catenin, and E-cadherin expression in patients with colorectal cancer: correlation with clinicopathologic features and its prognostic significance. J Surg Oncol 2010;102:433–42. [DOI] [PubMed] [Google Scholar]
- [36].Togo N, Ohwada S, Sakurai S, et al. Prognostic significance of BMP and activin membrane-bound inhibitor in colorectal cancer. World J Gastroenterol 2008;14:4880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen S, Liu J, Li G, et al. Altered distribution of beta-catenin and prognostic roles in colorectal carcinogenesis. Scand J Gastroenterol 2008;43:456–64. [DOI] [PubMed] [Google Scholar]
- [38].Bravou V, Klironomos G, Papadaki E, et al. ILK over-expression in human colon cancer progression correlates with activation of beta-catenin, down-regulation of E-cadherin and activation of the Akt-FKHR pathway. J Pathol 2006;208:91–9. [DOI] [PubMed] [Google Scholar]
- [39].Kim CJ, Cho YG, Park YG, et al. Pin1 overexpression in colorectal cancer and its correlation with aberrant beta-catenin expression. World J Gastroenterol 2005;11:5006–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bondi J, Bukholm G, Nesland JM, et al. Expression of non-membranous beta-catenin and gamma-catenin, c-Myc and cyclin D1 in relation to patient outcome in human colon adenocarcinomas. APMIS 2004;112:49–56. [DOI] [PubMed] [Google Scholar]
- [41].Ougolkov AV, Yamashita K, Mai M, et al. Oncogenic β-catenin and MMP-7 (matrilysin) cosegregate in late-stage clinical colon cancer. Gastroenterology 2002;122:60–71. [DOI] [PubMed] [Google Scholar]
- [42].Chung GG, Provost E, Kielhorn EP, et al. Tissue microarray analysis of beta-catenin in colorectal cancer shows nuclear phospho-beta-catenin is associated with a better prognosis. Clin Cancer Res 2001;7:4013–20. [PubMed] [Google Scholar]
- [43].Maruyama K, Ochiai A, Akimoto S, et al. Cytoplasmic beta-catenin accumulation as a predictor of hematogenous metastasis in human colorectal cancer. Oncology 2000;59:302–9. [DOI] [PubMed] [Google Scholar]
- [44].Wong SC, Lo ES, Chan AK, et al. Nuclear beta catenin as a potential prognostic and diagnostic marker in patients with colorectal cancer from Hong Kong. Mol Pathol 2003;56:347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lee SJ, Choi SY, Kim WJ, et al. Combined aberrant expression of E-cadherin and S100A4, but not beta-catenin is associated with disease-free survival and overall survival in colorectal cancer patients. Diagn Pathol 2013;8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jang KY, Kim YN, Bae JS, et al. Expression of Cyclin D1 is associated with beta-catenin expression and correlates with good prognosis in colorectal adenocarcinoma. Translational Oncol 2012;5:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Khiari M, Arfaoui A, Kriaa L, et al. The prognostic value of the immunohistochemical expression and mutational pattern of the key mediator of Wnt signaling: beta-catenin in Tunisian patients with colorectal carcinoma. Appl Immunohistochem Mol Morphol 2012;20:62–70. [DOI] [PubMed] [Google Scholar]
- [48].Kamposioras K, Konstantara A, Kotoula V, et al. The prognostic significance of WNT pathway in surgically-treated colorectal cancer: beta-catenin expression predicts for disease-free survival. Anticancer Res 2013;33:4573–84. [PubMed] [Google Scholar]
- [49].Boo YJ, Park JM, Kim J, et al. L1 expression as a marker for poor prognosis, tumor progression, and short survival in patients with colorectal cancer. Ann Surg Oncol 2007;14:1703–11. [DOI] [PubMed] [Google Scholar]
- [50].Salim T, Sjolander A, Sand-Dejmek J. Nuclear expression of glycogen synthase kinase-3beta and lack of membranous beta-catenin is correlated with poor survival in colon cancer. Int J Cancer 2013;133:807–15. [DOI] [PubMed] [Google Scholar]
- [51].Wang L, Zhang X, Li Z, et al. Overexpression of nuclear beta-catenin at invasive front in rectal carcinoma is associated with lymph node metastasis and poor prognosis. Clin Translat Oncol 2014;16:488–94. [DOI] [PubMed] [Google Scholar]
- [52].Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ (Clin Res ed) 2001;323:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gough NR. Focus issue: Wnt and beta-catenin signaling in development and disease. Sci Signaling 2012;5:eg2. [DOI] [PubMed] [Google Scholar]
- [54].Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell 2000;103:311–20. [DOI] [PubMed] [Google Scholar]
- [55].Guo YH, Wang LQ, Li B, et al. Wnt/beta-catenin pathway transactivates microRNA-150 that promotes EMT of colorectal cancer cells by suppressing CREB signaling. Oncotarget 2016;7:42513–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Qi J, Yu Y, Akilli Ozturk O, et al. New Wnt/beta-catenin target genes promote experimental metastasis and migration of colorectal cancer cells through different signals. Gut 2015;65:1690–701. [DOI] [PubMed] [Google Scholar]
- [57].Inomata M, Ochiai A, Akimoto S, et al. Alteration of beta-catenin expression in colonic epithelial cells of familial adenomatous polyposis patients. Cancer Res 1996;56:2213–7. [PubMed] [Google Scholar]
- [58].Chiurillo MA. Role of the Wnt/beta-catenin pathway in gastric cancer: An in-depth literature review. World J Exp Med 2015;5:84–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mao TL, Chu JS, Jeng YM, et al. Expression of mutant nuclear beta-catenin correlates with non-invasive hepatocellular carcinoma, absence of portal vein spread, and good prognosis. J Pathol 2001;193:95–101. [DOI] [PubMed] [Google Scholar]
- [60].Schmitt-Graff A, Ertelt V, Allgaier HP, et al. Cellular retinol-binding protein-1 in hepatocellular carcinoma correlates with beta-catenin, Ki-67 index, and patient survival. Hepatology (Baltimore, MD) 2003;38:470–80. [DOI] [PubMed] [Google Scholar]
- [61].Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997;275:1787–90. [DOI] [PubMed] [Google Scholar]
- [62].Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev 2006;16:51–9. [DOI] [PubMed] [Google Scholar]
- [63].Amary MF, Pauwels P, Meulemans E, et al. Detection of beta-catenin mutations in paraffin-embedded sporadic desmoid-type fibromatosis by mutation-specific restriction enzyme digestion (MSRED): an ancillary diagnostic tool. Am J Surg Pathol 2007;31:1299–309. [DOI] [PubMed] [Google Scholar]
- [64].Park WS, Oh RR, Park JY, et al. Frequent somatic mutations of the beta-catenin gene in intestinal-type gastric cancer. Cancer Res 1999;59:4257–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


