Abstract
The aim of this study was to assess the impact of mothers’ and newborns’ fat mass and obesity-associated gene (FTO) rs9939609 and leptin receptor (LEPR) rs1137101 gene polymorphisms on neonatal anthropometric parameters in order to identify a potential risk for developing obesity.
We performed a cross-sectional study on 355 mother–newborn couples in an Obstetrics Gynecology Tertiary Hospital from Romania, evaluated with regard to anthropometric parameters, clinical and laboratory parameters besides 2 genetic polymorphisms (FTO rs9939609 and LEPR rs1137101).
Newborns with mothers carrying variant AT or AA genotype for FTO rs9939609 presented lower BMI (P = 0.012) and lower MUAC (P = 0.029). There was a significant interaction effect between newborn and mother LEPR rs1137101 polymorphism on birth weight (P = 0.009) and BMI (P = 0.007). We noticed significantly increased birth weight and BMI in newborns carriers of AG + GG genotype, coming from mothers with AA genotype (P = 0.006). There was no evidence of significant interaction effect between newborn and mother FTO rs9939609 polymorphism on the studied anthropometrical data (P > 0.05). In addition, lower BMI scores (P = 0.042) were observed in newborns carriers of TT genotype whose mothers had AA + AT genotype. Lower MUAC scores (P = 0.041) were noticed in newborns carriers of AA + AT genotype whose mothers had AA + AT genotype for FTO rs9939609 gene polymorphism. Newborns carriers of the AG + GG genotype (P = 0.003) of LEPR rs1137101 coming from mothers with increased FMI (upper tertile) had significantly increased BMIs.
Presence of the variant A allele of FTO rs9939609 polymorphism in mothers decreased BMI and MUAC in newborns. The impact of LEPR rs1137101 polymorphism on BMI and birth weight in newborns differed depending on the presence/absence of the dominant LEPR allele in mothers. In addition, we noticed that maternal FMI presented a significant positive effect on newborns’ BMI by changing the effect of LEPR rs1137101.
We can conclude that mothers’ FTO rs9939609 and LEPR rs1137101 gene polymorphisms presented an impact on birth weight and newborns’ BMI, therefore being involved in the newborns’ nutritional status and in the design of a potential protocol.
Keywords: fat mass index, FTO rs9939609 and LEPR rs1137101 gene polymorphisms, mothers, newborns
1. Introduction
Birth weight is very important, representing a predictor not only for the perinatal health, but also for the development, growth, and the afterwards adult period. It is influenced by maternal (mother's weight, gestational weight gain [GWG]), obstetrical and gynecological, genetic, environmental but also socioeconomic factors.[1,2] Both low weight and overweight are associated with an increased risk of obstetrical and neonatal complications, but also metabolic and cardiovascular disorders later in life.[3,4] Excessive body mass index (BMI) and GWG before labor are strongly related with obstetrical and maternal risks.[5,6] Excessive GWG increased birth weight and obesity risk, further on in life.[7] There are also other important factors, such as genetic and environmental ones (mother or infant obesity-related genes).[7] High-quality diets during pregnancy are recommended in order to provide an adequate growth of embryo and fetus.[8,9]
Obesity is determined by the combined effect of genes, environment, lifestyle, and interactions of these factors.[10,11] The critical periods for the onset of obesity are: pregnancy,[11,12]‘adiposity rebound’ childhood development (age 3–6 years),[13]and puberty.[14] Identification of risk factors for obesity even since the first days of life and diminishment of obesity incidence in pregnant woman and child have a great impact also on the adult's health, preventing complications of this disorder.[15,16]
The determinism of adiposity, therefore of obesity and its complications is the result of the combination between lifestyle, genetic, and psychologic factors. Genetic factors include SNP genes that codify the regulating proteins, proinflammatory cytokines that are involved in the regulation of body composition.[17] Therefore, numerous genes involved in the determinism of obesity are known, one of the most frequently involved in the mechanism of this disease being the fat mass and obesity-associated (FTO) gene, even though its role in energy homeostasis and signaling pathways is not completely understood.[18–20] Although there are studies on mice that emphasized the ubiquity expression of this protein in both fetal and adult tissues, with the highest concentration in the hypothalamus, the role of this gene in the pathological mechanism of obesity is through modifications of the energetic balance, as a result of the alteration of FTO mRNA expression level in the hypothalamus.[19,21,22] Larder et al[23] underlined that FTO protein is a member of iron(II) and 2-oxoglutarate oxygenase. Some studies have shown that FTO gene is associated with a predisposition to obesity.[24] Labayen in Helena study showed that the A allele of FTO rs9939609 gene polymorphism is associated with elevated serum leptin levels in adolescents independent of potential confounders, like adiposity. Therefore we can say that leptin plays an important role between FTO rs9939609 gene polymorphism and adiposity.[19] These persons are at high risk for developing obesity, fact that leads to a mandatory and careful monitoring of these children by pediatricians and nutritionists, in order to issue recommendations for preventive dietary measurements and also in order to prevent or even treat potential complications. Also, it was proven that physical activity can attenuate the effect of the FTO rs9939609 polymorphism on adiposity in children.[25] In addition, it was observed that small birth weight is associated with metabolic disorders because these children present a smaller proportion of lean tissue mass later on in life, fact that provides a higher susceptibility to an increased nutritional intake in a certain period of life.[26] The genetic determinism is decisive in influencing birth weight (ponderal index [PI]) corresponding to the length and adult metabolic disorders, and vice versa.[27,28]
There are numerous mediators involved in appetite regulation such as insulin, gastrointestinal peptides (peptide Y, cholecystokinin, glucagon-like peptide-1), ghrelin that stimulates the appetite, leptin that decreases the appetite and increases the energy expenditure.[29] Leptin is a hormone synthetized in the white adipocytes, but also in other organs. It controls the dietary intake and energy expenditure through central and peripheral mechanisms. In obesity an endogenous leptin-resistance mechanism may be present, limiting the regulating effect, explaining the correlation between high leptin levels and body fat mass.[19,30] The leptin receptor gene (LEPR) is a biological pathway associated with obesity. During pregnancy, leptin is produced by the adipose tissue of the mother and fetus, but also by the placenta, the serum level of leptin in the umbilical cord being positively correlated with birth weight, in comparison to the maternal levels of leptin.[31,32] There are several polymorphisms in the LEPR gene involved in the mechanism of obesity, 3 single nucleotide polymorphisms being the most studied, namely: Q223R, K109R, and K656N. Recent studies sustain that LEPR rs1137101 (Gln223Arg) is the most frequent one associated with obesity.[33–35] Therefore, in some particular cases, it is important to assess these genetic factors, for example in obese pregnant women, those with excessive GWG, or even those who previously had newborns with high birth weight. In the everyday medical practice both FTO and LEPR polymorphisms can be important diagnostic tools, and even prevention ones if they are assessed in pregnant women at risk for having a macrosomic newborn, or a child with increased susceptibility for developing obesity later in life.
The aim of this study was to assess the impact of mothers’ and newborns’ FTO rs9939609 and LEPR rs1137101 gene polymorphisms on neonatal anthropometric parameters in order to identify a potential risk for developing obesity.
On the basis of the above mentioned facts, we considered the following objectives: to investigate the effect of neonatal and maternal FTO rs9939609 gene polymorphism on neonatal anthropometric parameters; to assess the effect of maternal FTO rs9939609 gene polymorphism on the relationship between neonatal FTO rs9939609 gene polymorphism and neonatal anthropometric parameters; to investigate the effect of neonatal and maternal LEPR rs1137101 gene polymorphism on neonatal anthropometric parameters; to assess the associations between maternal and neonatal LEPR rs1137101 gene polymorphism and neonatal anthropometric parameters; to evaluate the effect of maternal fat mass index (FMI) on the relationship between the newborn's polymorphisms and neonatal anthropometric parameters.
2. Materials and methods
A cross-sectional study was performed on 355 pairs of mothers–newborns in an Obstetrics Gynecology Tertiary Hospital from Romania, during a period of 9 months. The cases were included in the study as they presented in the clinic. The criteria for inclusion were: mothers with a single fetus that presented for labor in the above mentioned clinic, gestational age between 37 and 42 weeks. The exclusion criteria were: mothers and newborns with chronic diseases, patients with infectious processes, parity >8; fetuses diagnosed with intrauterine malformation, intrauterine growth retardation, patients with incomplete anthropometric, clinic or laboratory data (usual and genetic) or those who did not sign the informed consent.
All mothers gave written informed consent for them and their child before inclusion in the study and research was performed in compliance with the principles of the Helsinki Declaration, and was approved by the Ethics Committee of the University of Medicine and Pharmacy of Tîrgu Mureş (No. 32/March 16, 2015).
2.1. Anthropometric characteristics
A single trained person performed the measurements including the following: weight (kg), height (cm), MUAC (mid-upper arm circumference), and TST. Weight was measured with a daily calibrated scale (±10 g error), height with a pedometer calibrated in cm ± SD (0.1 cm error); MUAC was measured at the mid-point between shoulder tip and elbow, using a tape measure calibrated in centimeters and TST was determined on the posterior area of the upper arm, using a thickness caliper (http://www.who.int/childgrowth/training/jobaid_weighing_measuring.pdf). BMI was calculated by dividing weight (kg) to standing height squared (m2).
FMI of the mothers was calculated by dividing fat mass by height squared (m). This was an estimate for body size analogue with BMI. PI was computed in newborns as birth weight (kg) divided by birth length (m) cubed and in pregnant women being calculated as the ratio between the weight (kg) divided by height (m) cubed.
2.2. Genotyping description
Purified genomic DNA was obtained from whole blood by using the Zymo-Spin column technology with the aid of a Quick-gDNA MiniPrep kit (ZymoResearch, Irvine, California, USA). Genotyping of the LEPR rs1137101 (Gln223Arg) and FTO rs9939609 gene polymorphisms was accomplished using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique, as reported previously by Matsuoka et al[36] and López-Bermejo et al,[37] respectively.
2.3. Statistical analysis
For statistical analysis the advanced environment for statistical computing R (v.3.2.4, Vienna, Austria) and Statistics (StatSoft, v.6.0) were used. To assess the normality of neonatal and anthropometric newborn variables, the Shapiro–Wilk test, quantile Q–Q plot, and 95% confidence intervals (CIs) of univariate skewness and kurtosis were determined. All anthropometric variables were expressed as mean ± standard deviation while studied polymorphisms distributions were described with absolute and relative frequencies.
The possible associations between FTO rs9939609 and LEPR rs1137101 gene polymorphisms in mothers and newborns were tested by Chi-square test.
The individual main effect of each studied polymorphism was investigated by 1-way univariate ANOVA while interaction effects between maternal and neonatal polymorphisms were tested using 2-way univariate ANOVA analysis.
We considered the following 4 subgroups for testing neonatal and maternal FTO rs9939609 interaction: newborns carriers of TT genotype coming from mothers with the same genotype (TT), newborns carriers of TT genotype coming from mothers carrying the variant AA or AT (AA + AT) genotype, newborns carrying the AA or AT (AA + AT) whose mothers had TT genotype, and newborns carrying the AA or AT genotype with mothers carrying the same variant genotype (AA + AT).
We defined the following 4 subgroups for testing neonatal and maternal LEPR rs1137101 associations: newborns carriers of AA genotype whose mothers had the same genotype (AA) newborns carriers of AA genotype with mothers carrying the variant GG or AG (GG + AG) genotype, newborns carrying the GG or AG genotype whose mothers had AA genotype, and newborns carrying the GG or AG genotype whose mothers had GG + AG genotype.
Post hoc analysis regarding differences between any of the above defined 2 subgroups was realized using Student t test for independent samples.
To assess the possible interaction between the neonatal FTO rs9939609 and LEPR rs1137101 polymorphism and mother FMI on anthropometric variables, we applied the same 2-way univariate ANOVA analysis transforming the continuous FMI variable into a categorical one defined by tertiles: lower tertile: [0.578,6.36], middle tertile: [6.36,8.72], and upper: [8.72,20.5].
The level of statistical significance for all 2-sided tests was set to 0.05.
3. Results
Neonatal and clinical characteristics are shown in Table 1. In the studied samples 131 mothers were TT homozygous and 224 mothers carried the variant genotype (AA or AT) of FTO rs9939609, while 170 neonates were TT homozygous and 185 were newborns with variant genotype of the FTO rs9939609 polymorphism. Regarding the LEPR rs1137101 polymorphism, 107 mothers presented the AA genotype and 248 mothers carried the variant genotype (GG or AG), while 116 neonates had the AA genotype and 239 were newborns who presented the variant allele.
Table 1.
Descriptive characteristics of the newborns and mothers (number of subjects, percentages, arithmetic mean values, and standard deviations).
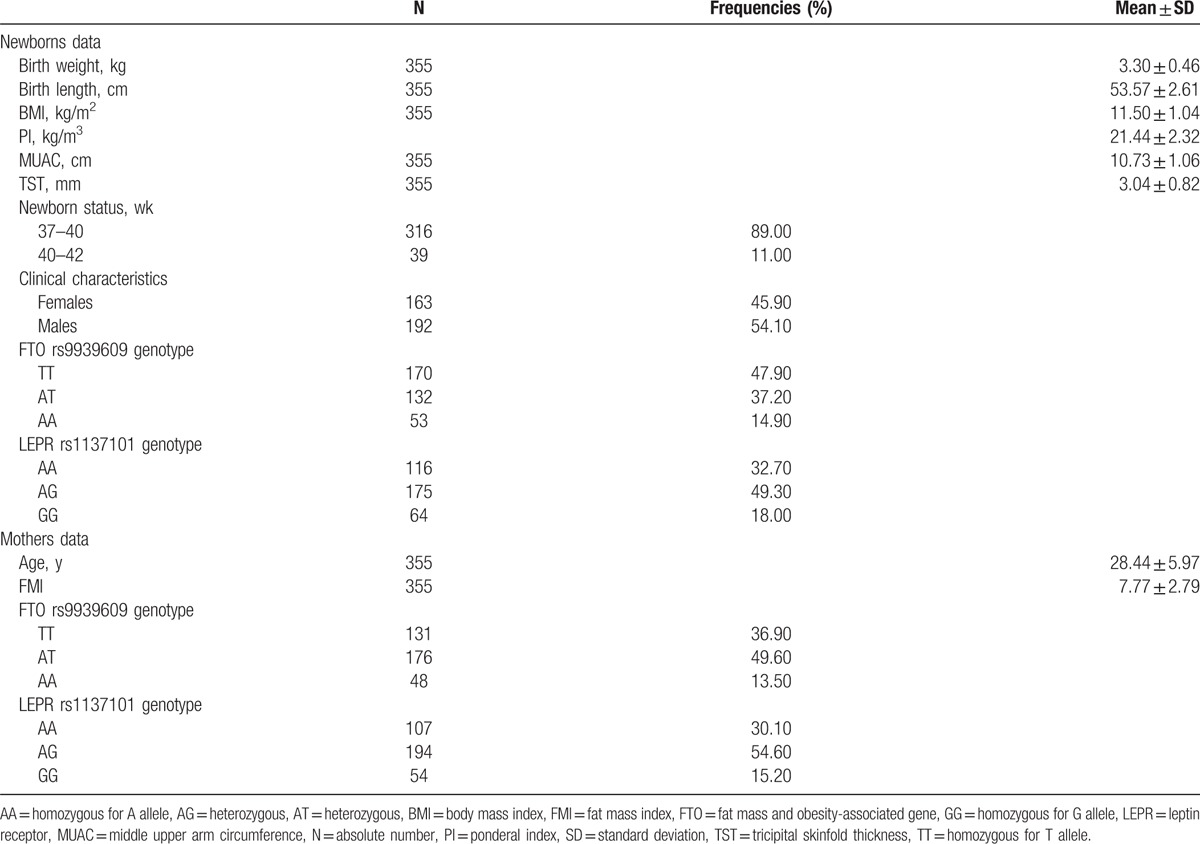
The anthropometrical characteristics (birth weight, birth length, BMI, PI, MUAC, TST) distribution was not influenced significantly by newborns’ gender (Student t test assuming equal variances, P > 0.05).
There was no significant difference in maternal age between the AA + AT and TT groups of the FTO rs9939609 polymorphism [Student t test assuming equal variances, t(353) = −1.56, P = 0.120] nor between newborns’ AA + AT and TT groups for the same polymorphism [Student t test assuming equal variances, t(353) = −1.13, P = 0.260]. The age variability was similar both in mothers’ GG + AG and AA genotype groups [t(353) = −0.04, P = 0.969] and newborns’ (P > 0.05) The distribution of male and female newborns was similar in the AA + AT and TT neonatal subgroups (Chi-square test, P = 0.400). The same result was noticed in GG + AG and AA neonatal genotype subgroups (Chi-square test, P = 0.774 and P = 0.258). We did not find any association between the presence/absence of the variant allele of FTO rs9939609 and LEPR rs1137101 neither in mothers (χ2(1) = 0.02, P = 0.902) nor in newborns (χ2(1) = 0.12, P = 0.726).
Table 2 describes the main effects of maternal and neonatal FTO rs9939609 polymorphisms on anthropometrical parameters. Newborns with mothers carrying the A allele (included in group AT + AA) presented lower BMI (P = 0.012) and lower MUAC (P = 0.029) than newborns from mothers with the TT genotype. We also noticed a tendency toward statistical significance regarding the association between newborns FTO rs9939609 polymorphism and BMI (P = 0.073) as well as MUAC (P = 0.054). We did not identify any significant effect of maternal or newborn LEPR rs1137101 polymorphism on anthropometrical characteristics (P > 0.05) (Table 2).
Table 2.
The main effects of neonatal/maternal FTO rs9939609 and LEPR rs1137101 polymorphisms on anthropometric variables.
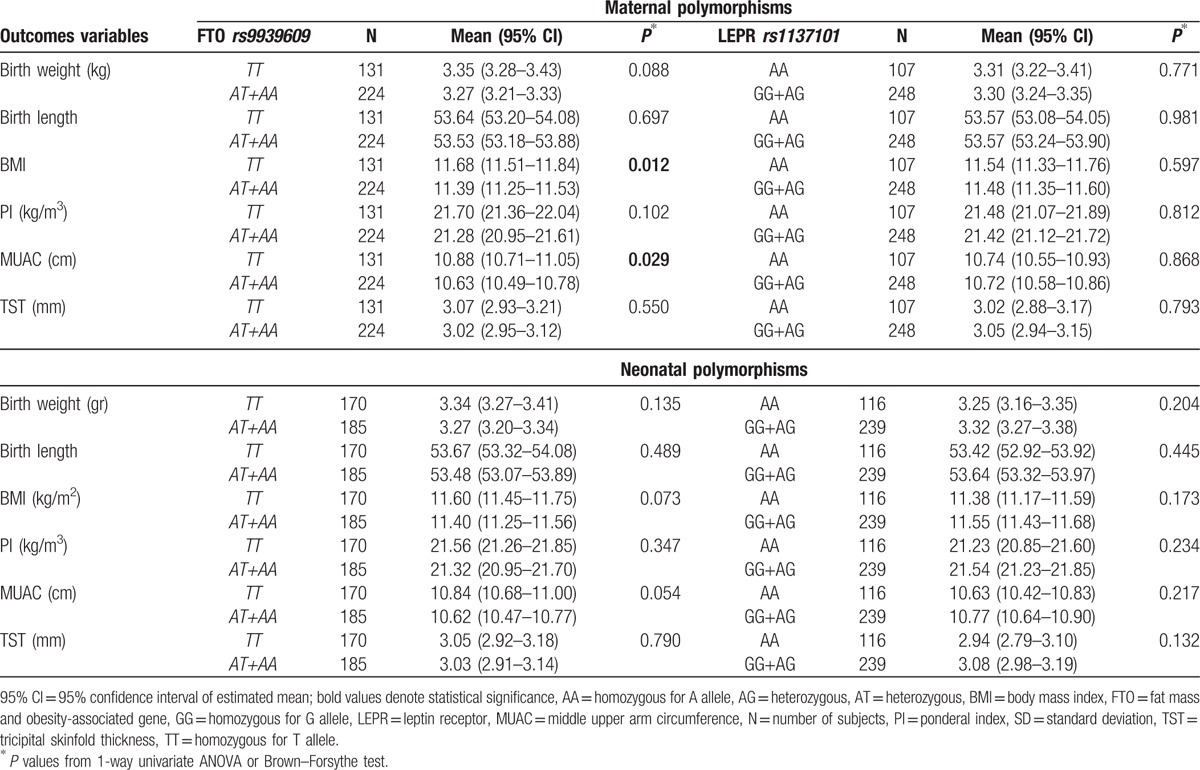
Table 3 describes the combined effect of maternal and neonatal studied polymorphisms on anthropometrical characteristics, the ANOVA analysis highlighting a significant interaction effect between neonatal and maternal LEPR rs1137101 gene polymorphism on birth weight (P = 0.009) and BMI (P = 0.007). We proved that the effect of newborns’ LEPR rs1137101 polymorphism on BMI and birth weight for GG + AG and AA maternal subgroups was not the same. In addition, a significant increase in birth weight and BMI was highlighted in newborns carriers of GG + AG genotype whose mothers had AA genotype versus newborns carriers of AA genotype whose mothers had the same AA genotype (Student t test, P = 0.006 and P = 0.004, respectively).
Table 3.
The interaction effect of neonatal and maternal FTO rs9939609/LEPR rs1137101 polymorphism on anthropometric variables.
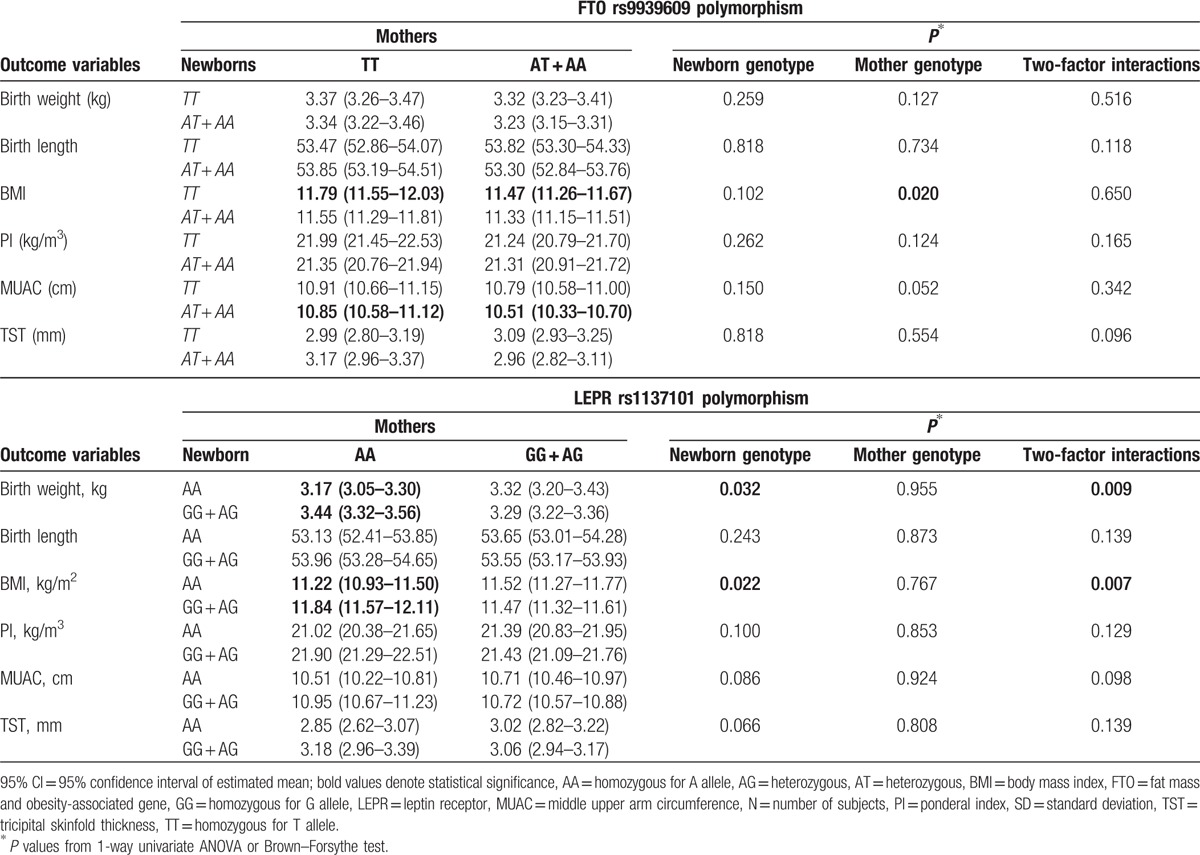
A tendency toward significance was observed for the interaction effect between neonatal and maternal variant allele of the LEPR rs1137101 polymorphism on MUAC (P = 0.098), the effect of the newborns’ variant allele on MUAC values being different between the subgroups of mothers with GG + AG and AA genotypes (Table 3).
There was no evidence of significant interaction effect between neonatal and maternal FTO rs9939609 gene polymorphism and the studied anthropometrical data (P > 0.05), but maternal variant allele significantly influenced BMI, while a tendency toward statistical significance was reached for MUAC. In addition, for the newborns carriers of the TT genotype lower BMI scores (Student t test, P = 0.042) were observed in mothers with AA + AT genotype, versus mothers with TT genotype. Also, for the newborns carriers of AA + AT, lower MUAC scores (P = 0.041) were noticed in the AA + AT genotype subgroup of mothers versus the TT genotype.
We also investigated the combined effect of the tested polymorphisms and maternal FMI on anthropometrical measurements; the ANOVA analysis revealed a significant interaction term between LEPR rs1137101 neonatal polymorphism and FMI tertiles on BMI (interaction P = 0.003).
For the mothers with increased FMI (upper tertile), there was a significantly increased BMI in newborns carriers of GG + AG genotype whose mothers had increased FMI (upper tertile), versus newborns with AA genotype coming from the same subgroup of mothers (Student t test, P = 0.003). The effect of neonatal LEPR rs1137101 polymorphism on BMI stratified by tertile of maternal FMI are present in Fig. 1.
Figure 1.
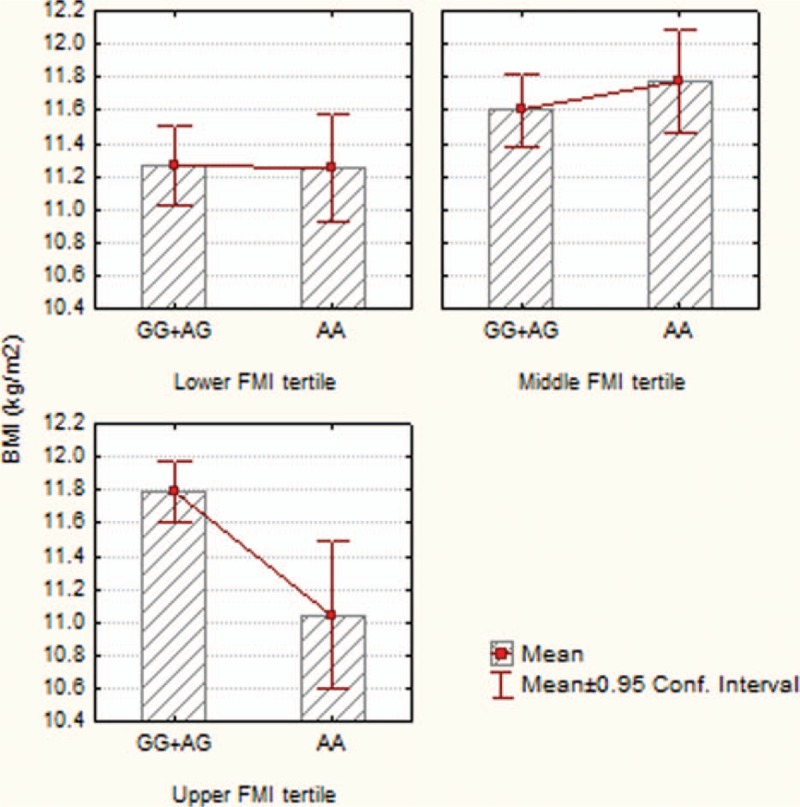
Interaction effect between neonatal LEPR rs1137101 polymorphism and maternal FMI tertiles on BMI. AA = homozygous for A allele, AG = heterozygous, BMI = body mass index, FMI = fat mass index, GG = homozygous for G allele.
We did not find significant interaction between the neonatal FTO rs9939609 polymorphism and maternal FMI (P > 0.05) on anthropometric data.
4. Discussions
4.1. Determinism of birth weight
Maternal body composition is a key determining factor for birth weight. Multiple studies assessed the maternal body composition through bioelectrical impedance studies and correlated the findings with birth weight. Therefore, some of them underlined the fact that maternal lean body mass is positively correlated with birth weight in comparison to maternal fat mass that seems to have no impact on birth weight.[3]
Obesity is a public health problem, therefore approximately 20% of mothers are obese at the beginning of the pregnancy.[38] In this group of women, excessive GWG was identified as a possible risk for an increased birth weight, while minimal GWG can lead to a decreased birth weight for gestational age.[39–41] Birth weight can lead to both, short-term and long-term complications. Thus, large-for-gestational-age newborns can present obstetrical complications (cesarean or operative delivery) and hypoglycemia, but they can also associate obesity during childhood, or metabolic syndrome later on in life.[42–44] In antithesis, small-for-gestational-age newborns, especially preterm ones, present an increased risk for different neurological or cardiovascular disorders, metabolic syndrome, or shorter height later on in life.[45–49]
Even though birth weight is determined by multiple factors and the interaction between them, we must always take under consideration the individual genetic susceptibility as an essential factor in its determinism.
4.2. Considerations according to the FTO rs9939609 gene polymorphism and anthropometric parameters of mothers and newborns
FTO (fat mass and obesity associated) is a very important protein, playing a role in the energetic metabolism and regulation of the organism's homeostasis,[23] although Larder underlined that there are still numerous unknown facts in relation to the role of FTO in adiposity. Thus, it was proven that the allele A of the FTO rs9939609 polymorphism is associated with increased leptin serum levels in adolescents, independent of other potential cofactors involved in adiposity. Gesteiro et al[9] underlined an association between an increased BMI and the AA homozygous genotype of the FTO rs9939609 polymorphism. Also, Frayling et al[20] noticed an increased risk of obesity in children with the age of 7 years carrying the mutant gene of this polymorphism. Leptin seems to own a relating role between the FTO rs9939609 polymorphism and adiposity.[19] Multiple studies proved that the damaging effect of FTO rs9939609 polymorphism is annihilated by 1 hour of physical activity,[18,50] fact proven also on a cohort of European teenagers.[25] Therefore, it is very useful for a pediatrician to assess this polymorphism and to know whether the child carries this genetic predisposition for obesity in order to insert adequate recommendations for physical activity. Nonetheless, few studies tried to establish an association between the role of FTO rs9939609 polymorphism in determining the risk of obesity and gestational age, GWG, and the same risk in newborns, respectively. Martins et al[51] noticed that the AA genotype of FTO rs9939609 gene polymorphism is positively associated with weight before birth, without being associated to GWG or postpartum weight. In our study the variant allele was also predominant in pregnant women (63.10%), in comparison with only 52.10% of the newborns. In addition, we proved that the newborns with mothers carrier of the A allele had lower BMI and MUAC than those with mothers carrying the T allele (P = 0.012, and P = 0.029, respectively). A tendency toward statistical significance was noticed between the association of BMI and MUAC in newborns and FTO rs9939609 polymorphism and BMI (P = 0.073, P = 0.054). Similar to our study Sovio et al[52] also proved that the presence of the A allele of FTO rs9939609 in infants is associated with a lower BMI, but the aspect changes during time, therefore above the age of 5 years the same allele is associated with a higher BMI. This is a tricky aspect for the pediatrician, favoring the development of obesity complications by missing the unexpected onset of this pathology. On the other hand, if the pediatrician is aware of the fact that the child is a carrier of the A allele of FTO rs9939609, he will be able to properly monitor the child, to assess its life style and adjust it in order to prevent the so called “inevitable” genetic predisposition.
Gesteiro et al in their study tried to assess the association of mother–newborn FTO rs9939609 gene polymorphism in order to identify the manner in which they correlate to anthropometric data, sensitivity to insulin, and lipids and lipoproteins serum levels. On 53 pairs of mothers and newborns they noticed that A allele predominated in 66% of cases, and that newborns from these mothers presented lower glucose levels, and that the newborns carrying the A allele had higher insulin and HOMA-IR.[9] In exchange, in our study we did not notice any association between the mothers’ and newborns’ alleles, but the mothers’ alleles significantly influenced neonatal BMI. In exchange, in the mothers subgroup, AA + AT × newborns TT had a more decreased BMI (P = 0.042), and for the mothers AA + AT subgroup × newborns AA + AT subgroup we obtained a more decreased MUAC (P = 0.041). Gesteiro et al[9] concluded that the Mediterranean diet can counterbalance the negative potential of the obesogenic A allele of FTO rs9939609 on glucose homeostasis in newborns. Perinatal complications can be an important problem for the neonatologist, leading to neurologic sequels or even death in case of repeated neonatal hypoglycemia. Therefore, if the obstetrician is aware of the maternal genetic predisposition by assessing whether the pregnant woman carries the A allele, he will be able to prevent these neonatal complications by recommending a proper diet during pregnancy and also to improve the neonatal outcome.
Labayen et al[18] observed in a study which involved 628 adolescents that the A allele of FTO rs9939609 was associated with increased BMI, body fat percentage, and FMI (P = 0.05), but not with PI. They also established that FMI was higher in teenagers with lower PI tertile carrying the A allele of FTO rs9939609. In our study, in exchange, we obtained no interaction between the neonatal FTO rs9939609 gene polymorphism and maternal FMI (P > 0.05), probably due to the small number of cases, but also due to the lack of longitudinal assessment of children at a distance from the moment of birth.
4.3. Considerations according to the LEPR rs1137101 gene polymorphism and anthropometric parameters of mothers and newborns
Leptin and ghrelin are 2 hormones with roles in the energetic balance. Leptin is a protein with hormone role, expressed in adipocytes, whose expression and secretion is correlated with body fat and adipocyte size,[53] being at the same time a long-term regulating mediator of the energetic balance that reduces dietary food intake and determines weight loss,[54] stimulating energy expenditure acting on the hypothalamic center of satiety.
Multiple studies assessed the role of LEPR rs1137101 gene polymorphism on the small child, adolescent, and adult nutritional status, but few of them tried to establish correlations with the newborn's birth weight or maternal LEPR gene.[31,55] It is well known the fact that leptin is produced by maternal and fetal adipose tissue, and is correlated with birth weight.[31] Souren et al,[31] in their study on 396 monozygotic and 232 dizygotic twins noticed that the subjects carrying the R allele of the Q223R SNP did not present a higher birth weight. In comparison to this study, the research of Rand et al[56] did not point out any correlation between the Q223R SNP in the maternal LEPR gene and birth weight, similar data with those obtained by us, meaning that in our study we did not observe the effects of maternal or newborn LEPR rs1137101 polymorphism on anthropometrical characteristics (P > 0.05). On the other hand, in our study we noticed that most of the mothers (69.80%) were A carriers, a similar percentage being also observed in newborns (67.30%). Guízar-Mendoza et al[11] showed that patients presenting the G allele of the LEPR rs1137101 gene polymorphism had higher body fat and leptin levels, correlated with MUAC, TST, and H/L, without being associated with BMI in the studied children. Also the studies of Mattevi et al[57] and Mergen et al[58] showed that the G allele is more frequent in overweight patients. In the review of Bender et al[59] it was underlined that some studies state that the G allele is associated with an increased risk of obesity, while others sustain that G allele owns a protector role, while other studies failed to identify any association. In a previous study of our team we did not find any correlation between the G allele and gender nor age.[35] Similar results reported by Pyrzak et al[60] highlighted the association between LEPR rs1137101 gene polymorphism and obesity. Even though the data are contradictory, in selected cases it is better to assess the LEPR gene polymorphism and leptin serum levels, taking under consideration the amount of obesity-related complications and their systemic impact.
In our study, trying to establish the combined effect of LEPR rs1137101 gene polymorphism in mothers and newborns, we noticed a significant interaction between their alleles with consequences on birth weight (P = 0.009) and BMI (P = 0.007), meaning the effect of newborns’ alleles on BMI and birth weight was not the same in the GG + AG and AA maternal genotype subgroups. In addition, we observed that birth weight and BMI were significantly higher in newborns carriers of variant GG + AG genotype whose mothers had AA genotype in comparison to newborns carriers of AA genotype whose mothers also had AA genotype (P = 0.006, and P = 0.004, respectively). Regarding the effect of maternal and neonatal LEPR rs1137101 gene polymorphism on MUAC, we obtained only a tendency toward significance for MUAC (P = 0.098), the effect of newborn allele on MUAC values differed between GG + AG and AA mothers genotype subgroups. In comparison to our study, in a previous study a higher frequency of the GG + AG genotypes was observed in obese patients, also correlated with leptin levels (P = 0.02) and indirectly proportional to adiponectin levels.[42]
In any case, in our study, similarly to Helena's study,[19] we tried to establish the combined effect of LEPR rs1137101 gene polymorphism and maternal FMI on anthropometrical measurements, and we obtained a significant interaction term between LEPR rs1137101 neonatal polymorphism and FMI tertiles on BMI (interaction P = 0.003). BMI was increased in newborns carriers of GG + AG genotype coming from mothers with increased FMI (upper tertile FMI) versus newborns with AA genotype (P = 0.003). Therefore, it seems that in practice it is mandatory to assess the combined effect of maternal FMI and LEPR gene polymorphism in newborns and to obtain a correlation between them, in order to identify the groups at risk for developing obesity and perform interventions at the right moment in time with adequate diet and life style recommendations in both pregnant women and neonates/children. Due to the increased incidence of childhood obesity and its large amount of complications during adulthood, it is mandatory for both obstetricians and pediatricians to collaborate in order to develop proper preventive medical measurements in cases of patients discovered to carry a high risk for developing obesity. Despite the presence of a genetic predisposition for developing obesity, it seems that certain interventions, such as a proper diet during pregnancy, or an ideal life style and physical effort can hinder the development of this condition. Nevertheless, it is very important to periodically monitor the children at risk for developing obesity because even though they have a normal BMI during their early childhood, obesity can appear later on, especially in the periods of “adiposity rebound.”
We must underline some limitations of our study, as the group comes from a single gynecology clinic, from a single geographic area of Romania, representing therefore a weakness of the study. It is very important to longitudinally follow-up the children at precisely set intervals until the preschool age and adolescent period, when the impact of the genetic profile of the studied polymorphisms is much higher. Other negative points of the study worth mentioning are the limitations related to the fact that this was a cross-sectional study, and also that there was no randomization of the samples. In addition, is important to provide the maternal caloric intake during pregnancy, to take into account food habits, environmental factors, and geographic ones that can interfere with the results. It is recommended to extend the study on a larger geographic area, on a higher number of cases, taking under consideration also the parental profile, birth weight of the genitors and other factors.
It is also very important to mention the strong points of our study, one of them being the very high estimation accuracy of the statistical parameters due to an adequate sample size. Another strength is represented by the fact that a single well trained person provided all the anthropometric data, measuring these parameters according to a well-established protocol. Both, mothers and newborns underwent all clinical and laboratory parameters, but also genotyping analysis. It should be noted that no data are available in the literature regarding the association between the anthropometric parameters in mothers and their newborns as well as FTO rs9939609 and LEPR rs1137101 gene polymorphisms and it may be considered a pilot study that needs to be expanded to a larger population. As far as we know, at the present moment there is no other study of this kind in Romania, but also in Europe. In this study multiple factors which may determine obesity in newborns were assessed. Even though these strengths present a great impact in the study, we must carry on the research, assessing the newborns further on in life, in clearly set periods in order to observe the impact of these parameters on the children's long-term nutritional status.
5. Conclusions
In our study we found that the presence of FTO rs9939609 variant A allele in mothers lead to the decrease of BMI and MUAC in newborns. A significant increase in BMI and birth weight was noticed in newborns carriers of GG + AG genotype whose mothers had AA genotype versus newborns with AA genotype coming from mothers with AA genotype. Maternal FMI presented a significant positive effect on newborns’ BMI by changing the effect of LEPR rs1137101.
We can conclude that mothers’ FTO rs9939609 and LEPR rs1137101 gene polymorphisms presented an impact on birth weight and newborns’ BMI, therefore being involved in the newborns’ nutritional status. Also in clinical practice it is very important to assess these genetic factors correlated with other factors supposed to trigger obesity in order to improve the outcome in selected cases by designing a potential protocol based on these parameters for determining the newborn's and child's risk for obesity. Further studies on larger groups and more extended geographical areas are needed, in order to accurately indicate the role of these 2 gene polymorphisms in predicting obesity.
Footnotes
Abbreviations: ADP = adiponectin, ALAT = alanine aminotransferase, ASAT = aspartate aminotransferase, BMI = body mass index, CDC = Centers for Disease Control and Prevention, Chol = cholesterol, CI = confidence interval, CRP = C-reactive protein, ELISA = enzyme-linked immunosorbant assay, FMI = fat mass index, FTO = fat mass and obesity-associated gene, GWG = gestational weight gain, H/L = height/length, HDL-chol = high-density lipoprotein cholesterol, HEI = healthy eating index, IL = interleukin, IL-6 = interleukin 6, IL-8 = interleukin 8, LDL = low-density lipoprotein, LDL-chol = low-density lipoprotein cholesterol, LEP = leptin, LEPR = leptin receptor, MUAC = mid-upper arm circumference, N = absolute number, OR = odds ratio, PCR = polymerase chain reaction, PI = ponderal index, SD = standard deviation, T-Chol = total cholesterol, TG = triglycerides, TST = tricipital skinfold thickness, W = weight.
Funding: This research was partially supported by the Research Grants of the University of Medicine and Pharmacy Tîrgu Mureş, Romania (Private Research Grant “Clinical implications of FTO rs9939609, LEPR rs1137101, and IL 6 174 C/G in the determination of obesity risk on newborns” no. 15299/24.10.2016).
CM and MI contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Sanin Aguirre LH, Reza-López S, Levario-Carrillo M. Relation between maternal body composition and birth weight. Biol Neonate 2004;86:55–62. [DOI] [PubMed] [Google Scholar]
- [2].Longnecker MP, Klebanoff MA, Zhou H, et al. Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet Lond Engl 2001;358:110–4. [DOI] [PubMed] [Google Scholar]
- [3].Kent E, O’Dwyer V, Fattah C, et al. Correlation between birth weight and maternal body composition. Obstet Gynecol 2013;121:46–50. [DOI] [PubMed] [Google Scholar]
- [4].Farah N, Stuart B, Donnelly V, et al. The influence of maternal body composition on birth weight. Eur J Obstet Gynecol Reprod Biol 2011;157:14–7. [DOI] [PubMed] [Google Scholar]
- [5].Castillo H, Santos IS, Matijasevich A. Relationship between maternal pre-pregnancy body mass index, gestational weight gain and childhood fatness at 6–7 years by air displacement plethysmography. Matern Child Nutr 2015;11:606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cedergren M, Brynhildsen J, Josefsson A, et al. Hyperemesis gravidarum that requires hospitalization and the use of antiemetic drugs in relation to maternal body composition. Am J Obstet Gynecol 2008;198:412.e1–5. [DOI] [PubMed] [Google Scholar]
- [7].Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet Lond Engl 2010;376:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gesteiro E, Rodríguez Bernal B, Bastida S, et al. Maternal diets with low healthy eating index or Mediterranean diet adherence scores are associated with high cord-blood insulin levels and insulin resistance markers at birth. Eur J Clin Nutr 2012;66:1008–15. [DOI] [PubMed] [Google Scholar]
- [9].Gesteiro E, Sánchez-Muniz FJ, Ortega-Azorín C, et al. Maternal and neonatal FTO rs9939609 polymorphism affect insulin sensitivity markers and lipoprotein profile at birth in appropriate-for-gestational-age term neonates. J Physiol Biochem 2016;72:169–81. [DOI] [PubMed] [Google Scholar]
- [10].Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am J Clin Nutr 2006;84:289–98. [DOI] [PubMed] [Google Scholar]
- [11].Guízar-Mendoza JM, Amador-Licona N, Flores-Martínez SE, et al. Association analysis of the Gln223Arg polymorphism in the human leptin receptor gene, and traits related to obesity in Mexican adolescents. J Hum Hypertens 2005;19:341–6. [DOI] [PubMed] [Google Scholar]
- [12].Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976;295:349–53. [DOI] [PubMed] [Google Scholar]
- [13].Rolland-Cachera MF, Deheeger M, Bellisle F, et al. Adiposity rebound in children: a simple indicator for predicting obesity. Am J Clin Nutr 1984;39:129–35. [DOI] [PubMed] [Google Scholar]
- [14].Mărginean C, Mărginean CO, Bănescu C, et al. Impact of demographic, genetic, and bioimpedance factors on gestational weight gain and birth weight in a Romanian population: a cross-sectional study in mothers and their newborns: the Monebo study (STROBE-compliant article). Medicine (Baltimore) 2016;95:e4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].World Health Organization, 2000. Obesity: preventing and managing the global epidemic. Available at: http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ Accessed October 29, 2016. [PubMed] [Google Scholar]
- [16].Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med 1993;119(7 Pt 2):655–60. [DOI] [PubMed] [Google Scholar]
- [17].Loos RJF, Rankinen T. Gene-diet interactions on body weight changes. J Am Diet Assoc 2005;105(5 suppl 1):S29–34. [DOI] [PubMed] [Google Scholar]
- [18].Labayen I, Ruiz JR, Ortega FB, et al. Body size at birth modifies the effect of fat mass and obesity associated (FTO) rs9939609 polymorphism on adiposity in adolescents: the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study. Br J Nutr 2012;107:1498–504. [DOI] [PubMed] [Google Scholar]
- [19].Labayen I, Ruiz JR, Ortega FB, et al. Association between the FTO rs9939609 polymorphism and leptin in European adolescents: a possible link with energy balance control. The HELENA study. Int J Obes (Lond) 2011;35:66–71. [DOI] [PubMed] [Google Scholar]
- [20].Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007;445:168–76. [DOI] [PubMed] [Google Scholar]
- [22].Timpson NJ, Emmett PM, Frayling TM, et al. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr 2008;88:971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Larder R, Cheung MKM, Tung YCL, et al. Where to go with FTO? Trends Endocrinol Metab 2011;22:53–9. [DOI] [PubMed] [Google Scholar]
- [24].Dina C, Meyre D, Gallina S, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 2007;39:724–6. [DOI] [PubMed] [Google Scholar]
- [25].Ruiz JR, Labayen I, Ortega FB, et al. Attenuation of the effect of the FTO rs9939609 polymorphism on total and central body fat by physical activity in adolescents: the HELENA study. Arch Pediatr Adolesc Med 2010;164:328–33. [DOI] [PubMed] [Google Scholar]
- [26].Labayen I, Ruiz JR, Vicente-Rodríguez G, et al. Early life programming of abdominal adiposity in adolescents: the HELENA Study. Diabetes Care 2009;32:2120–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Meirhaeghe A, Boreham CAG, Murray LJ, et al. A possible role for the PPARG Pro12Ala polymorphism in preterm birth. Diabetes 2007;56:494–8. [DOI] [PubMed] [Google Scholar]
- [28].Labayen I, Moreno LA, Marti A, et al. Effect of the Ala12 allele in the PPARgamma-2 gene on the relationship between birth weight and body composition in adolescents: the AVENA study. Pediatr Res 2007;62:615–9. [DOI] [PubMed] [Google Scholar]
- [29].Gahagan S. Kliegman RM, Stanton BF, St Geme JW, III, Schor NF. Overweight and obesity. Nelson's Textbook of Pediatrics, vol. 1 20th ed.Philadelphia: Elsevier; 2016. 307–16. [Google Scholar]
- [30].Moreno LA, González-Gross M, Kersting M, et al. Assessing, understanding and modifying nutritional status, eating habits and physical activity in European adolescents: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr 2008;11:288–99. [DOI] [PubMed] [Google Scholar]
- [31].Souren NY, Paulussen AD, Steyls A, et al. Common SNPs in LEP and LEPR associated with birth weight and type 2 diabetes-related metabolic risk factors in twins. Int J Obes (Lond) 2008;32:1233–9. [DOI] [PubMed] [Google Scholar]
- [32].Hauguel-de Mouzon S, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am J Obstet Gynecol 2006;194:1537–45. [DOI] [PubMed] [Google Scholar]
- [33].Cai G, Cole SA, Butte NF, et al. Genome-wide scan revealed genetic loci for energy metabolism in Hispanic children and adolescents. Int J Obes (Lond) 2008;32:579–85. [DOI] [PubMed] [Google Scholar]
- [34].Chagnon YC, Wilmore JH, Borecki IB, et al. Associations between the leptin receptor gene and adiposity in middle-aged Caucasian males from the HERITAGE family study. J Clin Endocrinol Metab 2000;85:29–34. [DOI] [PubMed] [Google Scholar]
- [35].Mărginean CO, Mărginean C, Voidăzan S, et al. Correlations between leptin gene polymorphisms 223 A/G, 1019 G/A, 492 G/C, 976 C/A, and anthropometrical and biochemical parameters in children with obesity: a prospective case-control study in a Romanian population—the nutrichild study. Medicine (Baltimore) 2016;95:e3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Matsuoka N, Ogawa Y, Hosoda K, et al. Human leptin receptor gene in obese Japanese subjects: evidence against either obesity-causing mutations or association of sequence variants with obesity. Diabetologia 1997;40:1204–10. [DOI] [PubMed] [Google Scholar]
- [37].López-Bermejo A, Petry CJ, Díaz M, et al. The association between the FTO gene and fat mass in humans develops by the postnatal age of two weeks. J Clin Endocrinol Metab 2008;93:1501–5. [DOI] [PubMed] [Google Scholar]
- [38].Chu SY, Kim SY, Bish CL. Prepregnancy obesity prevalence in the United States. Matern Child Health J 2009;13:614–20. [DOI] [PubMed] [Google Scholar]
- [39].Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynaecol Obstet 2006;93:269–74. [DOI] [PubMed] [Google Scholar]
- [40].Dietz PM, Callaghan WM, Sharma AJ. High pregnancy weight gain and risk of excessive fetal growth. Am J Obstet Gynecol 2009;201:51.e1–6. [DOI] [PubMed] [Google Scholar]
- [41].Nohr EA, Vaeth M, Baker JL, et al. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr 2008;87:1750–9. [DOI] [PubMed] [Google Scholar]
- [42].Ng S-K, Olog A, Spinks AB, et al. Risk factors and obstetric complications of large for gestational age births with adjustments for community effects: results from a new cohort study. BMC Public Health 2010;10:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Araz N, Araz M. Frequency of neonatal hypoglycemia in large for gestational age infants of non-diabetic mothers in a community maternity hospital. Acta Medica (Hradec Kralove) 2006;49:237–9. [DOI] [PubMed] [Google Scholar]
- [44].Boney CM, Verma A, Tucker R, et al. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290–6. [DOI] [PubMed] [Google Scholar]
- [45].O’Keeffe MJ, O’Callaghan M, Williams GM, et al. Learning, cognitive, and attentional problems in adolescents born small for gestational age. Pediatrics 2003;112:301–7. [DOI] [PubMed] [Google Scholar]
- [46].Lundgren EM, Tuvemo T. Effects of being born small for gestational age on long-term intellectual performance. Best Pract Res Clin Endocrinol Metab 2008;22:477–88. [DOI] [PubMed] [Google Scholar]
- [47].Meas T, Deghmoun S, Armoogum P, et al. Consequences of being born small for gestational age on body composition: an 8-year follow-up study. J Clin Endocrinol Metab 2008;93:3804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Levy-Marchal C, Jaquet D, Czernichow P. Long-term metabolic consequences of being born small for gestational age. Semin Neonatol 2004;9:67–74. [DOI] [PubMed] [Google Scholar]
- [49].Albertsson-Wikland K, Karlberg J. Postnatal growth of children born small for gestational age. Acta Paediatr Suppl 1997;423:193–5. [DOI] [PubMed] [Google Scholar]
- [50].Vimaleswaran KS, Li S, Zhao JH, et al. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. Am J Clin Nutr 2009;90:425–8. [DOI] [PubMed] [Google Scholar]
- [51].Martins MC, Trujillo J, Farias DR, et al. Association of the FTO (rs9939609) and MC4R (rs17782313) gene polymorphisms with maternal body weight during pregnancy. Nutrition 2016;32:1223–30. [DOI] [PubMed] [Google Scholar]
- [52].Sovio U, Mook-Kanamori DO, Warrington NM, et al. Association between common variation at the FTO locus and changes in body mass index from infancy to late childhood: the complex nature of genetic association through growth and development. PLoS Genet 2011;7:e1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Paracchini V, Pedotti P, Taioli E. Genetics of leptin and obesity: a HuGE review. Am J Epidemiol 2005;162:101–14. [DOI] [PubMed] [Google Scholar]
- [54].Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev 2007;8:21–34. [DOI] [PubMed] [Google Scholar]
- [55].Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998;395:763–70. [DOI] [PubMed] [Google Scholar]
- [56].Rand L, Winchester EC, Millwood IY, et al. Maternal leptin receptor gene variant Gln223Arg is not associated with variation in birth weight or maternal body mass index in UK and South Asian populations. Int J Obes Relat Metab Disord 2001;25:753–5. [DOI] [PubMed] [Google Scholar]
- [57].Mattevi VS, Zembrzuski VM, Hutz MH. Association analysis of genes involved in the leptin-signaling pathway with obesity in Brazil. Int J Obes Relat Metab Disord 2002;26:1179–85. [DOI] [PubMed] [Google Scholar]
- [58].Mergen H, Karaaslan C, Mergen M, et al. LEPR ADBR3 IRS-1 and 5-HTT genes polymorphisms do not associate with obesity. Endocr J 2007;54:89–94. [DOI] [PubMed] [Google Scholar]
- [59].Bender N, Allemann N, Marek D, et al. Association between variants of the leptin receptor gene (LEPR) and overweight: a systematic review and an analysis of the CoLaus study. PLoS ONE 2011;6:e26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pyrzak B, Wisniewska A, Kucharska A, et al. No association of LEPR Gln223Arg polymorphism with leptin, obesity or metabolic disturbances in children. Eur J Med Res 2009;14suppl 4:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


