Supplemental Digital Content is available in the text
Keywords: acute renal injury, cardiac surgery, intensive care unit, meta-analysis, renal replacement therapy
Abstract
This study aimed to investigate the outcomes of acute kidney injury (AKI) after cardiac surgery by the meta-analysis.
Electronic databases PubMed and Embase were searched for relative studies from December 2008 to June 2015. For eligible studies, the R software was conducted to meta-analyze outcomes of AKI patients (AKI group) and none-AKI patients after cardiac surgery (NO AKI group). The chi-square-based Q test and I2 statistic were used for heterogeneity analysis. P < 0.1 or I2 > 50% revealed significant heterogeneity among studies, and then a random effects model was used; otherwise a fixed effect model was performed. Egger's test was performed for publication bias assessment. Subgroup analysis was performed by stratifying AKI definitions and study type.
Totally 17 studies with 9656 subjects (2331 in the AKI group and 7325 in the NO AKI group) were enrolled. Significantly higher renal replacement therapy (RRT) (OR=23.67, 95%CI: 12.58–44.55), mortality (OR = 6.27, 95%CI: 3.58–11.00), serum creatinine (SMD = 1.42, 95%CI: 1.01–1.83), and hospital length of stay (LOS) (SMD = 0.45, 95%CI: 0.02–0.88) were shown in the AKI group compared with patients in the NO AKI group. Subgroup analysis showed that results of only 3 subgroups were reversed indicating that the definition of AKI did not affect its outcomes. Publication bias was only found among studies involving mortality and serum creatinine, but the 2 outcomes were not reversed after correction.
This meta-analysis confirmed the worse outcomes of AKI in patients after cardiac surgery, including higher RRT rates, mortality, and longer hospital LOS than those of NO AKI patients.
1. Introduction
Acute kidney injury (AKI) is described as a complex disease but no accepted standard definition for AKI is available up to now.[1] Generally, AKI occurs as a complication in patients with serious disease and may result in a raise of hospital costs and associate with the high mortality rates.[2] Thus, AKI is considered as an independent predictor of risk of death. Despite recent advances in knowledge of this disease, many aspects in this field, such as the cause, definition, diagnosis and treatment, remain controversial, and inconsistent.[3]
As one of the most common and serious complications of cardiac surgery, AKI is reported to occur in 5% to 33% of pediatric cardiac surgery and 1% to 30% of adult cardiac surgery depending on the definition strictness.[4,5] The mortality of AKI patients after cardiac surgery is as high as 40% to 60%. Several factors, such as age, weight, serum creatinine, have been identified to be associated with the incidence of AKI after cardiac surgery, and patient management during the perioperative period may also significantly affects AKI occurrence.[4,5] Many studies show that the development of AKI significantly influences the prognosis of cardiac surgery.[6] Besides, AKI patients after cardiac surgery are described to be characterized with the short-term mortality and long-term survival.[7–9] Dasta et al[9] found that the patients with AKI after cardiac surgery bear higher total costs, longer length of stay (LOS), and more requirement for renal replacement therapy (RRT). On the contrary, McIlroy et al[10] reported that compared to patients without AKI, AKI patients had neither increase in creatinine level, hospital mortality, RRT, length of ICU stay, or hospital LOS. The above conclusions make the disease much more controversial and elusive with respect to effects of AKI on patients after cardiac surgery.
On this premise, we conducted this systematic meta-analysis to investigate the outcomes of AKI after cardiac surgery, with an attempt to resolve the conflicting issue.
2. Materials and methods
2.1. Data sources
Databases of PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Embase (http://www.embase.com) were systematically searched for relative articles from December 2008 to June 2015. Key words used in this search strategy were “acute kidney injury, AKI, postcardiac surgery, postcardiotomy, after cardiac surgery, and postcardiac surgery.”
2.2. Inclusion criteria
Studies meeting the following criteria were included in this meta-analysis: (i) articles about AKI after cardiac surgery; (ii) articles providing the numbers of AKI patients (AKI group) and non-AKI patients (NO AKI group) who suffered from cardiac surgery; (iii) articles having outcomes such as RRT, mortality, serum creatinine, ICU stay and/or hospital LOS. Studies were excluded (1) if they reported the non-adults patients; (2) if they were reviews, reports, comments, or letters.
2.3. Data extraction and quality assessment
Two authors independently extracted the following data from the eligible studies: the first author, publication time, country, data of study, type of study, counts of patients in the AKI group and the NO AKI group, demographic data (sex, age, weight, or BMI) and the outcomes including RRT, mortality, serum creatinine, ICU stay, and hospital LOS. Any disagreement was resolved by discussion with a third investigator.
The quality of the eligible studies was independently assessed by 2 authors using the Newcastle-Ottawa Scale (NOS).[11] A third investigator was introduced for discussion if there was any disagreement.
2.4. Statistical analysis
Meta-analysis was performed by using the R software (version 3.12, meta package, R Foundation for Statistical Computing, http://cran.r-project.org/). The effect sizes (ES) for dichotomous data were odds ratio (OR) with its 95% CI, whereas standard mean difference (SMD) was used for continuous data. Heterogeneity assessment was conducted by using the Chi-square-based Q test[12] and I2 statistic. The P < 0.1 or I2 > 50% indicated the presence of significant heterogeneity, and then a random effects model was used to pool the ES; otherwise, a fixed effect model was performed.[13] Subgroup analyses for AKI definition and study type were performed. Publication bias was assessed by Egger's test.[14] A trim and fill method was used for correction if there were significantly publication bias.[15] Finally, sensitivity analysis was conducted by omitting each study at a time to estimate the effect of each study on the overall ES.[16]
3. Results
3.1. Characteristics of the included studies
The selection process of included studies for this meta-analysis was displayed in Fig. 1. According to the search strategy, 1937 articles were initially obtained. After removing the duplicates (n = 612), 1325 articles were included. Then, 1162 ones obviously irrelevant to this study were excluded by reviewing for title. After reading the abstracts, 60 articles were further excluded, including 25 reviews, 8 letters, and 27 case reports. Subsequently, the remaining 103 articles were further reviewed for full-texts, of which 35 lacked of necessary information; 23 were not about AKI and 28 were not related to cardiac surgery. Finally, a total of 17 studies [3,6,10,17–30] were enrolled in this meta-analysis.
Figure 1.
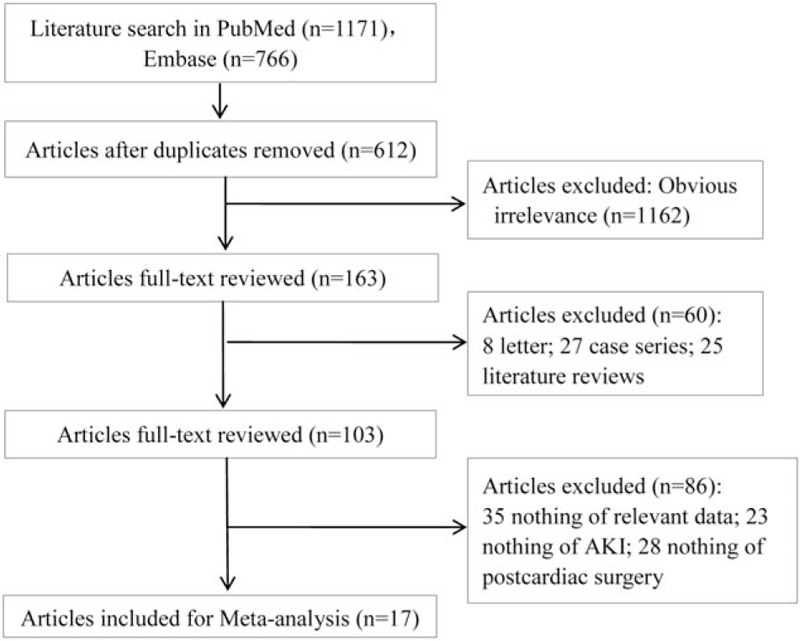
Process of study selection.
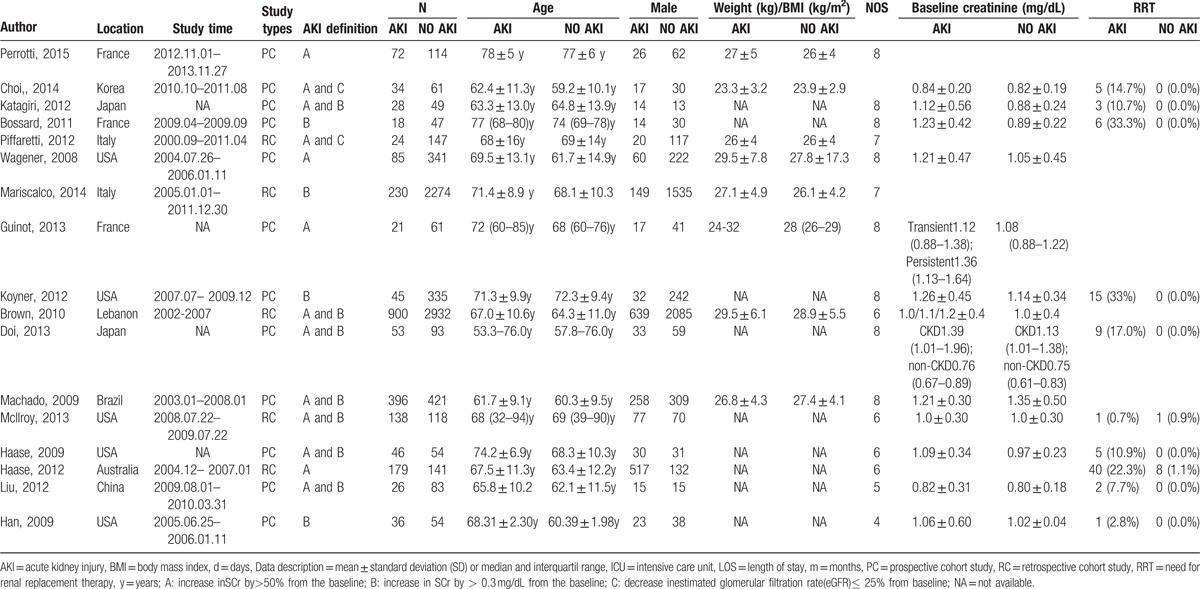
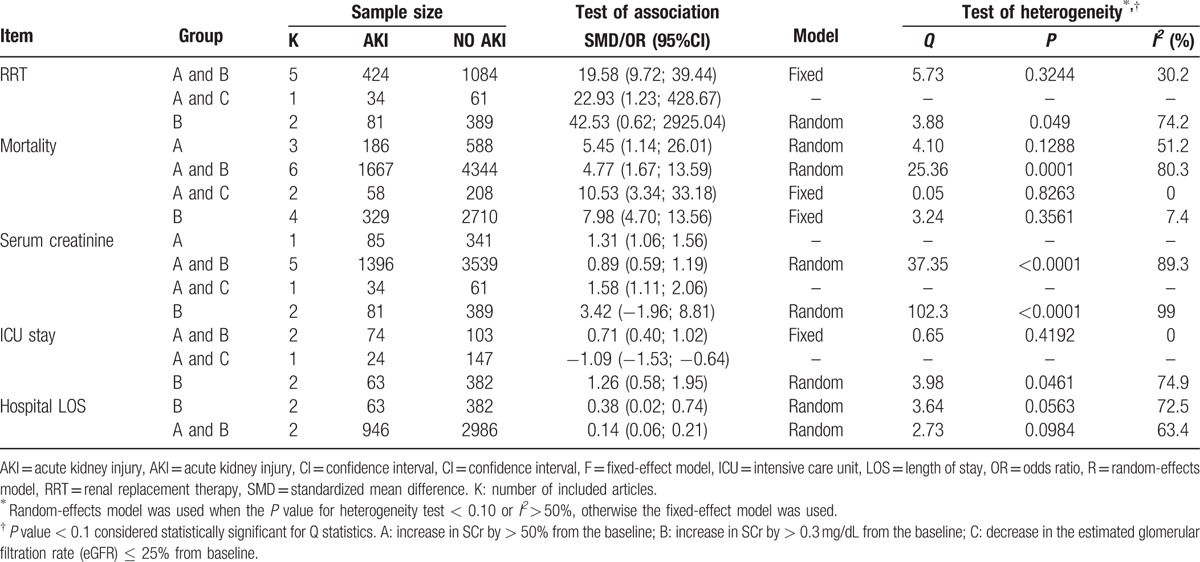
The characteristics of the included studies were listed in Table 1. Totally there were 9656 subjects, including 2331 in the AKI group and 7325 in the NO AKI group. The studies conducted during 2008–2015 were distributed in France, the United States, South Korea, Japan, Italy, and China. All of the eligible studies were designed as cohort studies, among which 5 were retrospective study and the other 12 were prospective study. Definitions of AKI in each study were not identical. There were 3 definitions about AKI; they were A—increase in SCr by > 50% from the baseline; B—increase in SCr by > 0.3 mg/dL from the baseline; C—decrease in the estimated glomerular filtration rate (eGFR) ≤ 25% from baseline. NOS quality assessment showed that all of the eligible studies scored from 4 to 8, indicating the relatively high quality.
Table 1.
Characteristics of the included studies.
3.2. Meta-analysis for the outcomes of AKI after cardiac surgery
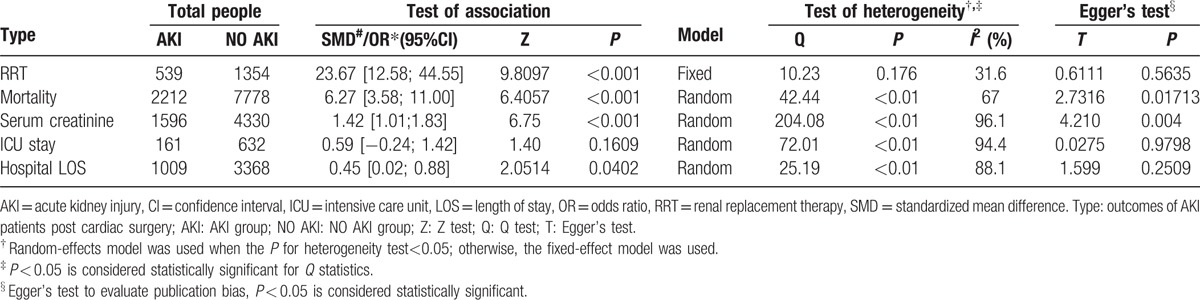
Outcomes of AKI patients post cardiac surgery were meta-analyzed, including RRT (Fig. 2A), mortality (Fig. 2B), serum creatinine (Fig. 2C), ICU stay (Fig. 2D), and hospital LOS (Fig. 2E). Significant heterogeneity was found among studies involving mortality, serum creatinine, ICU stay, and hospital LOS (P < 0.05); thus, the random effects model was performed for meta-analysis of these outcomes, whereas no heterogeneity was found for RRT (P > 0.05) and the fixed effect model was performed. Results showed that patients in the AKI group were characterized with significantly higher RRT use (OR = 23.67, 95%CI: 12.58–44.55, P < 0.001), mortality (OR = 6.27, 95%CI: 3.58–11.00 P < 0.001), serum creatinine (SMD = 1.42, 95%CI: 1.01–1.83, P < 0.001), and LOS (SMD = 0.45, 95%CI: 0.02–0.88, P = 0.04) than those in the NO AKI group. However, no significant differences were found in ICU stay (SMD = 0.59, 95%CI: -0.02–1.42, P < 0.05) between the AKI group and the NO AKI group (Table 2).
Figure 2.
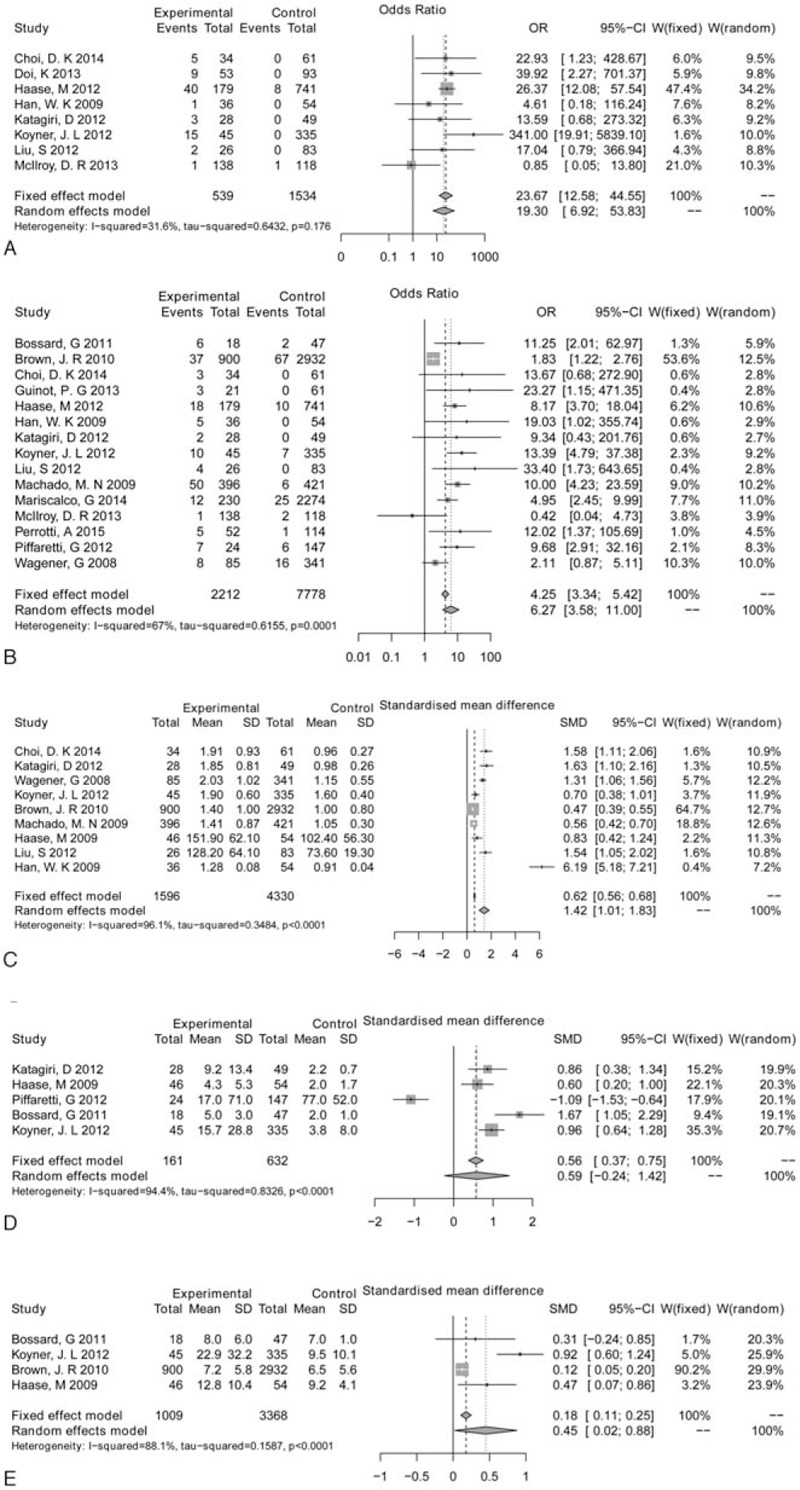
Forest plot of renal replacement therapy (RRT) (A), mortality (B), serum creatinine (C), intensive care unit (ICU) stay (D), and hospital length of stay (LOS) (E).ICU = intensive care unit, LOS = length of stay, RRT = renal replacement therapy.
Table 2.
Results of the meta-analysis.
3.3. Subgroup analysis based on AKI definition
In order to detect if the different AKI definitions are contributed to the outcome of meta-analysis, a subgroup analysis based on AKI definition was conducted in Table 3. Results of this subgroups analysis were inconsistent with the pooled results. For example, there were no significant differences of RRT use between patients in the AKI group and the NO AKI group based on AKI definition of B (OR = 42.53, 95% CI = 0.62–2925.04). For serum creatinine, no significant difference was found between the 2 groups based on definition of B (SMD = 3.42, 95% CI = –1.96–8.81). For ICU stay, even a less ICU stay duration was found in the AKI group than that in the No AKI group based on definition of A and C (OR = –1.09, 95% CI = –1.53; –0.64).
Table 3.
Subgroup analysis based on AKI definitions.
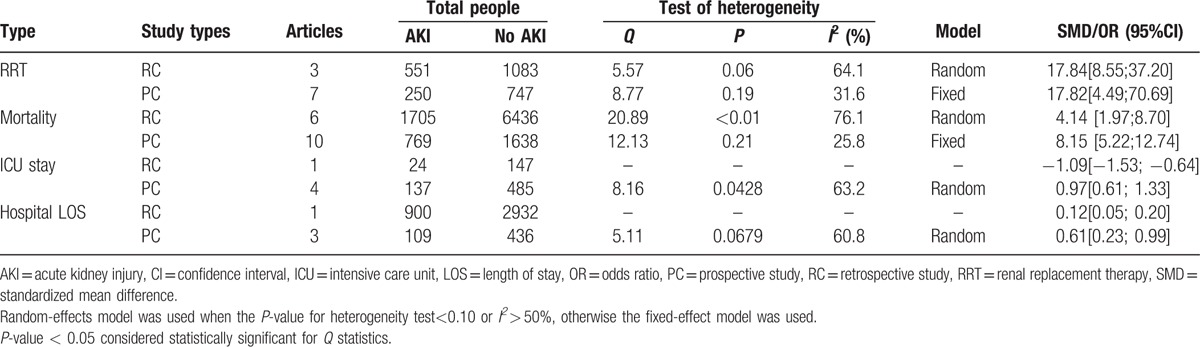
3.4. Subgroup analysis based on study type
The results of subgroup analysis based on the study type were shown in Table 4. Results of subgroups analysis for RRT, mortality, and hospital LOS were consistent with the pooled results, but the ICU stay in prospective study showed longer time.
Table 4.
Subgroup analysis based on study type.
3.5. Publication bias
Egger's test indicated potential publication biases among studies involving mortality (t = 2.7316, P = 0.017) and serum creatinine (t = 4.210, P = 0.004) but not among studies involving other outcomes (Table 2). Thus, trim and fill methods were used for correction of these 2 outcomes (see Figure, Supplemental Figures1 and 2, which illustrates the adjusted forest plot of mortality and serum creatinine by the trim and fill method). For mortality, a total of 6 articles were filled and the corrected OR was 4.08 (95% CI: 2.42–6.89, P < 0.001). However, for serum creatinine, 5 studies were filled and the corrected SMD was 0.55 (95%CI: 0.12–0.97, P < 0.001). Both of the 2 outcomes were not obviously changed after correction, which indicated the relatively credible of our results.
3.6. Sensitivity analysis
Sensitivity analyses obtained generally similar results with main analyses as to RRT, mortality, and serum creatinine (data not presented). However, for ICU stay and hospital LOS, the results were reversed when removing certain studies. Omitting the study by Piffaretti et al[24] (SMD = 0.34, 95% CI: 0.56–1.23) reversed the pooled effect of ICU stay on AKI; omitting the study by Bossard et al[20] and Haase et al[18] reversed the pooled effect of hospital LOS on AKI, indicating that the result for ICU stay and hospital LOS might be not robust (Fig. 3).
Figure 3.
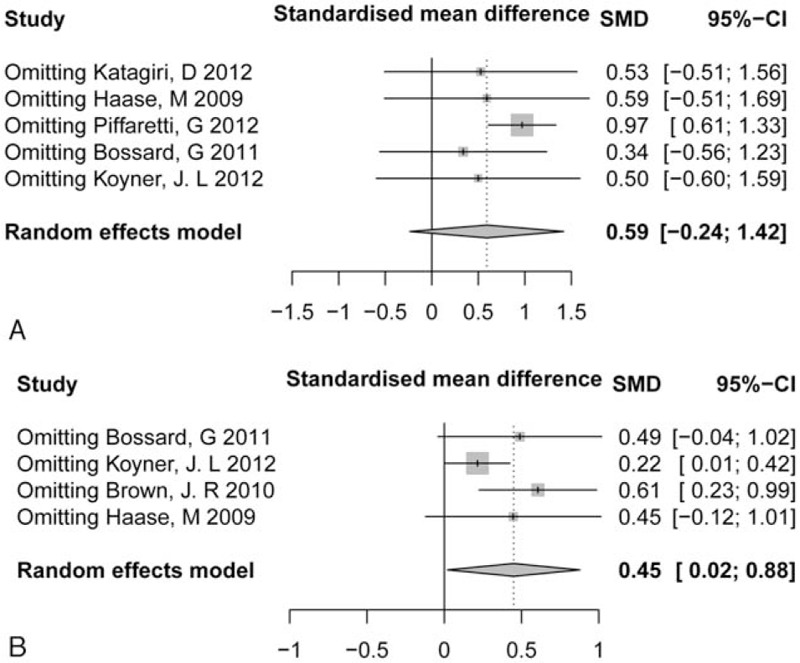
Sensitivity analysis of intensive care unit (ICU) stay (A) and hospital length of stay (LOS) (B). ICU = intensive care unit, LOS = length of stay .
4. Discussion
AKI is a common complication after cardiac surgery. Although continuous improvements have been made in understanding the pathophysiology and clinical treatment of AKI in recent years, the specific effects of AKI occurrence on patients with cardiac surgery remain inconsistent.[18] Thus, we investigated the AKI outcomes in patients with cardiac surgery, and the results supported the view that AKI after cardiac surgery involves in higher RRT, mortality, serum creatinine, and longer hospital LOS. However, AKI after cardiac surgery did not associate with the length of ICU. This hints that postcardiac surgery AKI is a serious complication that it should be prevented or suppressed.
A previous meta-analysis has assessed whether the definition of acute kidney disease modify the estimated association of AKI with cardiopulmonary bypass (CBP), and concluded that CBP-associated AKI is associated with a more than 2-fold increase in early mortality regardless of AKI definition.[31] This study was limited the cardiac surgery to CBP, and other types of heart surgery. In the present study, we systematically searched studies involving outcomes of AKI after all kinds of cardiac surgery. The results suggest that AKI were associated with a 6.19-fold of mortality with a 95%CI as 3.66–10.48 and as high as 19.73-fold of RRT use due to acute renal failure. Besides, AKI patients after heart surgery were found to be with higher serum creatinine and longer hospital LOS than those of NO AKI patients with SMD as 1.42, and 0.45 respectively. These results revealed significant influence of AKI on prognosis in patient with cardio-surgery.
Prediction of postcardiac surgery AKI has drawn insensitive attention due to its independent association with increased morbidity and mortality. Several risk factors have been reported as potential predictors for AKI after cardiac surgery, such as hemodilution during CBP and intraoperative renal regional oxygen desaturation, postoperative extubation time, congestive cardiac failure, and pulmonary complication.[28,32,33] Further on, exploring of the urinary biomarkers of postcardiac surgery AKI has achieved prominent results. For example, urinary neutrophil gelatinase-associated lipocalin (NGAL)or cystatin C alone [34] is acknowledged the early predictors of postcardiac surgery AKI.[7,8,17,18] However, the combination of NGAL with other biomarkers like kidney injury molecule-1,N-acetyl-β-D-glucosaminidase [6] and cystatin C [18] can enhance the sensitivity of early detection of postcardiac surgery AKI. By early detection of these biomarkers, we could predict the occurrence of AKI and take steps to suppress AKI, and to further improve the survival of the patients.
In respect to the AKI definition, no accepted standard definition for AKI is available up to now.[1] Therefore, we performed the subgroup analysis to detect if the AKI definition contributes to the heterogeneity of this meta-analysis. As a result, some subgroups showed different results from the pooled results, indicating that definitions might play an important role in affecting outcomes of AKI in patients after cardiac surgery. However, AKI is not a single lesion but rather a syndrome that comprising multiple clinical conditions. Outcomes of AKI rely on the underlying disease, the duration and the severity of renal impairment, as well as the patients’ renal baseline condition.[35] Therefore, the association between AKI definition and outcomes of AKI after cardiac surgery might be confounded by other factors.
Several disadvantages in this study have to be acknowledged. First, the high heterogeneity among studies was the prominent deficiency, which might result from the following sources: different determination methods for AKI or the different units for the indexes; different areas and ethnic lines; the influences of age, sex, and other confounding factors. Second, although subgroup analysis to some extent decreased the heterogeneity, it was insufficient due to the lack of original data. Third, meta-regression analysis did not find the exact causes inducing the high heterogeneity, due to the insufficient data in the original studies. Finally, the results for ICU stays and hospital LOS were not robust, so more clinical studies were needed.
Despite the disadvantages, the results in the present study are still important for future research and clinical prevention of AKI during cardiac surgery. In addition, this study systematically analyzed the data of 17 studies including 9656 subjects, which presented a high power of test.
In summary, postcardiac surgery AKI patients showed higher risk of RRT use and mortality, higher level of serum creatinine, and longer hospital LOS than NO AKI patients, indicating that close attention should be paid on this population.
Supplementary Material
Footnotes
Abbreviations: AKI = acute kidney injury, CBP = cardiopulmonary bypass, eGFR = estimated glomerular filtration rate, ES = effect sizes, LOS = length of stay, NGAL = neutrophil gelatinase-associated lipocalin, NOS = Newcastle–Ottawa Scale, OR = odds ratio, RRT = renal replacement therapy, SCr = serum creatinine, SMD = standard mean difference.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Bove T, Calabro MG, Landoni G, et al. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth 2004;18:442–5. [DOI] [PubMed] [Google Scholar]
- [2].Stafford-Smith M, Shaw A, Swaminathan M. Cardiac surgery and acute kidney injury: emerging concepts. Curr Opin Crit Care 2009;15:498–502. [DOI] [PubMed] [Google Scholar]
- [3].Machado MN, Miranda RC, Takakura IT, et al. Acute kidney injury after on-pump coronary artery bypass graft surgery. Arquivos Brasileiros Cardiol 2009;93:247–52. [DOI] [PubMed] [Google Scholar]
- [4].Li S, Krawczeski CD, Zappitelli M, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery–a prospective multicenter study. Crit Care Med 2011;39:1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Parolari A, Pesce LL, Pacini D, et al. Risk factors for perioperative acute kidney injury after adult cardiac surgery: role of perioperative management. Ann Thorac Surg 2012;93:584–91. [DOI] [PubMed] [Google Scholar]
- [6].Han WK, Wagener G, Zhu Y, et al. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 2009;4:873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wagener G, Jan M, Kim M, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 2006;105:485. [DOI] [PubMed] [Google Scholar]
- [8].Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 2012;23:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dasta JF, Kane-Gill SL, Durtschi AJ, et al. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008;23:1970–4. [DOI] [PubMed] [Google Scholar]
- [10].McIlroy DR, Argenziano M, Farkas D, et al. Incorporating oliguria into the diagnostic criteria for acute kidney injury after on-pump cardiac surgery: impact on incidence and outcomes. J Cardiothorac Vasc Anesth 2013;27:1145–52. [DOI] [PubMed] [Google Scholar]
- [11].Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. 2011[J] 2015. [Google Scholar]
- [12].Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Int Med 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [13].Feng R-N, Zhao C, Sun C-H, et al. Meta-analysis of TNF 308 G/A polymorphism and type 2 diabetes mellitus. PLoS One 2011;6:e18480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Luo AJ, Wang FZ, Luo D, et al. Consumption of vegetables may reduce the risk of liver cancer: Results from a meta-analysis of case-control and cohort studies. Clin Res Hepatol Gastroenterol 2014;39:45–51. [DOI] [PubMed] [Google Scholar]
- [15].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [16].Liu ZH, Ding YL, Xiu LC, et al. A meta-analysis of the association between TNF-alpha -308G>A polymorphism and type 2 diabetes mellitus in Han Chinese population. PLoS One 2013;8:e59421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wagener G, Gubitosa G, Wang S, et al. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis 2008;52:425–33. [DOI] [PubMed] [Google Scholar]
- [18].Haase M, Bellomo R, Devarajan P, et al. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg 2009;88:124–30. [DOI] [PubMed] [Google Scholar]
- [19].Brown JR, Kramer RS, Coca SG, et al. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg 2010;90:1142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bossard G, Bourgoin P, Corbeau JJ, et al. Early detection of postoperative acute kidney injury by Doppler renal resistive index in cardiac surgery with cardiopulmonary bypass. Brit J Anaesth 2011;107:891–8. [DOI] [PubMed] [Google Scholar]
- [21].Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012;27:153–60. [DOI] [PubMed] [Google Scholar]
- [22].Katagiri D, Doi K, Honda K, et al. Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann Thorac Surg 2012;93:577–83. [DOI] [PubMed] [Google Scholar]
- [23].Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 2012;23:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Piffaretti G, Mariscalco G, Bonardelli S, et al. Predictors and outcomes of acute kidney injury after thoracic aortic endograft repair. J Vasc Surg 2012;56:1527–34. [DOI] [PubMed] [Google Scholar]
- [25].Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care (London, England) 2013;17:R270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Guinot PG, Bernard E, Abou Arab O, et al. Doppler-based renal resistive index can assess progression of acute kidney injury in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 2013;27:890–6. [DOI] [PubMed] [Google Scholar]
- [27].Liu S, Che M, Xue S, et al. Urinary L-FABP and its combination with urinary NGAL in early diagnosis of acute kidney injury after cardiac surgery in adult patients. Biomarkers 2013;18:95–101. [DOI] [PubMed] [Google Scholar]
- [28].Choi DK, Kim WJ, Chin JH, et al. Intraoperative renal regional oxygen desaturation can be a predictor for acute kidney injury after cardiac surgery. J Cardiothorac Vasc Anesth 2014;28:564–71. [DOI] [PubMed] [Google Scholar]
- [29].Mariscalco G, Cottini M, Dominici C, et al. The effect of timing of cardiac catheterization on acute kidney injury after cardiac surgery is influenced by the type of operation. Int J Cardiol 2014;173:46–54. [DOI] [PubMed] [Google Scholar]
- [30].Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015;99:864–9. [DOI] [PubMed] [Google Scholar]
- [31].Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015;65:283–93. [DOI] [PubMed] [Google Scholar]
- [32].Doddakula K, Al-Sarraf N, Gately K, et al. Predictors of acute renal failure requiring renal replacement therapy post cardiac surgery in patients with preoperatively normal renal function. Interact Cardiovasc Thoracic Surg 2007;6:314–8. [DOI] [PubMed] [Google Scholar]
- [33].Karkouti K, Beattie W, Wijeysundera D, et al. Hemodilution during cardiopulmonary bypass is an independent risk factor for acute renal failure in adult cardiac surgery. J Thorac Cardiovasc Surg 2005;129:391–400. [DOI] [PubMed] [Google Scholar]
- [34].Hassinger AB, Backer CL, Lane JC, et al. Predictive power of serum cystatin C to detect acute kidney injury and pediatric-modified RIFLE class in children undergoing cardiac surgery. Pediatr Crit Care Med 2012;13:435–40. [DOI] [PubMed] [Google Scholar]
- [35].Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int 2011;81:819–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


