Supplemental Digital Content is available in the text
Keywords: continuous ambulatory peritoneal dialysis, patient survival, peritonitis, technique failure
Abstract
Peritonitis remains a major complication of peritoneal dialysis (PD). A high peritonitis rate (HPR) affects continuous ambulatory peritoneal dialysis (CAPD) patients’ technique survival and mortality. Predictors and outcomes of HPR, rather than the first peritonitis episode, were rarely studied in the Chinese population. In this study, we examined the risk factors associated with HPR and its effects on clinical outcomes in CAPD patients.
This is a single center, retrospective, observational cohort study. A total of 294 patients who developing at least 1 episode of peritonitis were followed up from March 1st, 2002, to July 31, 2014, in our PD center. Multivariate logistic regression was used to determine the factors associated with HPR, and the Cox proportional hazard model was conducted to assess the effects of HPR on clinical outcomes.
During the study period of 2917.5 patient-years, 489 episodes of peritonitis were recorded, and the total peritonitis rate was 0.168 episodes per patient-year. The multivariate analysis showed that factors associated with HPR include a quick occurrence of peritonitis after CAPD initiation (shorter than 12 months), and a low serum albumin level at the start of CAPD. In the Cox proportional hazard model, HPR was a significant predictor of technique failure. There were no differences between HPR and low peritonitis rate (LPR) group for all-cause mortality. However, when the peritonitis rate was considered as a continuous variable, a positive correlation was observed between the peritonitis rate and mortality.
We found the quick peritonitis occurrence after CAPD and the low serum albumin level before CAPD were strongly associated with an HPR. Also, our results verified that HPR was positively correlated with technique failure. More importantly, the increase in the peritonitis rate suggested a higher risk of all-cause mortality.
These results may help to identify and target patients who are at higher risk of HPR at the start of CAPD and to take interventions to reduce peritonitis incidence and improve clinical outcomes.
1. Introduction
Peritoneal dialysis (PD) is one of the main renal replacement treatments. Peritonitis remains the major complication and primary reason of technique failure in PD patients.[1] Peritonitis-related mortality was not significantly improved in recent years and varied from 2.8% to 8.8% of episodes according to several reports.[2–4] Despite the advanced prevention and therapy applied, peritonitis was still considered to play an important role in mortality of PD patients. It has been reported that frequent peritonitis accounts for a higher risk of mortality, independent of other factors.[5] A study identified that the peritonitis rate was associated with technique failure and predicted mortality.[6] Another study confirmed that episodes of peritonitis had a negative impact on long-term survival of PD patients.[7]
Peritonitis is harmful to the peritoneum. Long-term peritoneal dialysis causes structural changes in the peritoneal membrane, leading to peritoneal fibrosis,[8] and peritonitis accelerates this process.[9] The intensity of peritoneal inflammation and also the frequency of infection has an impact on the peritoneal function. Multiple or recurrent episodes could cause membrane permeability changes and ultrafiltration declines with time on PD, which finally leads to technique failure.
Predictors of peritonitis, such as lower education level, age, gender, diabetes mellitus, lower serum albumin level at the start of continuous ambulatory peritoneal dialysis (CAPD), have been previously identified.[10–12] Moreover, incidence and predictors of peritonitis differed based on the geographical region.[13]
A high peritonitis rate (HPR) was negatively correlated with technique failure and mortality according to current studies.[6,7] However, to our knowledge, there were fewer studies about the clinical outcomes and risk factors associated with an HPR in Chinese CAPD patients. To obtain an improved understanding of predictors of HPR and its impact on clinical outcomes, we conducted this retrospective study.
2. Materials and methods
2.1. Patient population
This retrospective study involved incidents of all the patients requiring CAPD followed up in our peritoneal dialysis (PD) Center, the First Affiliated Hospital of Zhejiang University, from March 1st, 2002 to July 31, 2014. All patients we studied remained on PD at least 90 days. The exclusion criteria were patients who started CAPD in other centers and followed up in our center, age less than 18 years, and remaining on PD less than 90 days. All data were derived from patient profiles from our center. Records from a total of 1473 CAPD patients were screened for study eligibility. Among these 1473 patients, 294 patients who developing at least 1 episode of peritonitis during the study period, were study objects. This study was approved by the Human Ethics Committee of the First Affiliated Hospital of Zhejiang University. The total peritonitis rate was 0.168 episodes per patient-year (294 patients presented 489 episodes of peritonitis during 2917.5 patient-years). According to the median of peritonitis rate (0.532/patient-year), we dichotomized study objects into 2 groups: low peritonitis rate (LPR, <0.532/patient-year, n = 147) and high peritonitis rate (HPR, ≥0.532/patient-year, n = 147). Patients were followed up until death, renal transplantation, switch to hemodialysis (HD), or the end day of the study on July 31, 2014. The baseline characteristics within 1 to 3 months after the initiation of PD therapy were collected, including demographic data (age, gender, education level), body mass index, Charlson comorbidity index (CCI) score, major comorbidities at the start of PD therapy (hypertension, diabetes, cardiovascular disease), time to first period of peritonitis, biochemical data, relevant PD adequacy indices, and microbiological characteristics of the first episode of peritonitis. Patients received a peritoneal equilibration test within the first 1 to 3 months after PD start.
2.2. Definition of peritonitis and clinical outcomes
The diagnosis of peritonitis must meet at least 2 of the following criteria according to the 2010 International Society for Peritoneal Dialysis (ISPD) guidelines: clinical symptoms, leukocytosis in peritoneal fluid effluent (white cell count at least 100/mm3, with at least 50% polymorphonuclear neutrophilic cells), and positive culture of PD fluid.[14]
The clinical outcomes in this study included all-cause mortality and technique failure. For the analysis of all-cause mortality, death was considered as the end-point event, whereas transfer to HD and renal transplantation were censored observations. The technique failure was defined as the switch from PD therapy to HD therapy permanently due to all kinds of operational problems, such as inadequate dialysis, exit-site infection, peritonitis, and so on. Those lost to follow-up are also seen as censored observations in analysis of clinical outcomes.
2.3. Statistical analysis
Results were expressed as means ± standard deviation for normally distributed data and median values with the interval from the 25th to the 75th percentile for skewed data. Categorical data were presented as frequency (n) and percentage (%). Differences between groups were analyzed by Student's t test for normally distributed data, the Wilcoxon rank sum test for skewed continuous data and Chi-square test or Fisher's exact test for categorical data. The Kaplan–Meier survival curve was conducted for the analysis of technique survival and patient survival. Univariate Cox proportional hazard regression was conducted to select significant variables associated with clinical outcomes. The inclusion criterion for variables selected to the final multivariate Cox model was P < 0.10. Collinearity of variables was tested. The multivariate logistic regression model was performed to select significant predictors for HPR and the inclusion criterion was also P < 0.10. A 2-tailed P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 19.0 (IBM SPSS Statistics 19. Inc.) software.
3. Results
3.1. Population and microbiologic characteristics
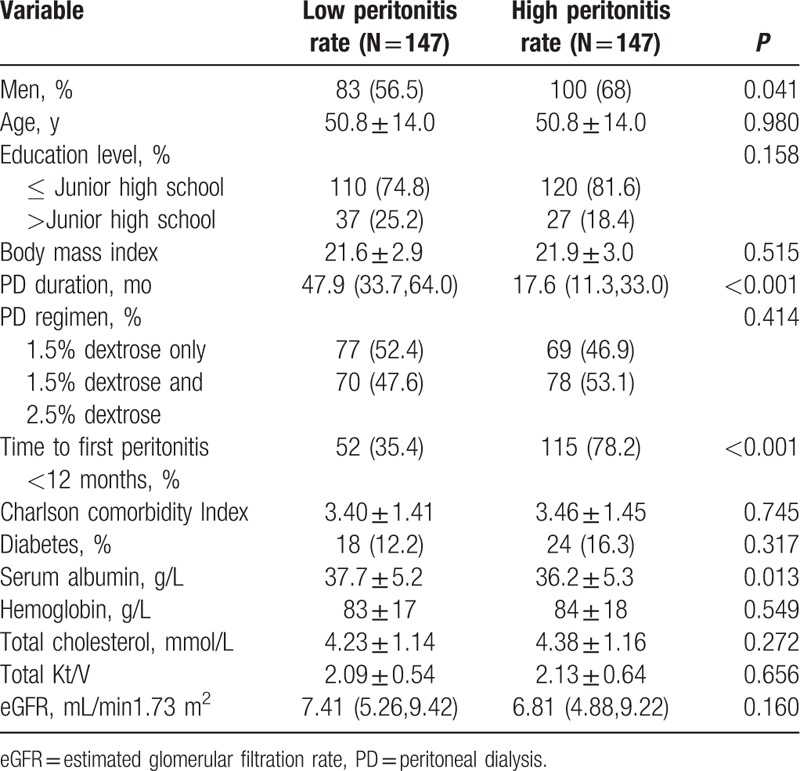
In a total of 1473 CAPD patients, 294 patients who were 18 years or older, stayed on PD at least 90 days and developed at least 1 episode of peritonitis, were eligible for the study. Conventional PD solutions were used by all of the patients (dianeal with 1.5% or 2.5% dextrose; Baxter Healthcare, Hangzhou, China). The systems utilized in PD patients were Y-sets and twin-bag. Mupirocin ointment was utilized in every PD patient to prevent exit infection. Each patient performed 3 to 4 cycles per 24 hours and the fill volume was usually 2 liters per 1.73 m2. The study population was followed up for a median of 33.3 months (interquartile range 17.3–52.8 months). The mean age of 294 peritonitis CAPD patients was 50.8 ± 14.0 years and 62.2% of patients were male. Clinical characteristics at commencement of the incident in 294 patients are shown in Table 1. Compared with the low peritonitis rate (LPR) patients group, HPR patients group had more male patients, shorter time to their first peritonitis, and lower levels of serum albumin (P < 0.05).
Table 1.
Baseline clinical characteristics and laboratory biochemistry data.
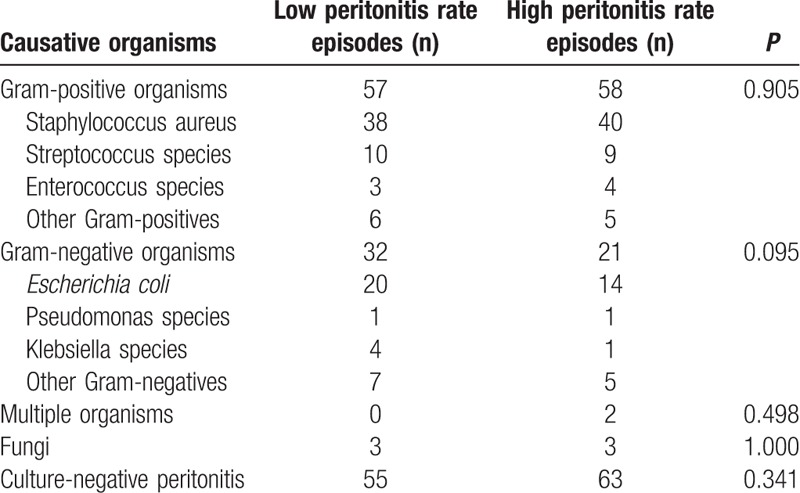
The causative organisms of the first episode of peritonitis of the 294 incidents are summarized below: 115 (39.1%) were due to gram-positive organisms, 53 (18.0%) were due to gram-negative organisms, 2 (0.7%) to multiple organisms, 6 (2.1%) to fungi and 118 (40.1%) culture-negative peritonitis were observed. Table 2 shows the microbiologic spectrum of the first episode of peritonitis according to the LPR and HPR groups. No significant microbiologic spectrum differences were found between LPR and HPR patient groups.
Table 2.
Details of causative organisms of the first peritonitis.
3.2. Technique failure
The univariate Cox analysis of risk factors for the technique failure is shown in the Supplementary Table 1. Adjusted for time to first peritonitis, diabetes, and serum albumin in the multivariate Cox proportional hazard model for technique failure, HPR was significantly associated with technique failure compared with LPR subjects, with hazard ratio (HR) of 4.512 (Table 3, P < 0.001). The cumulative technique survival was shown in Fig. 1.
Table 3.
Cox proportional hazard model for technique failure.
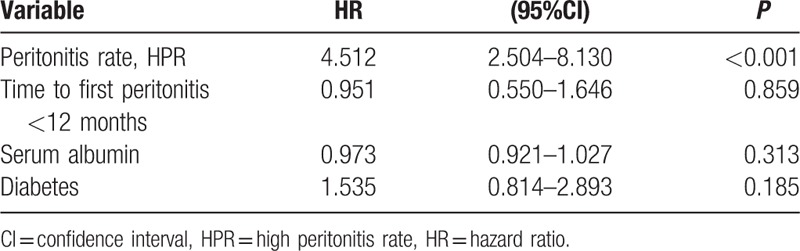
Figure 1.
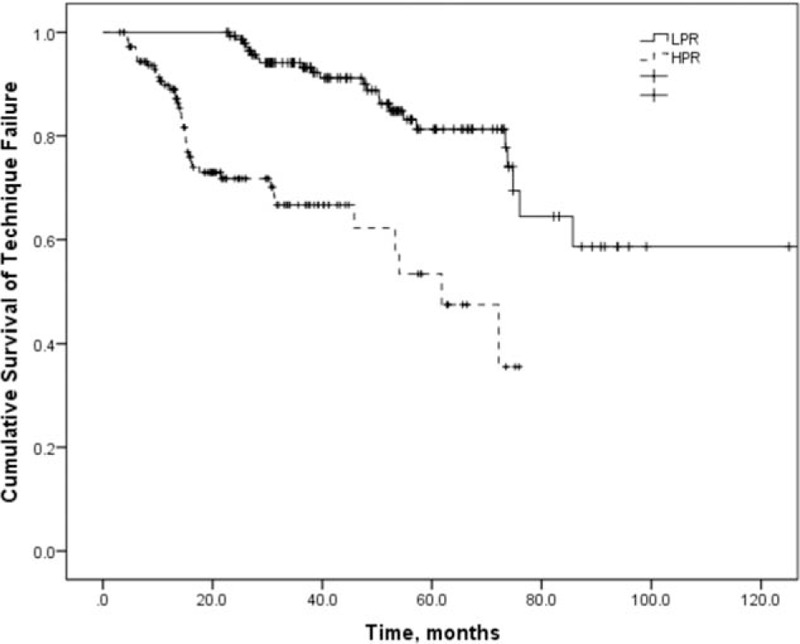
Technique survival of CAPD patients according to the peritonitis rate. CAPD = continuous ambulatory peritoneal dialysis.
3.3. All-cause mortality
In total, 19 patients died in the LPR group and 12 patients in the HPR group. The most common causes were cerebrovascular disease (29.0%) and infection (19.4%). All-cause mortality did not differ between LPR and HPR groups in the multivariate Cox proportional hazard model adjusted for age, time to first peritonitis, total cholesterol, diabetes, eGFR, CCI score, and serum albumin (Table 4 and Supplementary Table 2). There was a relative risk of 2.179 for all-cause mortality for the increase in every 1 episode/patient-year in the peritonitis rate (Table 5, P = 0.004). Figure 2 showed the patient survival according to HPR and LPR groups.
Table 4.
Cox proportional hazard model for mortality (by group).
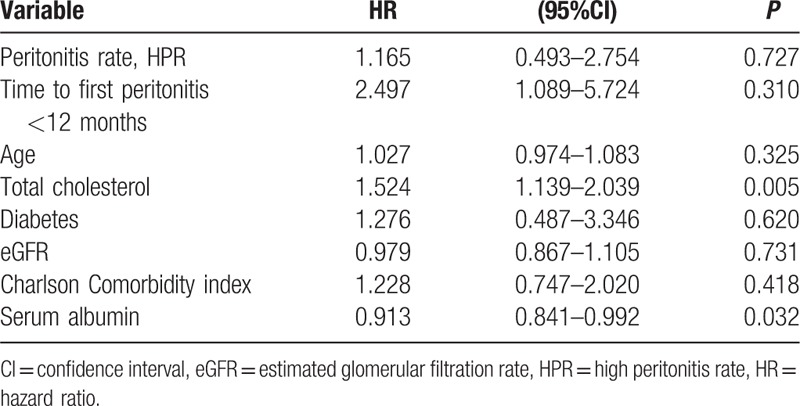
Table 5.
Cox proportional hazard model for mortality (by rate).
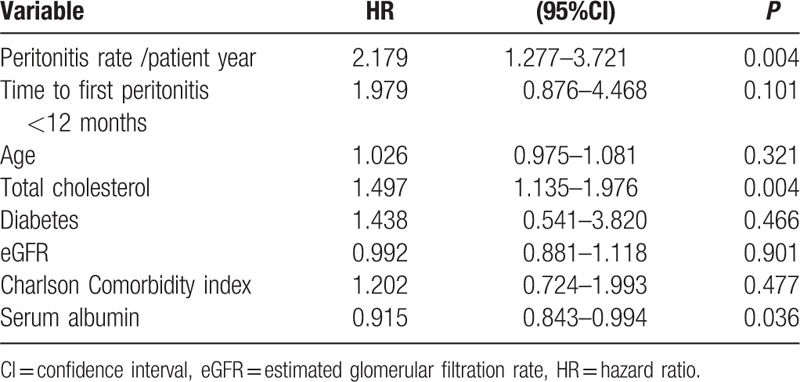
Figure 2.
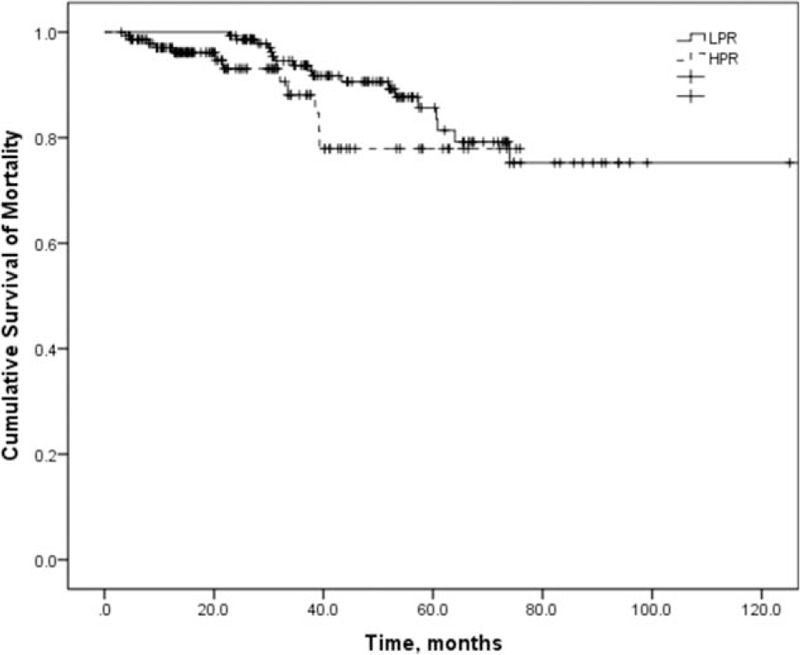
Patient survival according to the peritonitis rate.
3.4. Risk factors of high peritonitis rate
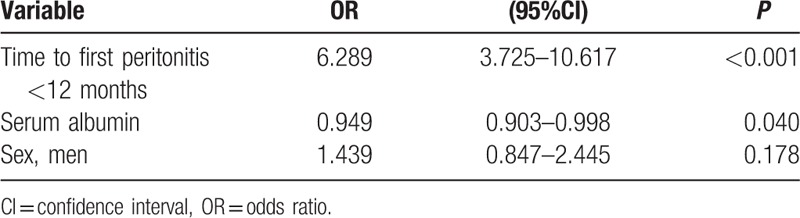
We constructed a multiple logistic regression model for the analysis of HPR. Variables used in this model were time to first peritonitis, serum albumin, and sex according to the simple logistic regression analysis of factors associated with HPR (Supplementary Table 3). Two independent factors were identified as risk factors for HPR, namely, time to first peritonitis shorter than 12 months and serum albumin at the start of CAPD (Table 6). Patients who had their first peritonitis episode <12 months after initiating CAPD were shown to have a significant increase in the risk of an HPR than those who had a longer time before the episode, with odds ratio (OR) of 6.289, P < 0.001. Serum albumin was another predictor of HPR. Every 1 g/L increase of serum albumin concentration at the start of CAPD lower the risk of HPR by 5.1% (OR 0.949, P = 0.04).
Table 6.
Multiple logistic regression analysis of factors associated with high peritonitis rate.
4. Discussion
Our retrospective study showed that HPR was still an independent predictor of technique failure. And a positive correlation was observed between the peritonitis rate and mortality. A quick occurrence of peritonitis after CAPD initiation (shorter than 12 months), and a low serum albumin level at the start of CAPD were associated with HPR.
Our results identified that first peritonitis occurred within 12 months after start of CAPD was an independent risk factor for HPR (OR 6.289, P < 0.001). There were few studies to investigate the relationship between the timing to first peritonitis and peritonitis rate. A retrospective study conducted by Hsieh et al[15] identified early peritonitis patients (< 20.28 months) were prone to have a higher peritonitis rate. They found an inverse correlation between the peritonitis rate and the timing to first peritonitis episode in PD patients. Late-onset peritonitis was referred to as a low peritonitis rate in that study, which is consistent with our results. A Greek researcher reported that patients who presented the first peritonitis episodes within the first 12 months on PD were more likely to present with repeated episodes of peritonitis and worse technique survival compared with those having the first peritonitis episodes later than 24 months on PD.[16] In elderly PD patients, the early peritonitis group (within 6 months after the initiation of PD) also had a higher peritonitis rate than the late peritonitis group, with 1 per 23.7 patient-months versus 1 per 36.4 patient-months. Similar results were shown in a recent published report from China.[17] According to their data, the higher occurrence rate of peritonitis was observed in early-onset peritonitis group (shorter than 3 months after PD initiation), compared to the late-onset group. In our study, there were more quick peritonitis occurrence patients in the HPR group than in the LPR group (P < 0.001).
Low baseline serum albumin had been reported as a novel predictor for peritonitis in previous studies. And our study reaffirmed its independent predictive value on HPR of CAPD patients. The risk of peritonitis increased by 74% and 67% with every 10 g/L decrease of serum albumin in studies from the United States[18] and Hong Kong.[12] In 1 study from the United States, Wang et al[18] found that patients with a baseline serum albumin level lower than 29 g/L were prone to have a higher peritonitis rate (2.5 times greater than patients with higher serum albumin levels). We believe serum albumin is a marker of malnutrition; thus, the serum albumin level is the reflection of nutritional status in PD patients. Hypoalbuminemia has been reported to be associated with malnutrition or inflammation in renal failure patients and may increase the risk of infection. The reduced albumin was also related to severe peritonitis and worse outcomes in CAPD patients.[19] The severity of peritonitis and number of peritonitis episodes were significantly related to malnutrition compared to patients with normal nutritional status.[20] Based on this evidence, we believe there is a strong correlation between peritonitis rate and low baseline serum albumin. In our study, every 1 g/L increase of serum albumin concentration at the start of CAPD decreased the risk of HPR by 5.1% (OR 0.949, P = 0.04). So more attention should be paid to malnutrition in CAPD patients during PD.
The correlation between peritonitis and technique failure has been investigated in previous studies.[21,22] Peritonitis is reported to be the leading cause of technique failure in PD patients. A recent study indicated that peritonitis-related technique failure did not decrease over time in Canada, and peritonitis is still the main reason for technique failure, though the peritonitis rate in PD patients declined over time.[23] In contrast, Nakamoto et al[24] reported that the main reason for technique failure had changed from peritonitis to overhydration, which may be partly explained by the low peritonitis rate in Japan. Peritonitis has a negative impact on the peritoneal membrane. Compared with a single episode of peritonitis, frequent peritonitis episodes may have significant effects on membrane permeability and ultrafiltration reduction, which leads to technique failure and withdrawal of PD.[25] A study in Scotland showed peritonitis accounted for 42.6% for all-cause technique failure in PD patients, and main pathogen of it was Coagulase-negative Staphylococcus.[26] Similar results were found in a study of the Tokai area of Japan. In that study, the main cause of PD patients transferring to HD from 2005 to 2007 was PD-related peritonitis (27%), followed by dialysis failure (21.3%).[27] The peritonitis rate was a strong predictor for technique failure in a Turkish retrospective study, with a relative risk of 3.22(P < 0.001).[6] In the present study, HPR was associated with a significant increase in the risk of technique failure compared with LPR subjects (HR 4.512, P < 0.001).
In our study, the main cause of death was cerebrovascular disease (29.0%), followed by infection (19.4%). It was reported that there was a strong correlation between peritonitis and death from cardiovascular or cerebrovascular disease.[28] Ghali et al[29] reported that peritonitis accounted for 2.6% of deaths in PD patients and many deaths occurred within 30 days of peritonitis due to cardiovascular disease and social reasons. A recent case-crossover study including 1316 PD patients in Australia and New Zealand found that the risk of death increased up to 120 days after peritonitis occurrence.[30] This could be explained by the persistent systemic inflammation after an episode of peritonitis, which may lead to cerebrovascular disease.[31] Our findings supported this hypothesis.
Previous studies reported that peritonitis was a risk factor for death in PD patients.[5,22] Fontan et al[2] found that the incidence of peritonitis was an independent predictor of overall mortality. A retrospective study from South Africa showed that frequent peritonitis, rather than a single episode, was significantly associated with adverse clinical outcomes including death in CAPD patients.[32] The risk of death increased by 87% with every 1 episode/patient-year increase in a report from Turkey.[6] de Bustillo et al[7] reported that each episode of peritonitis was significantly associated with increased risk of death in patients on PD. Consistent with previous studies, we found the peritonitis rate was a significant predictor for mortality with a hazard ratio of 2.179 for every 1 episode/patient-year increase (P = 0.004). Although there were no significant differences in all-cause mortalities between the HPR and LPR groups (P > 0.05), this result may be caused by inappropriate group division. Further studies should be done to find a better process for group division for mortality studies.
There was a high proportion of culture-negative episodes for the first peritonitis in the present study. This may be partly caused by early antibiotic use in a local hospital before patients transferred to our center. Also, we should check the methods used in our laboratory and improve technique protocols to optimize results in culture.
There are several limitations in our study. First, we did not include data on patients’ hygiene habits, whether the dialysis operated correctly throughout the process and nurse training, which could also present risk factors for HPR. Second, our study was retrospective and some drop-out reasons were missing. This could lead to biased results. Third, automated PD patients were not included in our study because only a small number of patients on this modality appeared in our center. Causal relations implicated in analysis cannot be extrapolated for these patients. Finally, our study was based on data from a single-center and the sample size was relatively small. Hence, further studies involving multicenter data are needed to enhance the validity of our study.
The strengths of the present study include its homogenous sampling limited exclusively to CAPD and long follow-up time over 10 years. Moreover, to our best knowledge, this is the largest Southern China study to address that the quick occurrence of the first peritonitis shorter than 12 months and low baseline serum albumin are predictors for HPR. In line with previous reports, HPR is significantly associated with technique failure and all-cause mortality in Chinese CAPD patients. These results could help in the early identification of patients with a high risk for HPR and suggest interventions to reduce this PD complication and improve clinical outcomes.
Acknowledgments
The authors appreciate Mrs Karen Wolf, from the University of Rochester Medical Center, Rochester, New York, for her help during the manuscript writing. They also thank Dr. Liang Shi, from Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, for offering valuable suggestions on revising the manuscript.
Supplementary Material
Footnotes
Abbreviations: CAPD = continuous ambulatory peritoneal dialysis, CCI = Charlson comorbidity index, CI = confidence interval, eGFR = estimated glomerular filtration rate, HD = hemodialysis, HPR = high peritonitis rate, HR = hazard ratio, ISPD = International Society for Peritoneal Dialysis, LPR = low peritonitis rate, OR = odds ratio, PD = peritoneal dialysis.
Funding: This work was supported by research programs from the National Natural Science Foundation of China (81170707) and the Joint Research Foundation from Health and Family Planning Commission of the Nation and Zhejiang Province (WKJ-ZJ-1610).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Davenport A. Peritonitis remains the major clinical complication of peritoneal dialysis: the London, UK, peritonitis audit 2002–2003. Perit Dial Int 2009;29:297–302. [PubMed] [Google Scholar]
- [2].Fontan MP, Rodriguez-Carmona A, Garcia-Naveiro R, et al. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 2005;25:274–84. [PubMed] [Google Scholar]
- [3].Oliveira LG, Luengo J, Caramori JC, et al. Peritonitis in recent years: clinical findings and predictors of treatment response of 170 episodes at a single Brazilian center. Int Urol Nephrol 2012;44:1529–37. [DOI] [PubMed] [Google Scholar]
- [4].Brown MC, Simpson K, Kerssens JJ, et al. Peritoneal dialysis-associated peritonitis rates and outcomes in a national cohort are not improving in the post-millennium (2000–2007). Perit Dial Int 2011;31:639–50. [DOI] [PubMed] [Google Scholar]
- [5].Fried LF, Bernardini J, Johnston JR, et al. Peritonitis influences mortality in peritoneal dialysis patients. J Am Soc Nephrol 1996;7:2176–82. [DOI] [PubMed] [Google Scholar]
- [6].Sipahioglu MH, Aybal A, Ünal A, et al. Patient and technique survival and factors affecting mortality on peritoneal dialysis in Turkey: 12 years’ experience in a single center. Perit Dial Int 2008;28:238–45. [PubMed] [Google Scholar]
- [7].de Bustillo EM, Borras F, Gomez-Roldan C, et al. Impact of peritonitis on long-term survival of peritoneal dialysis patients. Nefrologia 2011;31:723–32. [DOI] [PubMed] [Google Scholar]
- [8].An S, De Vriese SM, Norbert H, et al. What happens to the peritoneal membrane in long-term peritoneal dialysis? Perit Dial Int 2001;21suppl 3:S9–18. [PubMed] [Google Scholar]
- [9].Davies SJ, JB, Phillips L, Russell GI. Longitudinal changes in peritoneal kinetics the effects of peritoneal dialysis and peritonitis. Nephrol Dial Transplant 1996;11:498–506. [PubMed] [Google Scholar]
- [10].Chern YB, Ho PS, Kuo LC, et al. Lower education level is a major risk factor for peritonitis incidence in chronic peritoneal dialysis patients: a retrospective cohort study with 12-year follow-up. Perit Dial Int 2013;33:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kotsanas D, Polkinghorne KR, Korman TM, et al. Risk factors for peritoneal dialysis-related peritonitis: can we reduce the incidence and improve patient selection? Nephrology 2007;12:239–45. [DOI] [PubMed] [Google Scholar]
- [12].Chow KM, Szeto CC, Leung CB, et al. A risk analysis of continuous ambulatory peritoneal dialysis-related peritonitis. Perit Dial Int 2005;25:374–9. [PubMed] [Google Scholar]
- [13].Lim WH, Johnson DW, McDonald SP. Higher rate and earlier peritonitis in Aboriginal patients compared to non-Aboriginal patients with end-stage renal failure maintained on peritoneal dialysis in Australia: analysis of ANZDATA. Nephrology 2005;10:192–7. [DOI] [PubMed] [Google Scholar]
- [14].Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010;30:393–423. [DOI] [PubMed] [Google Scholar]
- [15].Hsieh YP, Wang SC, Chang CC, et al. The negative impact of early peritonitis on continuous ambulatory peritoneal dialysis patients. Perit Dial Int 2014;34:627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fourtounas C, Savidaki E, Dousdabanis P, et al. Peritonitis during the first year after commencement of peritoneal dialysis has an impact on technique survival and patient morbidity. Adv Perit Dial Conf Perit Dial 2006 2006;22:50–4. [PubMed] [Google Scholar]
- [17].Wu H, Huang R, Yi C, et al. Risk factors for early-onset peritonitis in Southern Chinese peritoneal dialysis patients. Perit Dial Int in Press. Published on May 4, 2016. doi:10.3747/pdi.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang Q, Bernardini J, Piraino B, et al. Albumin at the start of peritoneal dialysis predicts the development of peritonitis. Am J Kidney Dis 2003;41:664–9. [DOI] [PubMed] [Google Scholar]
- [19].Fox L, Tzamaloukas AH, Murata GH. Metabolic differences between persistent and routine peritonitis in CAPD. Adv Perit Dial 1992;8:346–50. [PubMed] [Google Scholar]
- [20].Narayan Prasad AG, Sharma Raj K, Sinha Archna, et al. Impact of nutritional status on peritonitis in CAPD patients. Perit Dial Int 2006;27:42–7. [PubMed] [Google Scholar]
- [21].Graham Woodrow JHT, Brownjohn Aleck M. Technique failure in peritoneal dialysis and its impact on patient survival. Perit Dial Int 1997;17:360–4. [PubMed] [Google Scholar]
- [22].Hsieh YP, Chang CC, Wang SC, et al. Predictors for and impact of high peritonitis rate in Taiwanese continuous ambulatory peritoneal dialysis patients. Int Urol Nephrol 2015;47:183–9. [DOI] [PubMed] [Google Scholar]
- [23].Perl J, Wald R, Bargman JM, et al. Changes in patient and technique survival over time among incident peritoneal dialysis patients in Canada. Clin J A Soc Nephrol 2012;7:1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hidetomo Nakamoto YK, Hiromichi Suzuki. Is technique survival on peritoneal dialysis better in Japan? Perit Dial Int 2006;26:136–43. [PubMed] [Google Scholar]
- [25].Brown EA. Peritonitis: limiting the damage. Nephrol Dial Transplant 2005;20:1539–41. [DOI] [PubMed] [Google Scholar]
- [26].Kavanagh D, Prescott GJ, Mactier RA, et al. Peritoneal dialysis-associated peritonitis in Scotland (1999–2002). Nephrol Dial Transplant 2004;19:2584–91. [DOI] [PubMed] [Google Scholar]
- [27].Mizuno M, Ito Y, Tanaka A, et al. Peritonitis is still an important factor for withdrawal from peritoneal dialysis therapy in the Tokai area of Japan. Clin Exp Nephrol 2011;15:727–37. [DOI] [PubMed] [Google Scholar]
- [28].Stenvinkel P, Chung SH, Heimburger O, et al. Malnutrition, inflammation, and atherosclerosis in peritoneal dialysis patients. Perit Dial Int 2001;21:S157–62. [PubMed] [Google Scholar]
- [29].Ghali JR, Bannister KM, Brown FG, et al. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit Dial Int 2011;31:651–62. [DOI] [PubMed] [Google Scholar]
- [30].Boudville N, Kemp A, Clayton P, et al. Recent peritonitis associates with mortality among patients treated with peritoneal dialysis. J Am Soc Nephrol 2012;23:1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Natalia S, Rost PAW, Carlos S Kase, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke 2001;32:2575–9. [DOI] [PubMed] [Google Scholar]
- [32].Isla RA, Mapiye D, Swanepoel CR, et al. Continuous ambulatory peritoneal dialysis in Limpopo province, South Africa: predictors of patient and technique survival. Perit Dial Int 2014;34:518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


