Abstract
Background:
The receptor for advanced glycosylation end products (RAGE) has been widely linked to diabetic atherosclerosis, but its effects on coronary artery disease (CAD) and ischemic stroke (IS) remain controversial. The Gly82Ser polymorphism is located in the ligand-binding V domain of RAGE, suggesting a possible influence of this variant on RAGE function. The aim of the present study is to clarify the association between the RAGE Gly82Ser polymorphism and susceptibility to CAD and IS.
Methods:
Eligible studies were identified through a comprehensive literature search. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to evaluate the association of Gly82Ser polymorphism with CAD and IS risk. Fixed- or random-effects model was used depending on the heterogeneity between studies. A funnel plot and Egger linear regression test were applied to assess publication bias. We also performed subgroup analyses to investigate potential sources of heterogeneity.
Results:
A total of 16 eligible articles containing 18 studies were analyzed. The pooled analysis indicated that the Gly82Ser polymorphism significantly increased CAD risk in recessive and homozygous genetic models (SS vs GS + GG: OR = 1.34, 95% CI = 1.09–1.64; SS vs GG: OR = 1.38, 95% CI = 1.12–1.71). A significant association between the Gly82Ser polymorphism and IS risk was observed in all tested models except the heterozygous genetic model (GS + SS vs GG: OR = 1.20, 95% CI = 1.04–1.38; SS vs GS + GG: OR = 2.20, 95% CI = 1.74–2.78; SS vs GG: OR = 2.23, 95% CI = 1.72–2.91; S vs G: OR = 1.32, 95% CI = 1.05–1.65). Subgroup analysis suggested an association between CAD and IS risk and the Gly82Ser polymorphism in the Chinese population, but not in the non-Chinese population.
Conclusions:
The current meta-analysis suggests that the RAGE Gly82Ser polymorphism is associated with an increased risk of CAD and IS, especially in the Chinese population. However, better-designed studies with larger sample sizes are needed to validate the results.
Keywords: coronary artery disease, Gly82Ser, ischemic stroke, meta-analysis, RAGE
1. Introduction
Coronary artery disease (CAD) and ischemic stroke (IS) share risk factors, frequently coexist, and remain major health burdens worldwide.[1,2] Both CAD and IS are manifestations of atherosclerosis.[2] Epidemiological studies have revealed that individuals with diabetes mellitus are more likely to suffer from atherosclerotic diseases.[3,4] Meanwhile, evidence has indicated that sustained hyperglycemia could result in the formation and accumulation of advanced glycosylation end products (AGEs), accelerating vascular dysfunction and atherogenesis.[5] However, these factors apply only to a proportion of the pathogenesis of atherosclerosis. Genetic evidence suggests that both CAD and IS are highly heritable and have substantial overlap of genetic risk.[4,6–8]
The receptor for AGEs (RAGE) is a member of the immunoglobulin superfamily of cell surface molecules.[9] RAGE expression occurs in most tissues, including the heart, brain, liver, and kidney.[10] The RAGE gene is located on chromosome 6p21.3 and is overexpressed in atherosclerotic plaques.[11,12] It was identified as a likely candidate gene associated with vascular and neurological complications, such as atherosclerosis, CAD, IS, and Alzheimer disease.[9,13,14] More than 50 polymorphisms have been identified in the region of the RAGE gene.[15] Of these, the RAGE Gly82Ser polymorphism (rs2070600), representing a glycine (G) to serine (S) substitution, is of interest because of its localization in the N-linked glycation site (position 82), which could influence AGE–RAGE interaction.[16] Some studies in humans and animals have yielded conflicting results concerning the potential role of the RAGE Gly82Ser polymorphism in the pathogenesis of atherosclerosis. Several studies have indicated that Gly82Ser polymorphism is a possible risk factor for the development of CAD,[17,18] while others failed to specifically link this variant to CAD.[19,20] Meanwhile, the role of Gly82Ser polymorphism in IS susceptibility remains controversial.[21,22]
Consequently, there appears a need for a meta-analysis to investigate the association between the RAGE Gly82Ser polymorphism and the risk of CAD and IS.
2. Methods and materials
2.1. Literature search strategy
A comprehensive search strategy was performed using electronic databases, including PubMed, Embase, Web of Science, ScienceDirect, Cochrane Library, Wanfang database, and the Chinese National Knowledge Infrastructure Database. Databases were searched using the following search terms: (“RAGE” OR “the receptor for advanced glycosylation end products” OR “Gly82Ser” OR “rs2070600”) and (“polymorphism” OR “genotype” OR “variant”) and (“cerebral infarction” OR “ischemic stroke” OR “coronary artery disease” OR “myocardial infarction” OR “angina” OR “atherosclerosis”). The deadline of publication for inclusion in the meta-analysis was March 2016. Manual searching was also performed to find potentially relevant records.
2.2. Inclusion and exclusion criteria
Studies included in our meta-analysis were qualified on the basis of the following criteria: studies on the association between the Gly82Ser polymorphism and susceptibility to CAD or IS; total CAD cases that were documented by angiographic evidence of at least 50% stenosis of 1 major coronary artery, myocardial infarction, a history of prior angioplasty, or coronary artery bypass surgery; studies in which magnetic resonance imaging or computed tomography was used to confirm the diagnosis of IS; studies in which the data in the references were sufficient for present estimation; and studies in which the publication language was English and/or Chinese. However, all reviews, abstracts, meta-analyses, letters to the editor, animal studies, case-only studies, and studies containing overlapping data were excluded.
2.3. Data extraction
The data were extracted by 2 independent reviewers (W-QM and YZ) according to the selection criteria. Disagreements were resolved through discussion, or by a third reviewer (N-FL). The following information was extracted from each study: author, publication date, region, country, total number of cases and controls, number of different genotypes in cases and controls, genotyping methods, and Hardy–Weinberg equilibrium (HWE) in controls.
2.4. Statistical analysis
Statistical analyses were performed with Stata software package (version 12.0; StataCorp, College Station, TX). Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess the strength of the association between susceptibility to CAD and IS and the RAGE Gly82Ser polymorphism. Pooled ORs were calculated for the dominant (GS + SS vs GG), recessive (SS vs GS + GG), homozygous (SS vs GG), heterozygous (GS vs GG), and allele (S vs G) genetic models. The Cochrane Q-test and index (I2) were used to measure heterogeneity between the studies. P > 0.10 in the Q-test or I2 < 50% indicated no heterogeneity among the studies. The fixed-effects model was used when there was no significant heterogeneity; otherwise, the random-effects model was applied. Subgroup analyses according to ethnicity (Chinese and non-Chinese populations), HWE status, and sample size (studies with >500 subjects were categorized as “large,” and studies with <500 subjects were categorized as “small”) were performed to detect sources of heterogeneity among the studies. To assess the stability of the results, sensitivity analysis was performed by removing each individual study at a time. Possible publication bias was tested by funnel plot and Egger linear regression test.
2.5. Ethics
Ethical approval was not required for the present meta-analysis.
3. Results
3.1. Selection and characteristics of studies
The initial literature search identified 1415 potentially relevant articles, of which 859 articles were excluded because they were duplicates. Twenty-six articles were obtained from the literature search after screening titles and abstracts. Then, 10 articles were excluded due to being reviews and meta-analyses, and including insufficient data. Finally, 16 articles meeting the inclusion criteria were preserved.[17–32] A flow diagram of the literature selection process is shown in Fig. 1.
Figure 1.
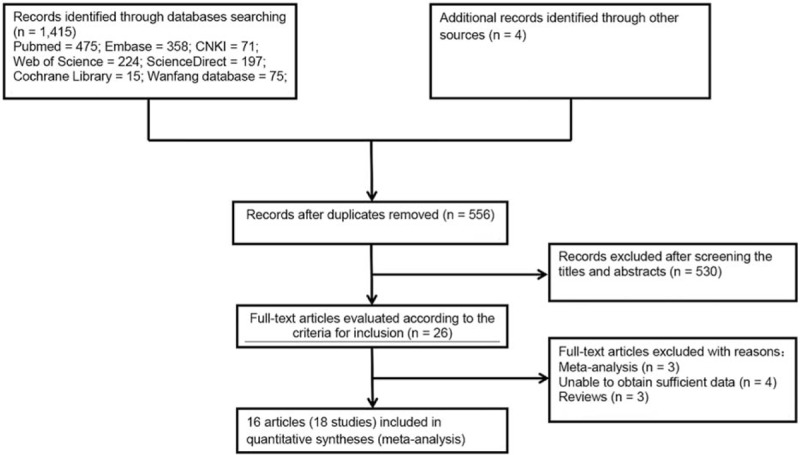
Flow diagram of the literature selection process. CNKI = Chinese National Knowledge Infrastructure Database.
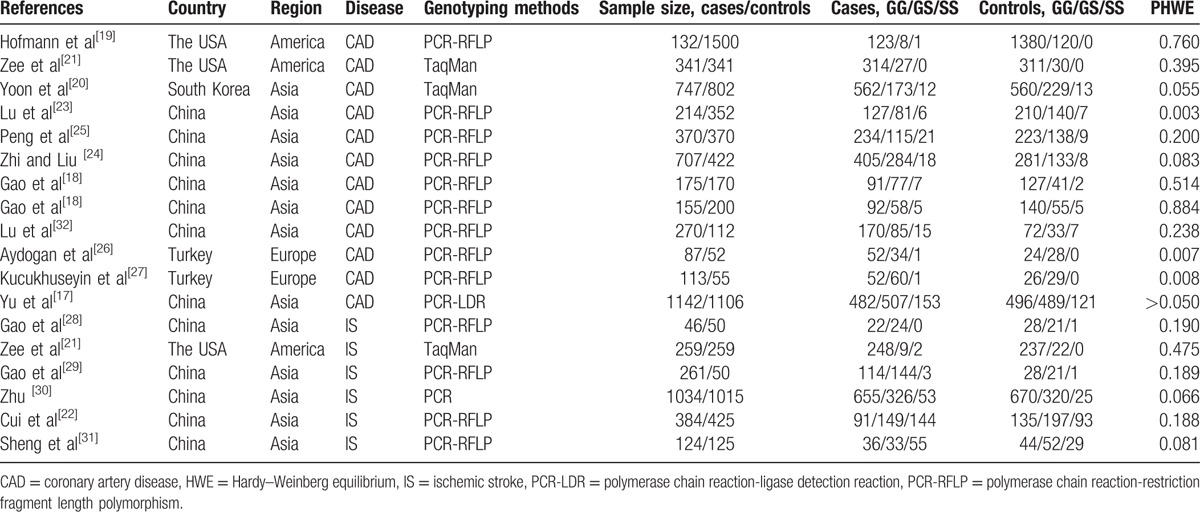
The main characteristics of the eligible studies are summarized in Table 1. A total of 18 studies, covered in 16 articles, were identified. One article reported 2 separate studies on the association between the RAGE Gly82Ser polymorphism and CAD susceptibility,[18] and another reported the association between the Gly82Ser polymorphism and susceptibility to CAD and IS.[21] Thus, 18 studies were eligible for our meta-analysis. There were 12 and 6 studies concerning the association between the Gly82Ser polymorphism and susceptibility to CAD and IS, respectively. The countries in which these studies were conducted included the United States, South Korea, China, and Turkey. Two articles reported 2 separate studies. Three studies did not satisfy the HWE for the control group.[23,26,27]
Table 1.
Characteristics of eligible studies included in the meta-analysis.
3.2. Association between the RAGE Gly82Ser polymorphism and susceptibility to CAD
All studies analyzed indicated an increased risk associated with the Gly82Ser polymorphism and CAD susceptibility in recessive and homozygous genetic models (SS vs GS + GG: OR = 1.34, 95% CI: 1.09–1.64; SS vs GG: OR = 1.38, 95% CI: 1.12–1.71). No significant associations were observed between the Gly82Ser polymorphism and CAD susceptibility in dominant, heterozygous, and allele genetic models. Heterogeneity inspection showed high between-study heterogeneity under dominant, heterozygous, and allele genetic models. Subgroup analysis stratified by simple size indicated that large sample sizes, rather than small sample sizes, showed a significant association between the Gly82Ser polymorphism and CAD susceptibility under recessive and homozygous genetic models. Stratification analysis stratified by ethnicity also suggested the existence of a significant association between the Gly82Ser polymorphism and CAD risk in the Chinese population under all tested models except the heterozygous genetic model (Table 2; Fig. 2).
Table 2.
Summary of meta-analysis of the association between the Gly82Ser polymorphism and coronary artery disease and ischemic stroke.
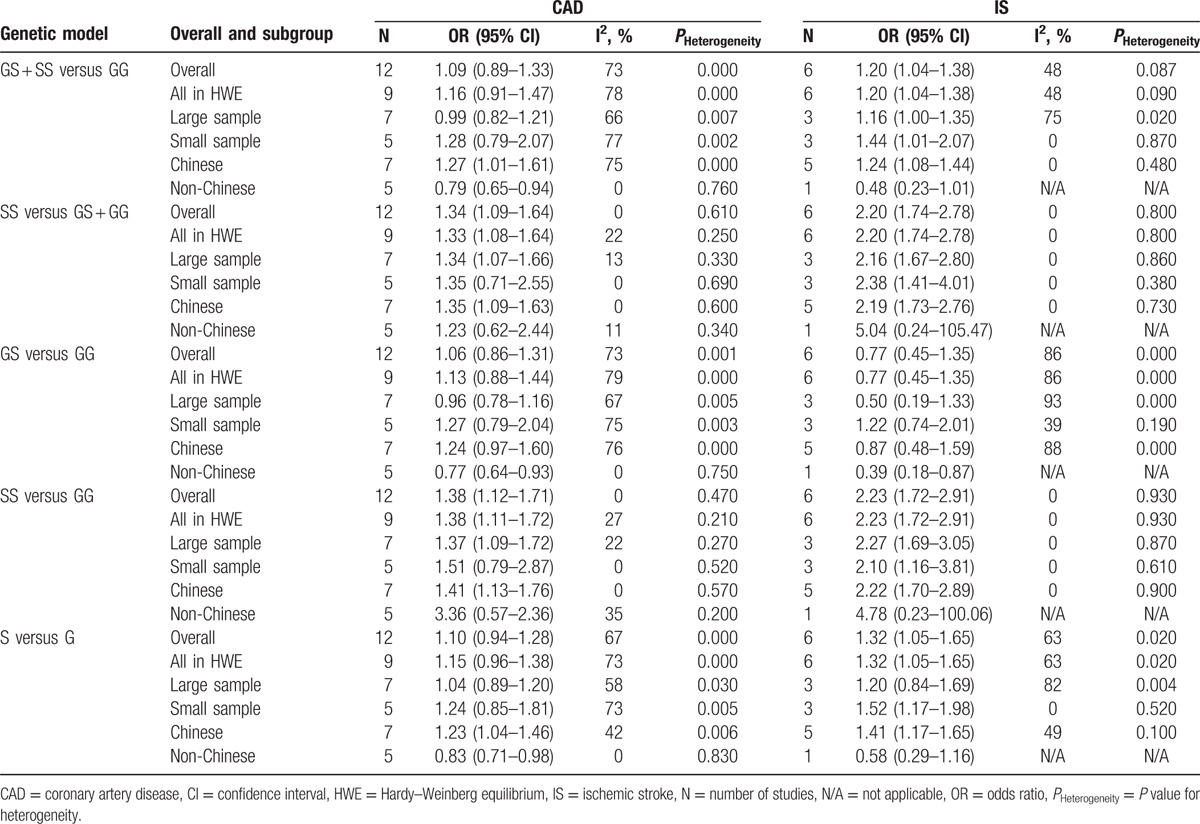
Figure 2.
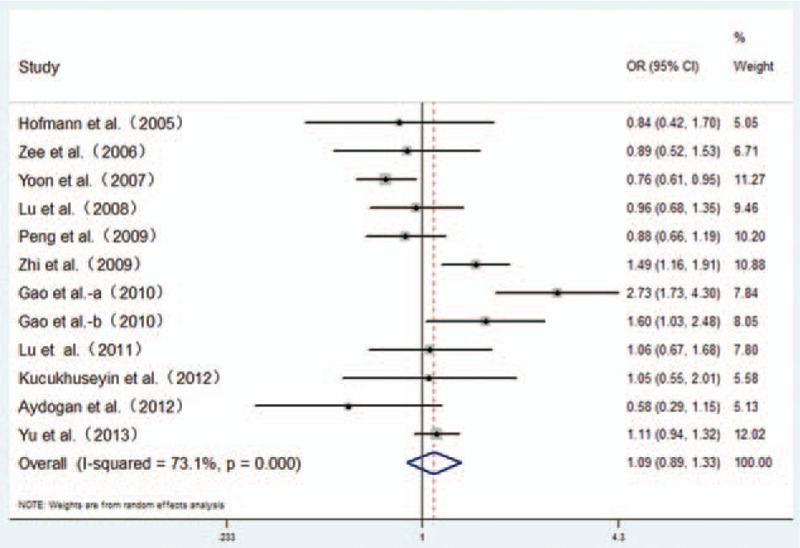
Forest plot from the meta-analysis for the association of the RAGE Gly82Ser polymorphism and CAD risk in dominant genetic model (GS + SS vs GG). Compared to the GG genotype, GS + SS genotypes showed no increased risk of CAD. CAD = coronary artery disease, CI = confidence interval, OR = odds ratio.
3.3. Association between the RAGE Gly82Ser polymorphism and susceptibility to IS
Meta-analysis of the RAGE Gly82Ser polymorphism showed an elevated risk of IS in all tested models except the heterozygous genetic model (GS + SS vs GG: OR = 1.20, 95% CI: 1.04–1.38; SS vs GS + GG: OR = 2.20, 95% CI: 1.74–2.78; SS vs GG: OR = 2.23, 95% CI: 1.72–2.91; S vs G: OR = 1.32, 95% CI: 1.05–1.65). Heterogeneity inspection showed high between-study heterogeneity under dominant, heterozygous, and allele genetic models. When we conducted subgroup analysis by ethnicity, significant associations were observed in the Chinese population under all tested models except heterozygous genetic model, with a reduction in heterogeneity. Stratification by sample size indicated that the polymorphism of Gly82Ser was significantly associated with IS risk for small sample sizes under dominant, recessive, homozygous, and allele genetic models, with low between-study heterogeneity (Table 2; Fig. 3).
Figure 3.
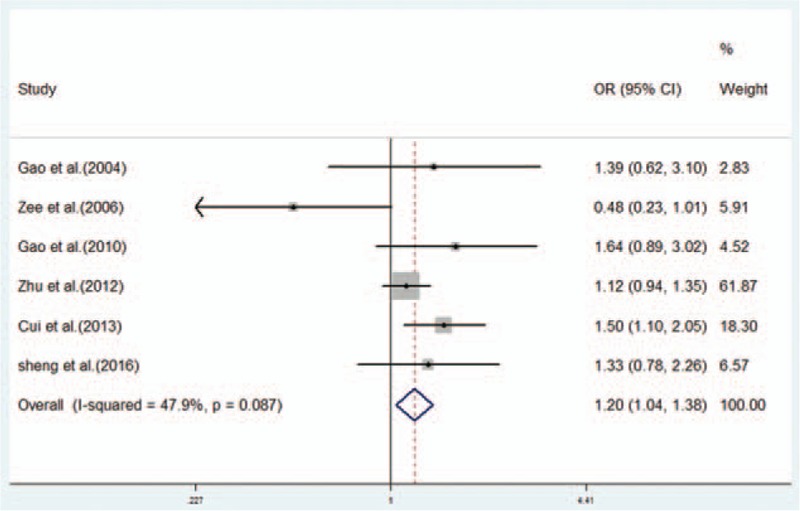
Forest plot from the meta-analysis on the association between the RAGE Gly82Ser polymorphism and IS risk in dominant genetic model (GS + SS vs GG). Compared to the GG genotype, GS + SS genotypes showed an increased risk of IS. CI = confidence interval, IS = ischemic stroke, OR = odds ratio.
3.4. Sensitivity analysis
The influence of each study on the pooled ORs and 95% CIs was evaluated by excluding each study at a time. No individual study significantly affected the OR (Fig. 4).
Figure 4.
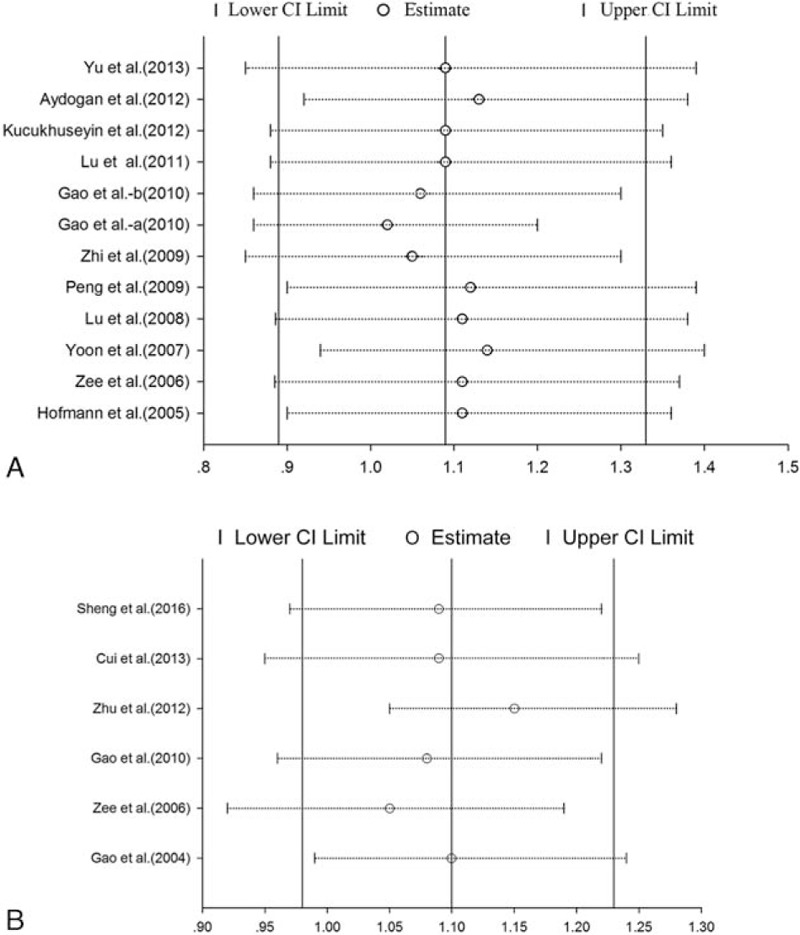
(A and B) Sensitivity analysis of pooled OR coefficients on the correlation between the RAGE Gly82Ser polymorphism and susceptibility to CAD (A) and IS (B). Results were evaluated by excluding each study 1 at a time. CAD = coronary artery disease, CI = confidence interval, IS = ischemic stroke, OR = odds ratio.
3.5. Publication bias
Funnel plots and Egger linear regression tests were performed to assess the publication bias of the included studies. The funnel plot appeared to be symmetrical (Fig. 5) and no statistically significant asymmetry was observed by Egger linear regression test for CAD (GS + SS vs GG, PEgger = 0.094). Because of the limited number of the studies included in IS, the power of the funnel plots for detecting bias will be low.[33] Consequently, only the Egger linear regression test was used to evaluate the publication bias for IS (GS + SS vs GG, P = 0.426).
Figure 5.
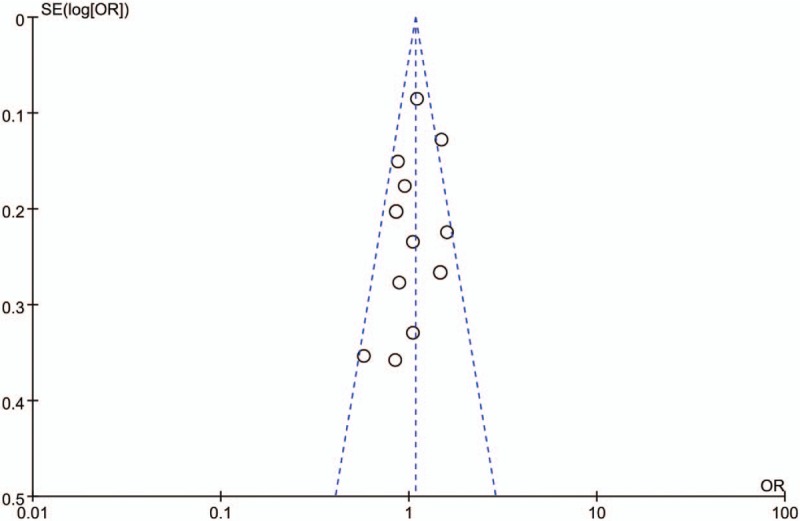
Funnel plot for studies investigating the effect of the RAGE Gly82Ser polymorphism on the risk of CAD under the domain model. The studies of the RAGE Gly82Ser polymorphism were symmetrically distributed within the funnel plot. CAD = coronary artery disease, OR = odds ratio; SE = standard error.
4. Discussion
The current meta-analysis has consolidated and reanalyzed 18 eligible studies of the effect of the Gly82Ser polymorphism on the incidence of CAD and IS. All of the results suggest that the RAGE Gly82Ser polymorphism is associated with an increased risk of CAD and IS. Furthermore, subgroup analysis indicated that the subjects with the Ser82 allele were at higher risk of CAD and IS in the Chinese population, than in the non-Chinese population. On the other hand, stratified analysis by ethnicity was successfully applied to relieve heterogeneity bias in the polymorphism analysis within the Chinese population, suggesting that ethnicity may potentially be the source of the heterogeneity. Although 3 studies did not comply with HWE in the non-CAD controls, removing these studies during the stratification analysis by HWE status did not alter the conclusions made in the meta-analysis. Sensitivity analysis also confirmed the stability of our results.
Atherosclerosis is the main cause of CAD and IS.[2] Although much of work has identified the significant association between RAGE gene polymorphisms and diabetic atherosclerosis,[34,35] it is difficult to draw a causal link between RAGE gene polymorphisms and the risk of CAD and IS. Sustained hyperglycemia could lead to the formation and accumulation of AGEs, and RAGE mediates most of the adverse effects of AGEs.[36] The AGE–RAGE interaction alters cellular signaling, promotes gene expression, and enhances the release of proinflammatory molecules, involved in the pathogenesis of vascular diabetic complications.[37] Previous studies have identified that significantly higher levels of RAGE are observed in human atherosclerotic plaques.[38] The Gly82Ser mutation exhibits potentially enhanced ligand-binding affinity, which may predispose or exasperate diabetic atherosclerosis.[16,39] Meanwhile, evidence has indicated that pharmacological blockage of RAGE, or genetic deletion, resulted in significant suppression of atherosclerosis progression.[34,40] In our study, we found its efforts on CAD and IS, especially in the Chinese population, and the reason may partly depend on genetic diversity among different ethnic groups.[41]
Although several relevant meta-analyses have analyzed the relationship between the Gly82Ser polymorphism and CAD susceptibility,[42,43] there are some differences. More eligible studies were included in our study and 5 genetic models gave us a comprehensive understanding of the relationship between the Gly82Ser polymorphism and susceptibility to CAD. Furthermore, the present meta-analysis is the first one to assess the association between the Gly82Ser polymorphism and IS risk.
Several limitations of our meta-analysis should be mentioned. First, heterogeneity in our study may influence the reliability of our results, although subgroup analysis was performed to detect the source of heterogeneity and sensitivity analysis was used to assess the stability of the results. Second, the data extracted from each record were based on unadjusted estimates, which may lead to misleading results. Third, the language of eligible studies was limited to English and Chinese, and despite no evidence of publication bias from our statistical tests, some may remain.
In conclusion, our meta-analysis supports an association between the RAGE Gly82Ser polymorphism and the risk of CAD and IS, especially in the Chinese population. Although subgroup analysis was used to investigate the significant heterogeneity among studies, these results should be interpreted with caution. Further well-designed studies with large sample sizes are needed to validate the present results.
Footnotes
Abbreviations: AGE = advanced glycosylation end product, CAD = coronary artery disease, CI = confidence interval, HWE = Hardy–Weinberg equilibrium, IS = ischemic stroke, OR = odds ratio, RAGE = receptor for advanced glycosylation end products.
Authors’ contributions: W-QM and N-FL designed the study and wrote the protocol. W-QM, N-FL, and YZ managed the literature searched and performed the analysis. Q-RQ and YZ undertook the statistical analysis, and W-QM wrote the first draft of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016;133:e38–60. [DOI] [PubMed] [Google Scholar]
- [2].Conforto AB, Leite CC, Nomura CH, et al. Is there a consistent association between coronary heart disease and ischemic stroke caused by intracranial atherosclerosis? Arq Neuropsiquiatr 2013;71:320–6. [DOI] [PubMed] [Google Scholar]
- [3].Mukherjee D. Peripheral and cerebrovascular atherosclerotic disease in diabetes mellitus. Best Pract Res Clin Endocrinol Metab 2009;23:335–45. [DOI] [PubMed] [Google Scholar]
- [4].Dichgans M, Malik R, Konig IR, et al. Shared genetic susceptibility to ischemic stroke and coronary artery disease: a genome-wide analysis of common variants. Stroke 2014;45:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Soro-Paavonen A, Zhang WZ, Venardos K, et al. Advanced glycation end-products induce vascular dysfunction via resistance to nitric oxide and suppression of endothelial nitric oxide synthase. J Hypertens 2010;28:780–8. [DOI] [PubMed] [Google Scholar]
- [6].Markus H. Stroke genetics: prospects for personalized medicine. BMC Med 2012;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lanktree MB, Hegele RA. Gene–gene and gene–environment interactions: new insights into the prevention, detection and management of coronary artery disease. Genome Med 2009;1:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kessler T, Erdmann J, Dichgans M, et al. Shared genetic aetiology of coronary artery disease and atherosclerotic stroke—2015. Curr Atheroscler Rep 2015;17:498. [DOI] [PubMed] [Google Scholar]
- [9].Hudson BI, Schmidt AM. RAGE: a novel target for drug intervention in diabetic vascular disease. Pharm Res 2004;21:1079–86. [DOI] [PubMed] [Google Scholar]
- [10].Brett E, Schmidt AM, Yan SD, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol 1993;143:1699–712. [PMC free article] [PubMed] [Google Scholar]
- [11].Sugaya K, Fukagawa T, Matsumoto K, et al. Three genes in the human MHC class III region near the junction with the class II: gene for receptor of advanced glycosylation end products, PBX2 homeobox gene and a notch homolog, human counterpart of mouse mammary tumor gene int-3. Genomics 1994;23:408–19. [DOI] [PubMed] [Google Scholar]
- [12].Johnson LL, Tekabe Y, Kollaros M, et al. Imaging RAGE expression in atherosclerotic plaques in hyperlipidemic pigs. EJNMMI Res 2014;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Traherne JA. Human MHC architecture and evolution: implications for disease association studies. Int J Immunogenet 2008;35:179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hudson BI, Wendt T, Bucciarelli LG, et al. Diabetic vascular disease: it's all the RAGE. Antioxid Redox Signal 2005;7:1588–600. [DOI] [PubMed] [Google Scholar]
- [15].Torres MC, Beltrame MH, Santos IC, et al. Polymorphisms of the promoter and exon 3 of the receptor for advanced glycation end products (RAGE) in Euro- and Afro-Brazilians. Int J Immunogenet 2012;39:155–60. [DOI] [PubMed] [Google Scholar]
- [16].Osawa M, Yamamoto Y, Munesue S, et al. De-N-glycosylation or G82S mutation of RAGE sensitizes its interaction with advanced glycation endproducts. Biochim Biophys Acta 2007;1770:1468–74. [DOI] [PubMed] [Google Scholar]
- [17].Yu X, Liu J, Zhu H, et al. An interactive association of advanced glycation end-product receptor gene four common polymorphisms with coronary artery disease in northeastern Han Chinese. PLoS One 2013;8:e76966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gao J, Shao Y, Lai W, et al. Association of polymorphisms in the RAGE gene with serum CRP levels and coronary artery disease in the Chinese Han population. J Hum Genet 2010;55:668–75. [DOI] [PubMed] [Google Scholar]
- [19].Hofmann MA, Yang Q, Harja E, et al. The RAGE Gly82Ser polymorphism is not associated with cardiovascular disease in the Framingham offspring study. Atherosclerosis 2005;182:301–5. [DOI] [PubMed] [Google Scholar]
- [20].Yoon S-J, Park S, Shim CY, et al. Association of RAGE gene polymorphisms with coronary artery disease in the Korean population. Coron Artery Dis 2007;18:1–8. [DOI] [PubMed] [Google Scholar]
- [21].Zee RY, Romero JR, Gould JL, et al. Polymorphisms in the advanced glycosylation end product-specific receptor gene and risk of incident myocardial infarction or ischemic stroke. Stroke 2006;37:1686–90. [DOI] [PubMed] [Google Scholar]
- [22].Cui X, Chen H, Zheng Z. Polymorphism of the RAGE affects the serum inflammatory levels and risk of ischemic stroke in a Chinese population. Cell Physiol Biochem 2013;32:986–96. [DOI] [PubMed] [Google Scholar]
- [23].Lu L, Pu LJ, Chen QJ, et al. Increased glycated albumin and decreased esRAGE concentrations are associated with in-stent restenosis in Chinese diabetic patients. Clin Chim Acta 2008;396:33–7. [DOI] [PubMed] [Google Scholar]
- [24].Zhi H, Liu NF. Molecular Epidemiological Study of Risk and Prognosis of Coronary Heart Disease in Chinese Ethnic Han Population. 2009;Jiangsu Province of China: Southeast University, 1–78. [Google Scholar]
- [25].Peng WH, Lu L, Wang LJ, et al. RAGE gene polymorphisms are associated with circulating levels of endogenous secretory RAGE but not with coronary artery disease in Chinese patients with type 2 diabetes mellitus. Arch Med Res 2009;40:393–8. [DOI] [PubMed] [Google Scholar]
- [26].Aydogan HY, Kuuckhuseyin O, Tekeli A, et al. Associations of receptor for advanced glycation end products −374 T/A and Gly82 Ser and peroxisome proliferator-activated receptor gamma Pro12Ala polymorphisms in Turkish coronary artery disease patients. Genet Test Mol Biomarkers 2012;16:134–7. [DOI] [PubMed] [Google Scholar]
- [27].Kucukhuseyin O, Yilmaz-Aydogan H, Isbir CS, et al. Is there any association between GLY82 ser polymorphism of rage gene and Turkish diabetic and non diabetic patients with coronary artery disease? Mol Biol Rep 2012;39:4423–8. [DOI] [PubMed] [Google Scholar]
- [28].Gao SS, Li J, Zhou C. A study on the association of RAGE gene Gly82Ser polymorphism with cerebral infraction patients. Chin J Neuromed 2004;3:37–40. [Google Scholar]
- [29].Gao SS, Li J, Han PH, et al. Association of advanced glycation end products gene Gly82Ser polymorphism with cerebral infarction groups by TOAST. Chin J Postgrad Med 2010;33:13–7. [Google Scholar]
- [30].Zhu J. The Association Study Between hmgb-1 and RAGE Gene Polymorphisms and its Interaction With Ischemic Stroke. 2012;Guangdong Province of China: Guangdong Medical University, 1–58. [Google Scholar]
- [31].Sheng M, Liu H-X, Zhang Y-L. Association of genetic polymorphism in the advanced glycation endproduct gene with genetic susceptibility to ischemic stroke. Chin J Geriatr 2016;35:174–8. [Google Scholar]
- [32].Lu WX, Xie GP, Feng B, et al. Association of RAGE gene polymorphisms with the presence and the severity of coronary artery disease in non-diabetic Han populations. J Suzhou Univ Med Sci 2011;31:454–8. [Google Scholar]
- [33].Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046–55. [DOI] [PubMed] [Google Scholar]
- [34].Bucciarelli LG. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein e-null mice. Circulation 2002;106:2827–35. [DOI] [PubMed] [Google Scholar]
- [35].Soro-Paavonen A, Watson AM, Li J, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 2008;57:2461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Barlovic DP, Soro-Paavonen A, Jandeleit-Dahm KA. RAGE biology, atherosclerosis and diabetes. Clin Sci (Lond) 2011;121:43–55. [DOI] [PubMed] [Google Scholar]
- [37].Del TS, Basta G. An update on advanced glycation endproducts and atherosclerosis. Biofactors 2012;38:266–74. [DOI] [PubMed] [Google Scholar]
- [38].Cipollone F, Iezzi A, Fazia M, et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation 2003;108:1070–7. [DOI] [PubMed] [Google Scholar]
- [39].Hofmann M, Monteiro J, Gregersen P, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun 2002;3:123–35. [DOI] [PubMed] [Google Scholar]
- [40].Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci 2011;1243:88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hudson BI, Stickland MH, Grant PJ. Identification of polymorphisms in the receptor for advanced glycation end products (RAGE) gene: prevalence in type 2 diabetes and ethnic groups. Diabetes 1998;47:1155–7. [DOI] [PubMed] [Google Scholar]
- [42].Peng F, Hu D, Jia N, et al. Association of four genetic polymorphisms of AGER and its circulating forms with coronary artery disease: a meta-analysis. PLoS One 2013;8:e70834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu L, Qiu XB. Association between the receptor for advanced glycation end products gene polymorphisms and coronary artery disease. Mol Biol Rep 2013;40:6097–105. [DOI] [PubMed] [Google Scholar]


