Abstract
In prosthesis-based breast reconstruction, drains are used to prevent seroma formation and to reduce the risk of infection. However, prolonged drainage increases the risk of ascending infection. Although the volume often accepted for drain removal is ≤30 mL per day, the optimal timing to remove the drain for best clinical outcome remains controversial.
We did a retrospective cohort study of 569 patients of prosthesis-based breast reconstruction with infection rate as the outcome variable; drain duration and last daily drainage volume as the main independent variables. Data on age, smoking history, diabetes mellitus history, body mass index, breast weight, tissue expander size, drain size, number of retrieved lymph nodes, tumor size, number of metastatic lymph nodes, tumor stage, mastectomy type, reconstruction type, submuscular implantation, skin defect, operative time, duration of antibiotics use, chemotherapy, and radiotherapy were collected as covariates. Multivariable logistic regression analysis was used to control for confounding.
The total infection rate was 5.1% (29/569). The daily drainage volume ≥30 mL/d at the time of drain removal was not found associated with increased infection rate (P = 0.32). Of the various cutoff values of last daily drainage volume, none was found to be a determinant for drain removal where the risk of infection was concerned. By contrast, drain duration over 21 days significantly increased infection rate (P = 0.001). The multivariable logistic regression analysis showed an increase of 76.2% in the infection rate with each additional week of drain retention (P = 0.001). Breast weight also had a significant influence on risk of infection. Chemotherapy and drain size showed borderline effect on risk of infection whereas the last daily drainage volume was not associated with risk of infection
In summary, our study revealed that drain duration, rather than the last daily drainage volume, significantly affects the infection rate in prosthesis-based breast reconstruction. We recommend that the drain is better removed no longer than 3 weeks postoperatively and can be removed as early as postoperative day 7, even when the drainage is over 30 mL in a 24-hour period.
Keywords: drain duration, drain removal, drainage volume, infection, prosthesis-based breast reconstruction
1. Introduction
More than 70% of breast reconstruction is done by prosthesis-based method.[1] One of its most unwanted complications of prosthesis-based breast reconstruction is infection, which usually results in reconstruction failure.[2] A closed suction drain in the surgical field can decrease seroma formation and possibly reduces the risk of infection.[3,4] However, prolonged drain duration may increase infection rate due to ascending infection.[5–7] The optimal timing of drain removal after prosthesis-based breast reconstruction is still unclear. Most surgeons (87.4%) recommend drain removal when the daily drainage volume at the time of drain removal (defined as last daily drainage volume) drops below 30 mL.[3,8,9] Some recommend 20 mL, or 50 mL.[3,10,11]
The object of this study is to identify the optimal timing of drain removal in terms of infection control. We hypothesized that drain duration plays a bigger role than last daily drainage volume in determining the timing of drain removal.
2. Materials and methods
This retrospective cohort study was approved by the Institutional Review Board at the Koo Foundation Sun Yat-Sen Cancer Center, Taipei, Taiwan. The records of patients who underwent immediate prosthesis-based breast reconstruction in Koo Foundation Sun Yat-Sen Cancer Center from 1998 to 2013 were collected. Autologous breast reconstruction and delayed breast reconstruction were excluded. The goal of this study was to evaluate the influence of drain duration and last daily drainage volume on the infection rate of prosthesis-based breast reconstruction. Those who did not use any drain after breast reconstruction and those with incomplete record of drain duration or last daily drainage volume were excluded from this study.
The outcome variable was infection. Infection herein was defined when the tissue expander was removed due to clinical symptoms and signs of infection, or when the seroma was positive for bacterial culture, yet the prosthesis was salvaged by antibiotic treatment. In 2-stage breast reconstruction, infection was counted only when tissue expander was still in place. In 1-stage breast reconstruction, infection was counted within 1 year of surgery. Infections sometimes might happen several years after reconstruction, which was less likely related to the drain and was not counted in this study.
The main independent variables were drain duration and last daily drainage volume. Covariates in the analysis included age, history of diabetes mellitus (DM), history of smoking, body mass index (BMI), breast weight, size of tissue expander, size of drain, number of retrieved lymph node (LN), tumor size, number of metastasized lymph node, tumor stage, mastectomy type, reconstruction type, submuscular implantation, skin defect, operative time, duration of antibiotics use, chemotherapy, and radiotherapy.
We do 1-stage prosthesis-based breast reconstruction when the excised breast weight is relatively small and the preserved skin is enough to close the wound without tension. Although our practice is to always preserve as much skin as possible when a breast reconstruction is considered, the term “skin sparing mastectomy” is not used. In this cohort, no patient received radical mastectomy. The mastectomy type is hence categorized into modified radical mastectomy, simple mastectomy with or without sentinel lymph node biopsy. Skin defect, in this study, is defined as skin necrosis or wound dehiscence that required surgical intervention. When the serratus anterior muscle was elevated from the ribs and the tissue expander was placed under pectoralis major muscle as well as serratus anterior muscle, it was classified as submuscular group. The rest of the tissue expanders were placed under pectoralis major muscle only.
Chemotherapy referred only to the postoperative chemotherapy when the tissue expander was still in place. Neither the preoperative chemotherapy nor chemotherapy after the second stage reconstruction was counted. In the 1-stage reconstruction, only chemotherapy within 1 year of surgery was counted. Radiotherapy referred to the radiation given when the tissue expander was in place or before reconstruction. Those who had radiotherapy before reconstruction were the patients who had undergone breast conserving surgery and radiotherapy before and received a mastectomy due to local recurrence later. In the 1-stage reconstruction, radiotherapy within 1 year of surgery was counted.
In the univariable analysis, χ2 test and Fisher exact test were used for categorical variables and univariable logistic regression test for continuous variable.
Multicollinearity existed among the covariates where the breast weight, BMI, and size of tissue expander were highly correlated; mastectomy types were correlated with the number of retrieved LN; and the stage represented tumor size and number of metastatic LN. Therefore, multivariable logistic regression using the forward stepwise method was performed to identify the significant variables associated with infection rate. All statistical analyses were performed using IBM SPSS, version 20.0 (IBM, Armonk, NY). P value less than 0.05 was used for statistical significance of variables.
3. Surgical methods
After completion of mastectomy, axillary sentinel lymph node sampling, or radical axillary lymph node dissection, the plastic surgeon takes over for the reconstruction procedure. The skin around the surgical field is disinfected again, and a new set of sterile surgical instruments is served. The plane under pectoralis major is opened. When the serratus anterior is not elevated, we laterally advance and suture the lateral part of the pectoralis major muscle to the lateral edge of the wound so the tissue expander is shielded from the skin wound by a muscle layer. In this study cohort, no acellular dermal matrix (ADM) was used.
After hemostasis is achieved, the pocket is irrigated with diluted aqua betadine solution. One gram of cephalosporin is spread onto the surgical field before the tissue expander is placed. Around 20 to 100 mL normal saline is instilled into the tissue expander. A closed suction drain is placed which exits through a separate skin incision at least 5 cm distal to the inferior edge of the pocket for a 5 cm subcutaneous tunnel to prevent or delay ascending infection. We place only 1 drain at the lateral part of the pocket. In the early period of this cohort, prophylactic antibiotics were used until the drain was removed. We gradually shortened the duration of antibiotics after embracing the Surgical Care Improvement Project.[12] In the middle period of this cohort, postoperative prophylactic antibiotic was used for only 1 day postoperatively. In recent years, only 1 dose 30 minutes before operation was used.
Almost all patients were discharged 24 hours after the operation with a drain in situ and then returned for a follow-up visit within a week. The drain was removed when the daily drainage volume reached below 30 mL in the early period of the cohort. However, this practice gradually changed to having the drain removed during the first follow-up visit around postoperative day 7 even if the last daily drainage volume was more than 100 mL. We usually began injecting the tissue expander on or around postoperative day 14, which was the second follow-up visit when the fluid accumulation in the pocket had decreased. In a few patients, expansion would be delayed to around postoperative day 21, because their fluid is still accumulating during the postoperative week 2. Postoperative rehabilitation of the shoulder is not encouraged until the fluid accumulation decreases.
When symptoms and signs of infection occur, the surgical wound is usually opened to evaluate the condition inside the pocket and the fluid is sent for gram stain. If the fluid is turbid or gram stain is positive for bacteria, the tissue expander is removed in addition to adequate antibiotic treatment.
4. Results
From 1998 to 2013, there were 569 immediate breast reconstruction fulfilling the inclusion criteria. The minimal follow-up duration of all patients was 2 years. Infection occurred in 29 breasts. The total infection rate was 5.1%. There was only 1 patient whose tissue expander was preserved as the fluid was clear and gram stain was negative. Culture of the fluid of this patient did come back positive on a later day. She was successfully treated with antibiotics only.
The mean ± standard deviation (SD) and median of drain duration were 13.6 ± 7.6 days and 12 days, ranging from 3 to 54 days. The mean ± SD and median of last daily drainage volume were 42.8 ± 22.9 and 38 mL, ranging from 1 to 170 mL.
As most surgeons removed drain when the last daily drainage volume was less than 30 mL, we divided the cohort into 2 groups by the last daily drainage volume equal to or less than versus more than 30 mL. χ2 test showed no significant difference in the infection rate between these 2 groups (P = 0.32). We further looked at the last daily drainage volume of various cutoff values of 10, 20, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, and 150 mL. All of which showed no significant difference in the infection rate (P = 0.67, P = 0.57, P = 0.17, P = 0.20, P = 0.22, P = 0.14, P = 0.43, P = 0.29, P = 0.30, P = 0.27, P = 0.19, P = 0.10, P = 0.10, and P = 0.10 respectively). We divided patients into 5 groups based on the volume of last daily drainage volume at 20 mL incremental difference, that is, ≦20, ≦40, ≦60, ≦80, and >80 mL. The results show no significant difference (P = 0.30, Fig. 1).
Figure 1.
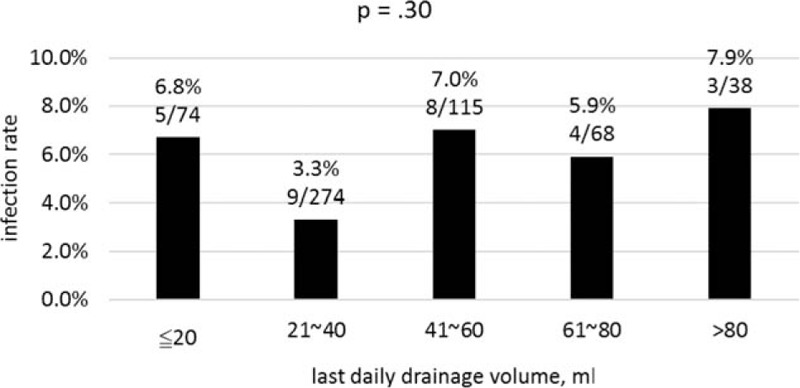
Infection rate stratified by last daily drainage volume in 20 mL intervals.
By contrast, when this cohort was divided into 2 groups based on drain duration, a significant statistical difference on infection rate was found between 2 groups with drain duration equal to or shorter than versus longer than 21 days (3.8% vs 14.3%, P = 0.001). In the later period of this cohort, we almost always removed the drain on the first follow-up visit, which was around postoperative day 7. Therefore, we divided the patients into 2 groups by drain duration of equal to or shorter than versus longer than 7 days. We did not find a significant difference of infection rate between the 2 groups (4.3% vs 5.3%, P = 0.82). We divided the cohort into 5 groups based on the duration of drain retention with an incremental interval of 1 week, that is, ≦1, ≦2, ≦3, ≦4, and >4 weeks. Figure 2 shows significant difference of infection rate in these 5 groups (P = 0.005).
Figure 2.
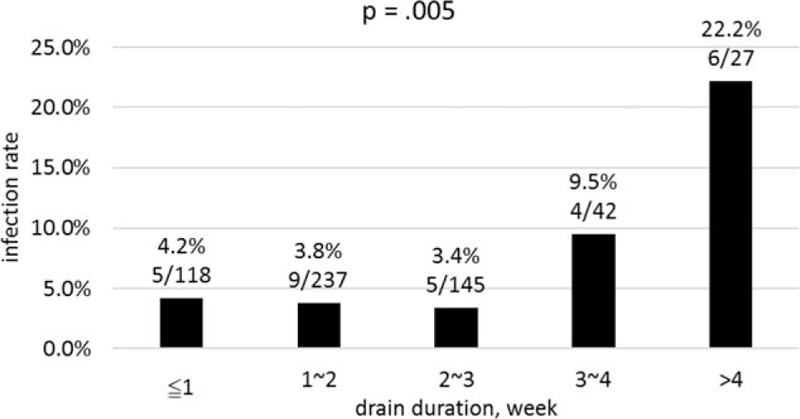
Infection rate stratified by drain duration in week-long intervals.
Table 1 shows the characteristics of the patients and their diseases that were uncontrollable factors that might affect the infection rate. Between the infected and noninfected groups, the breast weight, BMI, and number of retrieved LN were significantly different, but age, tumor size, number of metastasized LN, and stage of the disease showed no significant difference. Mastectomy type showed only a borderline effect on the infection rate. This could be due to the difference in the number of axillary LN retrieved. No infection was seen in the 44 patients who underwent simple mastectomy.
Table 1.
Patients and diseases characteristics.
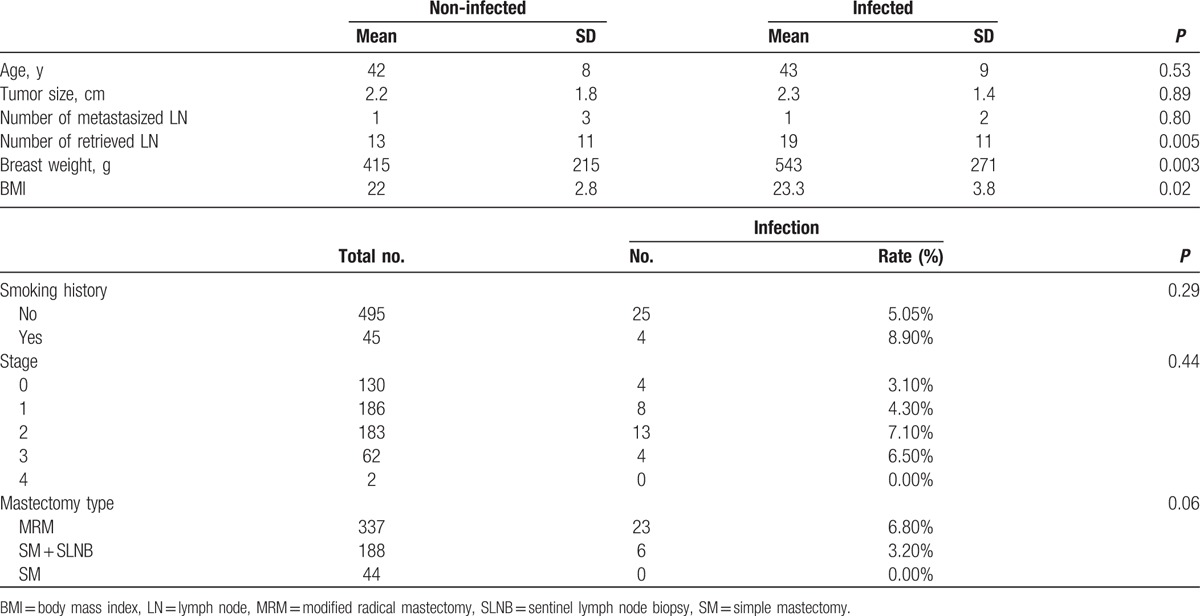
In this cohort, DM did not increase the infection rate. Only 3 (0.5%) patients were diabetic in this cohort which may have compounded the analysis. The mean excised breast weight in the 1-stage reconstruction group is 383 g as opposed to 424 g in the 2-staged reconstruction group (P = 0.33).
Table 2 shows the characteristics of operation and postoperative treatments which were relatively controllable factors. In the univariable logistic regression analysis, when both the drain duration and last daily drainage volume were considered continuous variables measured by day and milliliter, respectively, the drain duration significantly influenced the infection rate (P = 0.001), but the last daily drainage volume did not (P = 0.16). Chemotherapy significantly increases the infection rate (P = 0.01), but radiotherapy does not increase the infection rate (P = 0.16). Tissue expander size significantly influenced the infection rate (P = 0.03). Tissue expander size is highly correlated with breast weight and BMI. Among these, breast weight is the most distinct clinical factor. One-stage or 2-stage reconstruction, submuscular implantation, skin defect, operative time, and duration of postoperative antibiotic use did not significantly influence the infection rate.
Table 2.
Operative and postoperative characteristics.
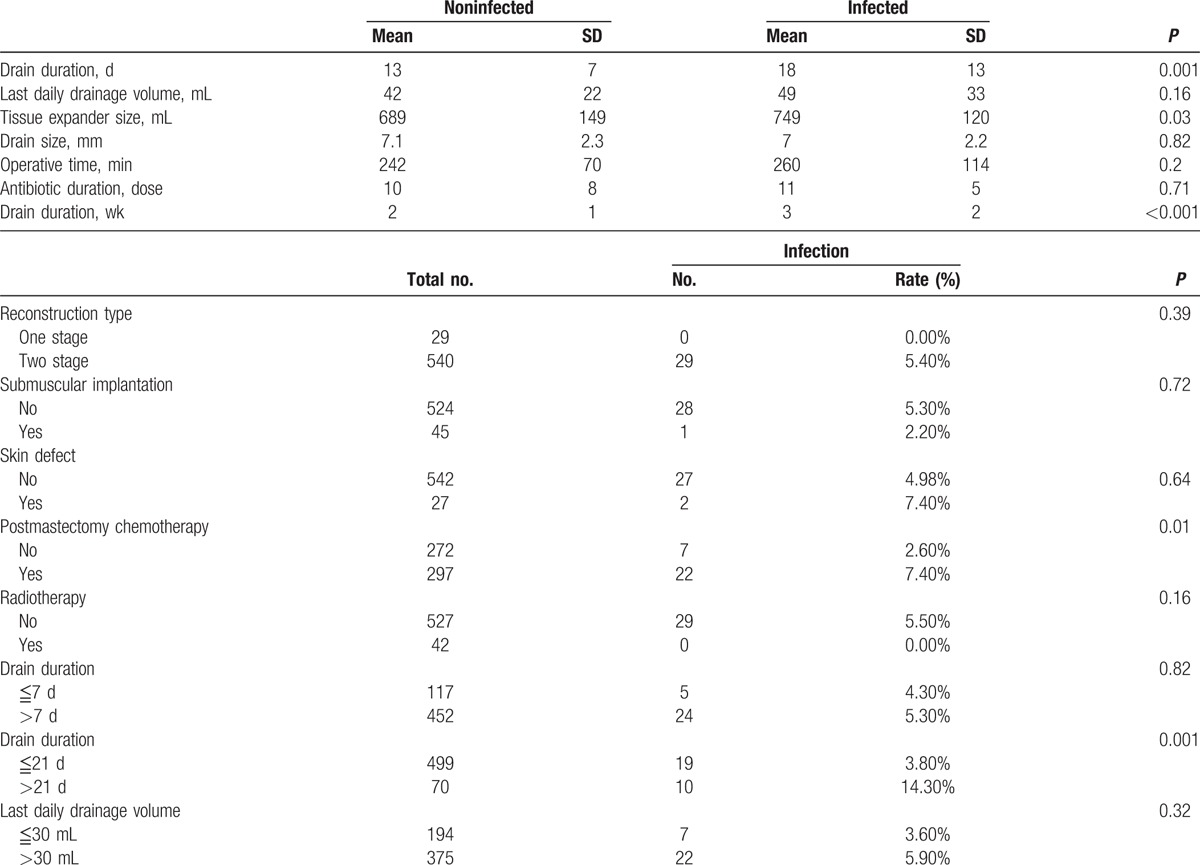
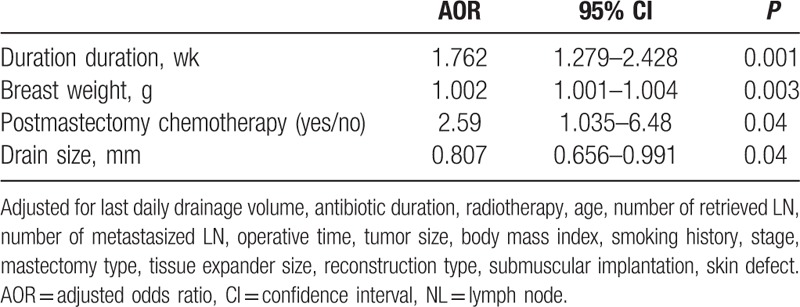
In the multivariable logistic regression analysis using the forward stepwise method, the drain duration was the first variable that was selected into the model followed by breast weight and chemotherapy. We found that the odds ratio of infection increased by 76.2% with each additional week of drain duration (P < 0.001), or by 8.1% with each additional day of drain duration (P = 0.001, not shown in tables), after adjusting for breast weight, antibiotic duration, chemotherapy, radiotherapy, drain size, age, number of retrieved LN, number of metastasized LN, operative time, tumor size, mastectomy type, tissue expander size, reconstruction type, submuscular implantation, skin defect (Table 3). Breast weight and chemotherapy also showed significant influence on odds ratio of infection (P = 0.006, P = 0.04 respectively). By contrast, last daily drainage volume did not affect the infection rate in multivariable analysis. (P = 0.28, not shown in Table 3). When this categorical variable of last daily drainage volume (≦30 mL vs >30 mL) was changed to continuous variable, using milliliter as a unit, there was still no significant association between last daily drainage volume and infection rate in multivariable logistic regression analysis (P = 0.30, not shown in Table 3).
Table 3.
Multiple logistic regression analysis evaluating risk factors for infection rate.
5. Discussion
In this study, we found that drain duration was an important factor on infection rate of prosthesis-based breast reconstruction. The odds ratio of infection increases by 76.2% with each additional week of drain duration. A drain duration over 21 days is significantly more likely to cause infection. Our current practice dictates drain removal on postoperative day 7, even if the last daily drainage volume is relatively high. This cohort study showed no significant correlation between infection rate and the last daily drainage volume. Therefore, the findings support the safety guideline of our current practice of removing the drain on or around postoperative day 7 even if the daily drainage is more than 30 mL.
Traditionally, draining is a routine procedure after modified radical mastectomy.[13] In most cases, 1 or 2 drains are placed.[3,8] Because early drain removal may increase seroma formation, it is common to wait until the last daily drainage volume falls below 20, or 30 mL before removing the drain.[14–17] The purpose of postmastectomy draining is to detect postoperative bleeding that usually stops within 48 hours. It is also used to decrease the amount of fluid accumulation. Fluid accumulation prevents the skin flaps from adhering to the underlying pectoralis major muscle and delays healing of the surgical pocket after removing breast tissue. Seroma formation is positively correlated with infection rate.[3] In addition, a large seroma may distend the overlying skin and jeopardize the circulation of the skin flaps leading to wound dehiscence or marginal necrosis with increased risk of infection.[18]
This common practice of postmastectomy drain management has been adopted for postreconstruction care. However, such a rationale is debatable when a tissue expander is placed in the surgical field. A tissue expander occupies most of the surgical space as an intention to create the desired pocket by preventing healing around the tissue expander. A seroma around the tissue expander does increase the volume of dead space but may not significantly increase the risk of infection compared with the scenario where seroma occurs in the mastectomy wound in the absence of a tissue expander. A foreign body per se increases the susceptibility of infection.[19] Prosthesis-based breast reconstruction carries more infection risks than mastectomy alone. Olsen et al[20] reported a 12.4% incidence of surgical site infection following mastectomy with immediate implant reconstruction, but 4.4% following mastectomy only. In the event of bacterial contamination during the operation, the existence of a foreign body alone, namely the tissue expander, may result in infection.[21] The benefit of prolonged drain duration is to reduce seroma, thereby reducing the risk of infection. This benefit may be subdued by the increased risk of ascending infection due to prolonged drain duration. This may explain the conflicting findings that prolonged use of drain reduces seroma formation but does not decrease the infection rate. A Cochrane review concluded that drains reduced seroma (OR = 0.46, 95% CI = 0.23–0.91) with no effect on infection.[22]
Distended skin may cause marginal necrosis and wound dehiscence. Concerning possible bacterial transfer from the skin wound into the pocket, lateral advancement of the lateral part of the pectoralis major muscle is pursued as often as possible, using the muscle layer to protect the tissue expander from the overlying skin wound. Our goal is not to cover the whole tissue expander with muscle, but only to add a muscle barrier for the tissue expander to keep out infection from the skin wound.
Concerning high tension on the overlying skin, we fill the tissue expander with 20 to 100 mL intraoperatively, which is less than 20% of the full volume of the tissue expander. Greater initial tissue expander fill increased infection rate.[23] Crosby et al reported that for every 10% increase in saline fill volume, complication risk increased 1.15 times in univariable analysis (P = 0.018). In their study,[24] the mean percentage of intraoperative tissue expander saline fill volume was 68%.
We routinely instruct our patients to limit their shoulder exercise until the drain is removed. Shamley et al[25] reported a meta-analysis that showed delaying exercises significantly decreased seroma formation (OR = 0.4; 95% CI 0.2–0.5; P = 0.00001).
After removing the drain on or around postoperative day 7, fluid accumulation may occur. We seldom aspirate the seroma. Instead, we injected the tissue expander on postoperative day 14. Hanna et al[9] recommended early expansion of tissue expander on postoperative day 21 to reduce infectious complications. They also found an association between infection and drain duration greater than 21 days. After drain removal, we are concerned that fluid accumulation may cause leakage from the surgical wound. In our cohort, there was no fluid leakage from around the pocket to the skin.
In this study, radiotherapy did not significantly increase the infection rate. Overall, there were 42 patients who received radiation. None of them had infection. This is counterintuitive. Radiation renders the tissue more vulnerable to the bacteria.[21,26] By contrast, in our study, patients who underwent chemotherapy had a higher infection rate (7.4%) than those who did not receive chemotherapy (2.6%) (AOR = 2.59, P = 0.04).
In this study, age did not affect the risk of infection. It is a common belief that the elderly are more susceptible to infection.[23,27] However, the mean age of our cohort was 42.3 ± 7.8 years old, ranging from 19 to 69, and 99% of them were younger than 60. This relatively low average age might explain why age did not influence the risk of infection in this study.
Observational study is the limitation of this study. This makes the interpretation of causality difficult. Some may argue that prolonged drain duration might have been the result rather than the cause of infection. To my knowledge, it is uncertain if the drainage volume would increase or decrease in the event of an infection. The above speculation could only be established if the drainage volume increases during infection. Assuming the daily drainage volume increased during infection, it would delay the timing of drain removal. Hence, it is likely that the drain would not be removed until the tissue expander is removed. However, in this study, it only occurred in 1 of the 29 infected patients. In the remaining 28 patients, the drain was removed before the tissue expander was. This evidence decreases the probability that infection is the cause of prolonged drain duration.
ADM, as an additional foreign body, may affect the risk of seroma formation, skin necrosis, and infection.[18,28,29] The object of this study is to analyze the association of infection with drain duration versus that with last drainage volume to determine the timing of drain removal. The absence of ADM in this study helped avoid a very significant confounder which might have contaminated the analysis
The exact last daily drainage volume is rarely reported. Most of the articles deemed last daily drainage volume dichotomous for statistical analysis. We used various cutoff values of last daily drainage volume and still did not find any significant association with the infection rate. The finding is robust even when the last daily drainage volume was analyzed as a continuous variable, using milliliter as a unit.
In conclusion, in this study, we showed evidence that the infection rate increased with longer drain duration and greater breast weight. The infection rate is not significantly related to the last daily drainage volume. We recommend that the drain is better removed no longer than 3 weeks postoperatively and can be removed as early as postoperative day 7, even when the drainage is over 30 mL in a 24-hour period.
Footnotes
Abbreviations: ADM = acellular dermal matrix, AOR = adjusted odds ratio, BMI = body mass index, CI = confidence interval, DM = diabetes mellitus, LN = lymph node, MRM = modified radical mastectomy, SD = standard deviation, SLNB = sentinel lymph node biopsy, SM = simple mastectomy.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Plastic Surgery Statistics. Available at: http://www.plasticsurgery.org/news/plastic-surgery-statistics/2013.html (accessed May 18, 2016). [Google Scholar]
- [2].Yii NW, Khoo CT. Salvage of infected expander prostheses in breast reconstruction. Plast Reconstr Surg 2003;111:1087–92. [DOI] [PubMed] [Google Scholar]
- [3].Jordan SW, Khavanin N, Kim JY. Seroma in prosthetic breast reconstruction. Plast Reconstr Surg 2016;137:1104–16. [DOI] [PubMed] [Google Scholar]
- [4].Baker E, Piper J. Drainless mastectomy: is it safe and effective? Surgeon 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [5].Degnim AC, Hoskin TL, Brahmbhatt RD, et al. Randomized trial of drain antisepsis after mastectomy and immediate prosthetic breast reconstruction. Ann Surg Oncol 2014;21:3240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rotstein C, Ferguson R, Cummings KM, et al. Determinants of clean surgical wound infections for breast procedures at an oncology center. Infect Control Hosp Epidemiol 1992;13:207–14. [DOI] [PubMed] [Google Scholar]
- [7].Xue DQ, Qian C, Yang L, et al. Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol 2012;38:375–81. [DOI] [PubMed] [Google Scholar]
- [8].Phillips BT, Wang ED, Mirrer J, et al. Current practice among plastic surgeons of antibiotic prophylaxis and closed-suction drains in breast reconstruction: experience, evidence, and implications for postoperative care. Ann Plast Surg 2011;66:460–5. [DOI] [PubMed] [Google Scholar]
- [9].Hanna KR, Tilt A, Holland M, et al. Reducing infectious complications in implant based breast reconstruction: impact of early expansion and prolonged drain use. Ann Plast Surg 2016;76suppl 4:S312–5. [DOI] [PubMed] [Google Scholar]
- [10].Unalp HR, Onal MA. Analysis of risk factors affecting the development of seromas following breast cancer surgeries: seromas following breast cancer surgeries. Breast J 2007;13:588–92. [DOI] [PubMed] [Google Scholar]
- [11].Ganske I, Verma K, Rosen H, et al. Minimizing complications with the use of acellular dermal matrix for immediate implant-based breast reconstruction. Ann Plast Surg 2013;71:464–70. [DOI] [PubMed] [Google Scholar]
- [12].Hunter JG. Appropriate prophylactic antibiotic use in plastic surgery: the time has come. Plast Reconstr Surg 2007;120:1732–4. [DOI] [PubMed] [Google Scholar]
- [13].Terrell GS, Singer JA. Axillary versus combined axillary and pectoral drainage after modified radical mastectomy. Surg Gynecol Obstet 1992;175:437–40. [PubMed] [Google Scholar]
- [14].Barton A, Blitz M, Callahan D, et al. Early removal of postmastectomy drains is not beneficial: results from a halted randomized controlled trial. Am J Surg 2006;191:652–6. [DOI] [PubMed] [Google Scholar]
- [15].Clegg-Lamptey JN, Dakubo JC, Hodasi WM. Comparison of four-day and ten-day post-mastectomy passive drainage in Accra, Ghana. East Afr Med J 2007;84:561–5. [DOI] [PubMed] [Google Scholar]
- [16].Baas-Vrancken Peeters MJ, Kluit AB, Merkus JW, et al. Short versus long-term postoperative drainage of the axilla after axillary lymph node dissection. A prospective randomized study. Breast Cancer Res Treat 2005;93:271–5. [DOI] [PubMed] [Google Scholar]
- [17].Droeser RA, Frey DM, Oertli D, et al. Volume-controlled vs no/short-term drainage after axillary lymph node dissection in breast cancer surgery: a meta-analysis. Breast 2009;18:109–14. [DOI] [PubMed] [Google Scholar]
- [18].Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 2010;125:429–36. [DOI] [PubMed] [Google Scholar]
- [19].Zimmerli W, Lew PD, Waldvogel FA. Pathogenesis of foreign body in fection. Evidence for a local granulocyte defect. J Clin Invest 1984;73:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Olsen MA, Chu-Ongsakul S, Brandt KE, et al. Hospital-associated costs due to surgical site infection after breast surgery. Arch Surg 2008;143:53–60. [DOI] [PubMed] [Google Scholar]
- [21].Olsen MA, Lefta M, Dietz JR, et al. Risk factors for surgical site infection after major breast operation. J Am Coll Surg 2008;207:326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thomson DR, Sadideen H, Furniss D. Wound drainage after axillary dissection for carcinoma of the breast. Cochrane Database Syst Rev 2013;10:CD006823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Avraham T, Weichman KE, Wilson S, et al. Postoperative expansion is not a primary cause of infection in immediate breast reconstruction with tissue expanders. Breast J 2015;21:501–7. [DOI] [PubMed] [Google Scholar]
- [24].Crosby MA, Dong W, Feng L, et al. Effect of intraoperative saline fill volume on perioperative outcomes in tissue expander breast reconstruction. Plast Reconstr Surg 2011;127:1065–72. [DOI] [PubMed] [Google Scholar]
- [25].Shamley DR, Barker K, Simonite V, et al. Delayed versus immediate exercises following surgery for breast cancer: a systematic review. Breast Cancer Res Treat 2005;90:263–71. [DOI] [PubMed] [Google Scholar]
- [26].Nahabedian MY, Tsangaris T, Momen B, et al. Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg 2003;112:467–76. [DOI] [PubMed] [Google Scholar]
- [27].Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg 2010;125:1606–14. [DOI] [PubMed] [Google Scholar]
- [28].Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg 2012;68:346–56. [DOI] [PubMed] [Google Scholar]
- [29].Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:1049–58. [DOI] [PubMed] [Google Scholar]


