Abstract
To investigate the incidence and outcome of major complication following conventional transarterial embolization/chemoembolization (TAE/TACE) therapy for hepatocellular carcinoma (HCC).
From May 2010 to May 2016, all patients with major complication following conventional TAE/TACE for HCC were included. Major complication was defined as admission to a hospital for therapy, an unplanned increase in the level of care, prolonged hospitalization, permanent adverse sequelae, or death after conventional TAE/TACE therapy by Society of Interventional Radiology.
During the study period, a total of 2863 TAE/TACE procedures were performed among 1120 patients, and a total of 24 patients (21 male and 3 female) developed major complication with the incidence of 2.1% (24/1120) per patient and 0.84% (24/2863) per TAE/TACE procedure. The major complications were liver rupture (n = 6), liver abscess (n = 5), femoral artery pseudoaneurysm (n = 3), cholecystitis (n = 2), biloma (n = 2), pulmonary embolism (n = 2), and 1 each of the following: cerebral lipiodol embolism, tumor lysis syndrome, partial intestinal obstruction, gallbladder perforation. The mean interval from last TAE/TACE procedure to the diagnosis of major complication was 11.1 ± 7.7 days. The treatments of the complications were conservative treatment (n = 12), conservative treatment plus percutaneous drainage (n = 3), ultrasound-guided thrombin injection (n = 3), conservative treatment plus TAE (n = 2), and conservative treatment plus surgery (n = 2). Of the 24 patients, 20 patients were recovered, and remaining 4 patients were died of major complications; therefore, the mortality rate of major complication was 16.7% (4/24).
Major complication following conventional TAE/TACE therapy is uncommon; the outcomes are benign of most major complications, but some are mortality.
Keywords: complication, hepatocellular carcinoma, transarterial chemoembolization
1. Introduction
Conventional transcatheter arterial embolization/chemoembolization (TAE/TACE) has been widely accepted as a choice of treatment for unresectable hepatocellular carcinoma (HCC) and hepatic metastases and has been considered as an effective treatment modality.[1–3] However, major complications can occur, which include but not limit to hepatic failure, liver abscess, liver ruptrue, biliary tract injury, renal failure, necrotizing pancreatitis, cerebral lipiodol embolism, and hepatic encephalopathy.[4–9]
The major complications of conventional TAE/TACE were defined as admission to a hospital for therapy, an unplanned increase in the level of care, prolonged hospitalization, permanent adverse sequelae, or death after the procedures by Society of Interventional Radiology,[10] which can cause a significantly morbidity.[7,9,11] However, the incidence and outcome are largely unknown.[9] The aim of this study was to investigate the incidence and outcome of major complication following conventional TAE/TACE in patients with HCC.
2. Materials and methods
2.1. Study design
This study was approved by both institutional review boards. From May 2010 to May 2016, retrospective reviewing of major complications following conventional TAE/TACE therapy for patients with HCC was conducted in 2 different medical centers. Patients with hepatic metastases were excluded from this study. Cases were identified through the departmental procedural logs. Patient demographics, clinical information, procedural data, and management of major complications following TAE/TACE therapy were gathered from patients’ medical records. The imaging data were gathered from Picture Archiving and Communications System of our institutions.
2.2. TAE/TACE procedure
All conventional TAE/TACE procedures were performed according to the current practice guidelines.[12] TAE/TACE was performed using a 2.7-Fr micro-catheter (Progreat; Terumo, Tokyo, Japan). Lipiodol (Lu Yin Pharmaceutial Co. Ltd., Yantai, Shandong province, China), gelatin sponge particles (GSP), and polyvinyl alcohol (PVA) were used as embolic agents. All patients were admitted after the TAE/TACE procedures for post procedure supportive treatment and observing potential complications. Routine managements include hydration, antiemetic, pain control, and monitoring liver function changes.
According to the follow-up protocol established at 2 different hospitals, routine survey of procedure-related complications was carried out on postdischarged from day 5 to 9 with telephone contact, and additional telephone calls or clinic visits as needed. Immediate clinical follow-up was required if there was suspicious for any major complications of TAE/TACE therapy.
2.3. Diagnosis of major complication
The diagnosis of major complication was based on patients symptoms, signs, laboratory testing, imaging evaluation, or interventions. The imaging evaluation include contrast enhanced computed tomography (CT) scan, magnetic resonance imaging (MRI), or ultrasound examines; the interventions include percutaneous drainage, ultrasound guided thrombin injection, TAE, or surgery.
2.4. Treatment of major complication
Once the diagnosis of major complication was established, conservative therapy was routinely initiated in patients as need, and further treatment intervention was based on the major complication, patient's condition.
2.5. Clinical follow-up
Clinical follow-up was scheduled on the first, second, and third months after discharge, and every 3 months thereafter. During follow-up, contrast-enhanced CT or MRI and routine laboratory work up were obtained, including complete blood count, liver enzymes and bilirubin, and serum alpha-fetoprote.
3. Results
3.1. Patients
From May 2010 to May 2016, a total of 1341 patients with HCC underwent conventional TAE/TACE procedures among 2 participant hospitals. Of the 1341 patients, 221 (221/1341, 16.5%) patients were excluded due to 1) major complication evaluation not available (n = 154), 2) combination of TAE/TACE and radiofrequency ablation (n = 43), and 3) combination of TAE/TACE and percutaneous ethanol injection (n = 24), and the remaining 1120 patients with clinical follow-up were reviewed. Of the 1120 patients, a total of 2863 TAE/TACE procedures were performed, and a total of 24 patients were diagnosed of major complication following conventional TAE/TACE therapy and included in this study (Fig. 1). Therefore, the incidences of major complication were 2.1% (24/1120) per patient and 0.84% (24/2863) per TAE/TACE procedure.
Figure 1.
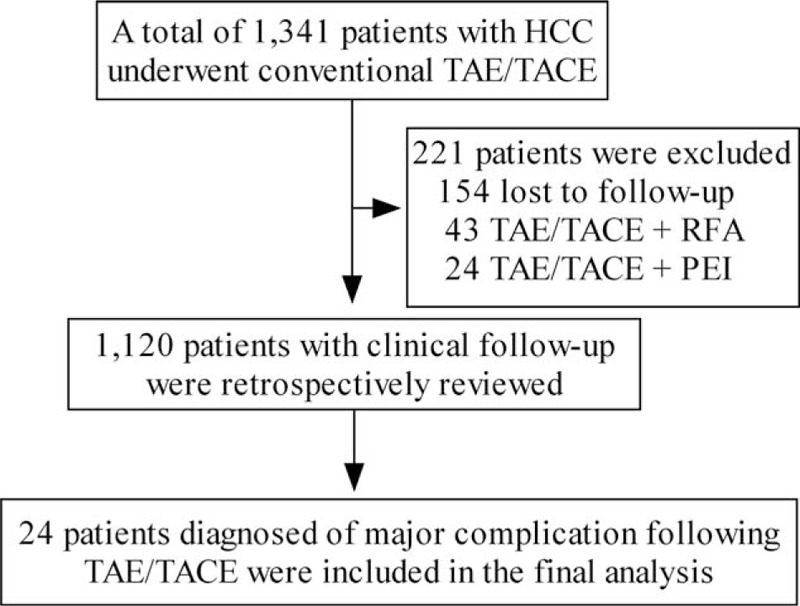
Selection of the patients.
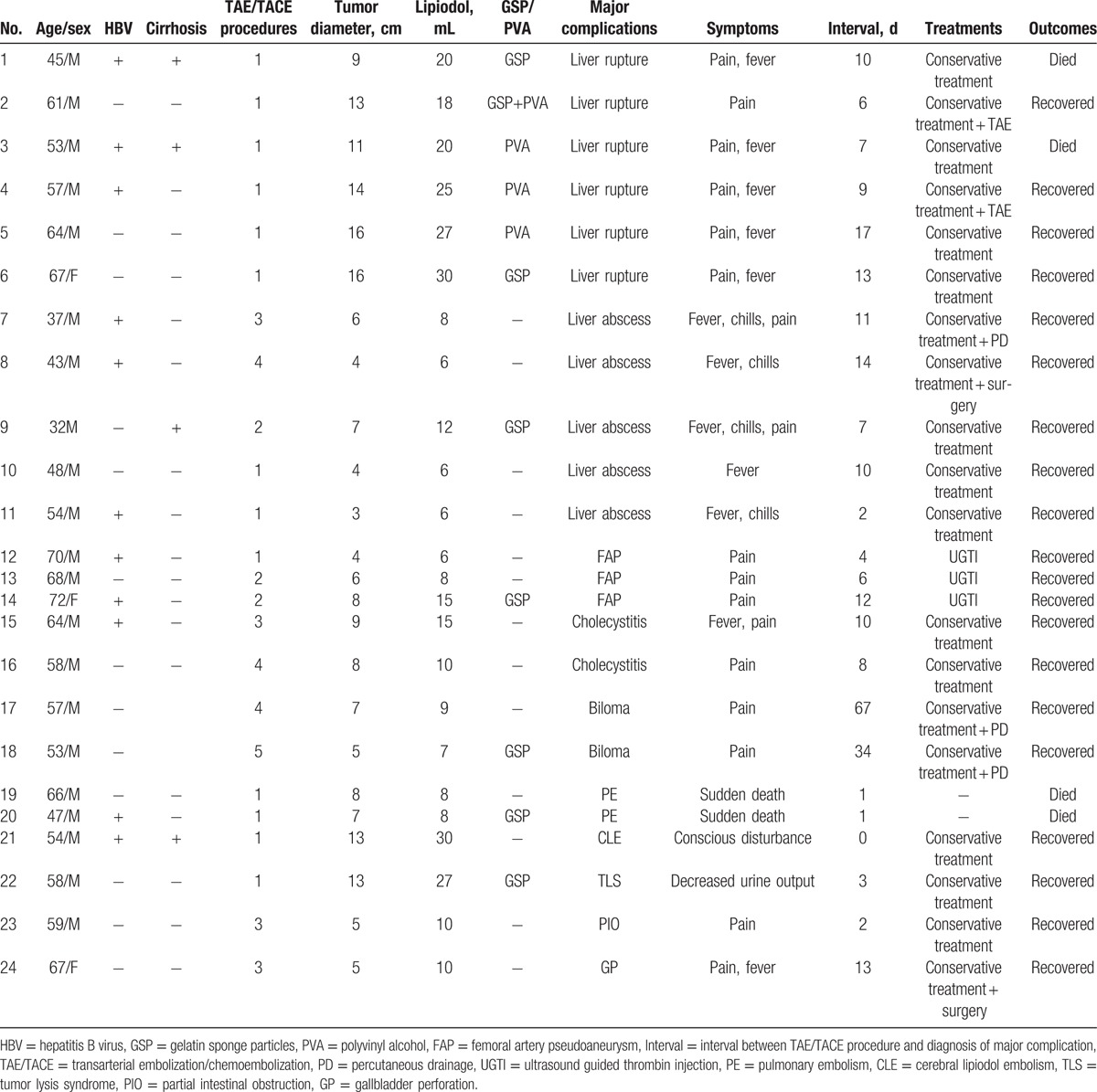
Of the 24 patients, there were 21 male and 3 female, with mean age of 56.4 ± 8.2 years (range, 32–72 years). The mean tumor diameter was 8.4 ± 3.2 cm (range, 3–16 cm), and lipiodol as an embolic agent was used in all patients (mean 14.2 ± 7.1 mL, range, 6–30 mL), GSP plus PVA were used in 1 patient, and GSP or PVA was used in 7 and 3 patients, respectively. A total of 48 TAE/TACE procedures were performed among the 24 patients, with mean of 2.0 ± 1.1 (range, 1–5) procedures for each patient. Table 1 summarizes the demographic information, baseline characteristics, treatments, and outcomes of the 24 patients.
Table 1.
The demographic information, baseline characteristics, treatments, and outcomes of the 24 patients.
3.2. Major complications
The major complications were liver rupture (n = 6), liver abscess (n = 5, Fig. 2), femoral artery pseudoaneurysm (n = 3), cholecystitis (n = 2), biloma (n = 2, Fig. 3A), pulmonary embolism (PE, n = 2), and 1 each of the following: cerebral lipiodol embolism (Fig. 3B), tumor lysis syndrome, partial intestinal obstruction (Fig. 3C and D), gallbladder perforation (Table 1). The mean interval between TAE/TACE procedure and diagnosis of major complications was 11.1 ± 7.7 days (range, 0–67 days). The most frequently reported symptom was pain (70.8%, 17/24), followed by fever (50%, 12/24).
Figure 2.
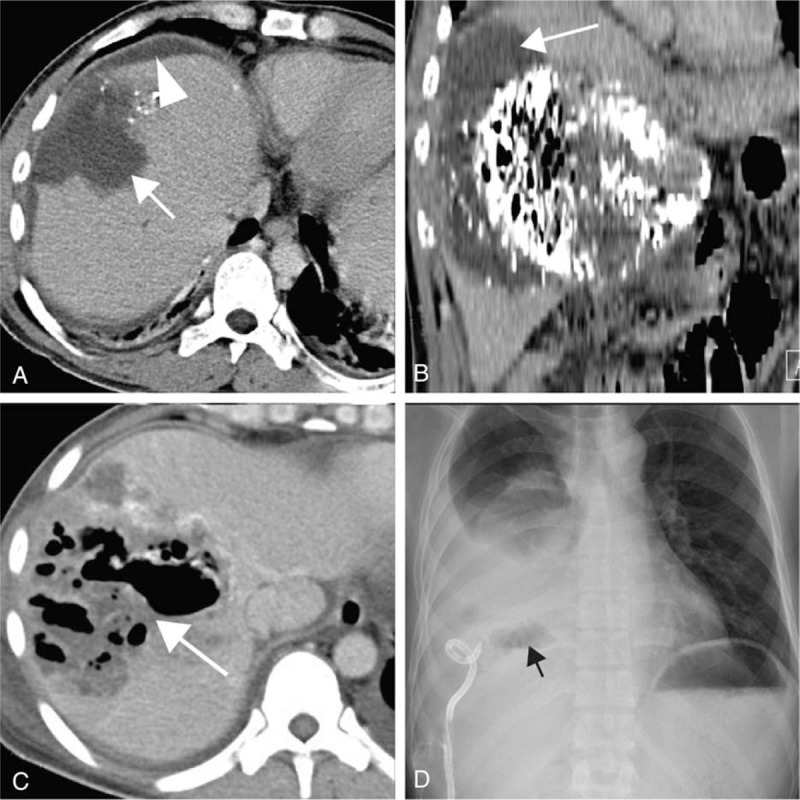
(A and B) A 61-year-old man presented with sudden abdominal pain 6 days after transarterial embolization (TACE) treatment. Computed tomography (CT) scan showed liver rupture following TACE. Hematoma in the liver (arrow) and subcapsular hematoma (arrow head); (C and D) a 37-year-old man presented with fever, chills, and abdominal pain 11 days after TACE treatment for approximately 8 days. CT scan showed large gas (arrow) contained cavity within the liver, near the residual lipiodol area; the liver abscess was identified by percutaneous drainage, and “air–fluid levels” can be seen (black arrow).
Figure 3.
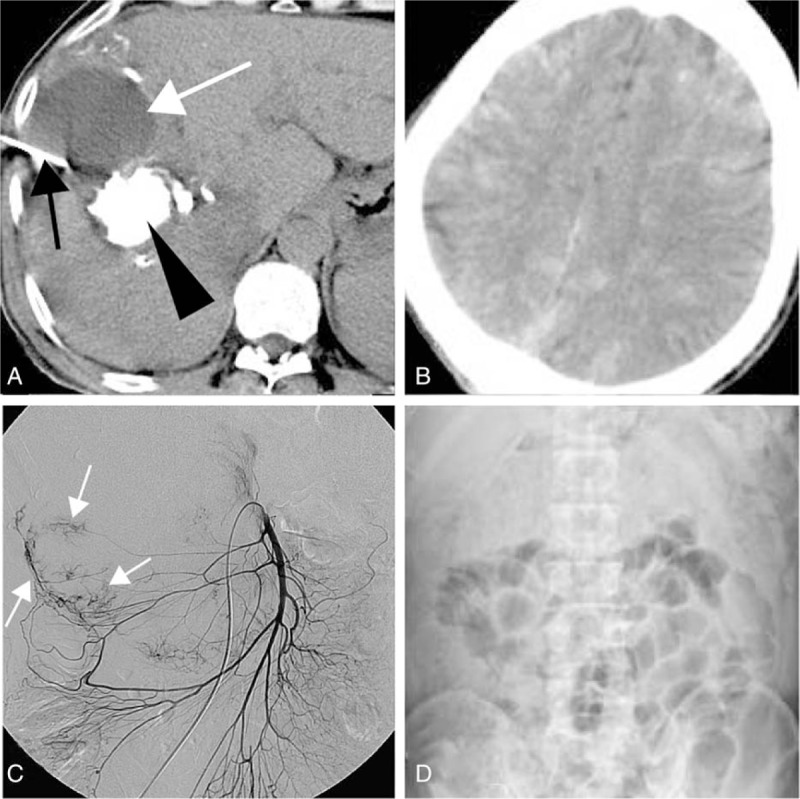
(A) Computed tomography (CT) scan showed a hypodense area (white arrow). Biloma was identified by percutaneous drainage (black arrow). Lipiodol accumulated in the tumor (black arrow head); (B) noncontrast-enhanced CT scan showed multiple disseminated hyperdense lesions in the brain, consistent with deposition of lipiodol; (C and D) the superior mesenteric angiography showed tumor was fed by middle and right colic arteries (white arrow), and transarterial embolization (TACE) was performed via those arteries; partial intestinal obstruction was occurred 2 days post-TACE, and dilatation of the small bowel can be seen in the abdominal X-ray.
3.3. Treatments and outcomes
Of the 24 patients, 22 patients were received treatment for major complications, and remaining 2 patients were died suddenly. Among the 22 patients, the treatments were conservative treatment (n = 12), conservative treatment plus percutaneous drainage (n = 3), ultrasound guided thrombin injection (n = 3), conservative treatment plus TAE (n = 2), conservative treatment plus surgery (n = 2) (Table 1).
Of the 24 patients, 20 (83.3%, 20/24) patients were recovered after treatments, and remaining 4 (16.7%, 4/24) patients were died. Of the 4 patients who died of major complications, 2 were died of liver decompensation on 28 and 47 days after liver rupture, and 2 were died suddenly due to PE on the next morning (Table 1).
4. Discussion
This study demonstrated the incidences of major complication were 2.1% per patient and 0.84% per TAE/TACE procedure; the most frequently reported major complication was liver rupture; conservative treatment can be applied successfully in about half patients, and other interventions were needed in about half patients; the mortality rate of major complication following TAE/TACE was 16.7%.
TAE/TACE is a widely accepted treatment option for unresctable HCC.[13] With the advancement of TAE/TACE technologies, therapeutic effects have improved, whereas complications have decreased.[14,15] However, TAE/TACE therapy may still cause several major complications.[9,16,17] It was reported the incidence of major complication was 2.7% per TAE/TACE procedure.[18] However, the incidence of major complication was 0.84% per TAE/TACE procedure of this study, which was significantly lower. The low incidence of major complication was due to hepatic artery spasm/occlusion was excluded from this study.
There are many risk factors of major complications following TAE/TACE therapy. The risk factors of liver rupture are giant tumor (exceed 10 cm in diameter)[19], tumor located superficially on the surface of the liver, initial and complete embolization of the feeding artery, especially to the patients who additionally used GSP and/or PVA.[9,18] The risk factors of liver abscess are bacterial infection superimposed upon liver embolization and necrosis; infection during the TACE procedure, the immunosuppressant effect of chemotherapeutic agents leading to decreased immunity, patients who have diabetes mellitus, and history of bilioenteric anastomosis.[11] The risk factors of femoral artery pseudoaneurysm are atherosclerosis and hypertension. The risk factors of pulmonary oil or thrombus embolism are arteriovenous shunt, long time of lying in bed following TAE/TACE, and compression of the femoral vein.[20] The risk factor of bile duct injuries is the ischemic injuries of the peribiliary plexus due to repeated TAE/TACE treatment.[21–23] The risk factor of cerebral lipiodol embolism is the existence of hepatic arterio-pulmonary vein shunt. The risk factors of tumor lysis syndrome are large tumor size, rapid tumor growth, chemo-sensitive tumors, large area of tumor necrosis, and pretreatment renal dysfunction.[24] This study showed all 6 patients who developed liver rupture at their initial TACE procedure with giant tumor, and 2 patients who developed biloma after 4.5 TACE procedures. Although 24 patients were included in this study, statistical analyses of risk factors were not possible because of the small sample and various major complications.
The prevention of major complication following TAE/TACE therapy is critical. To prevent the major complications, following points should be paid attention: the interventional radiologists should keep in mind the risk factors mentioned above; avoid nontarget embolization; lipiodol along other than emulsion of lipiodol and chemotherapeutic agents (GSP/PVA) should be used if super-selective embolization cannot be achieved; also, adjunctive maneuvers should be performed to protect extrahepatic parenchy; the dose of lipiodol should not exceed 30 mL for the patients with giant lesions; postprocedure supportive treatments, including hydration, antiemetic, and pain control, are important to reduce the incidence of major complications.
Delayed recognition of major complications and subsequent ones can cause significant morbidity and mortality.[11] The present study suggested that the most frequently reported symptom was pain, followed by fever, however, which were also frequently encountered in patients without major complications. Contrast-enhanced CT, MRI, and abdominal ultrasonography are useful to differentiate major complications formation from tumor necrosis syndrome. Also, the present study suggested that most major complications occurred within 2 weeks of TAE/TACE therapy.
When major complications following TAE/TACE therapy are suspected, prompt treatment is important as major complication is uniformly fatal.[11] The management strategies for major complications following TAE/TACE therapy are various and dependent on major complication itself and patient's condition. Although initial conservative treatment should be carried out immediately for most patients after the occurrences of major complications; some major complications need treatment interventions, like TAE in the treatment of liver rupture, surgery in the treatment of gallbladder perforation, and percutaneous drainage in the treatment of liver abscess or biloma. The present study proved that conservative treatment can be applied successfully in about half the patients, and treatment interventions, including TAE, percutaneous drainage, and surgery, were needed in about half patients.
The mortality of major complications following TAE/TACE therapy is unreported. The early diagnosis and therapeutic strategies for major complications following TAE/TACE therapy have been reported differently due to individual patients’ critical pathophysiological status, such as advanced tumors, hepatic dysfunction, ascites, and dyscrasia.[11] The present study suggested that the mortality rate of major complication following TAE/TACE was 16.7%.
The major limitation of this study is that its retrospective nature, and the true incidence of major complication, is likely to be even higher due to under reporting in the patients; all of which may affect the results.
In conclusion, major complication following conventional TAE/TACE therapy is uncommon; the outcomes are benign of most major complications, but some were mortality.
Footnotes
Abbreviations: CT = computed tomography, GSP = gelatin sponge particles, HCC = hepatocellular carcinoma, MRI = magnetic resonance imaging, PVA = polyvinyl alcohol, TAE/TACE = transarterial embolization/chemoembolization.
JT and ZJ have contributed equally to the article.
Funding/support: This study was supported by Program for Science and Technology Department of Zhejiang Province (2010C33113), and High-Level Medical Talents Training Project of Changzhou (2016CZBJ009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Dig Liver Dis 2016;48:571–7. [DOI] [PubMed] [Google Scholar]
- [2].Zou JH, Zhang L, Ren ZG, et al. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis 2016;17:510–7. [DOI] [PubMed] [Google Scholar]
- [3].Suciu BA, Gurzu S, Marginean L, et al. Significant shrinkage of multifocal liver metastases and long-term survival in a patient with rectal cancer, after trans-arterial chemoembolization (TACE): a case report. Medicine (Baltimore) 2015;94:e1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bae SI, Yeon JE, Lee JM, et al. A case of necrotizing pancreatitis subsequent to transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin Mol Hepatol 2012;18:321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Miyayama S, Yamashiro M, Okuda M, et al. Main bile duct stricture occurring after transcatheter arterial chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2010;33:1168–79. [DOI] [PubMed] [Google Scholar]
- [6].Chu HJ, Lee CW, Yeh SJ, et al. Cerebral lipiodol embolism in hepatocellular carcinoma patients treated with transarterial embolization/chemoembolization. PLoS One 2015;10:e0129367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Toro A, Bertino G, Arcerito MC, et al. A lethal complication after transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. Case Rep Surg 2015;2015:873601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pietrosi G, Miraglia R, Luca A, et al. Arterial chemoembolization/embolization and early complications after hepatocellular carcinoma treatment: a safe standardized protocol in selected patients with Child class A and B cirrhosis. J Vasc Interv Radiol 2009;20:896–902. [DOI] [PubMed] [Google Scholar]
- [9].Jia Z, Tian F, Jiang G. Ruptured hepatic carcinoma after transcatheter arterial chemoembolization. Curr Ther Res Clin Exp 2013;74:41–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2003;14:S199–202. [DOI] [PubMed] [Google Scholar]
- [11].Lv WF, Lu D, He YS, et al. Liver abscess formation following transarterial chemoembolization: clinical features, risk factors, bacteria spectrum, and percutaneous catheter drainage. Medicine (Baltimore) 2016;95:e3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brown DB, Nikolic B, Covey AM, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 2012;23:287–94. [DOI] [PubMed] [Google Scholar]
- [13].Takayasu K, Arri S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma. Gastroenterology 2006;131:461–9. [DOI] [PubMed] [Google Scholar]
- [14].Matsui O, Kadoya M, Yoshikawa J, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology 1993;188:79–83. [DOI] [PubMed] [Google Scholar]
- [15].Takayasu K, Muramatsu Y, Maeda T, et al. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR Am J Roentgenol 2001;176:681–8. [DOI] [PubMed] [Google Scholar]
- [16].Chung JW, Park JH, Im JG, et al. Pulmonary oil embolism after transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology 1993;187:689–93. [DOI] [PubMed] [Google Scholar]
- [17].Chung JW, Park JH, Han JK, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology 1996;198:33–40. [DOI] [PubMed] [Google Scholar]
- [18].Xia J, Ren Z, Ye S, et al. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol 2006;59:407–12. [DOI] [PubMed] [Google Scholar]
- [19].Bara T, Gurzu S, Jung I, et al. Giant cavernous hepatic hemangioma diagnosed incidentally in a perimenopausal obese female with endometrial adenocarcinoma: a case report. Anticancer Res 2016;36:769–72. [PubMed] [Google Scholar]
- [20].Lee DH, Hwang JC, Lim SM, et al. Pleural and pulmonary staining at inferior phrenic arteriography mimicking a tumor staining of hepatocellular carcinoma. Cardiovasc Intervent Radiol 2000;23:109–13. [DOI] [PubMed] [Google Scholar]
- [21].Kobayashi S, Nakanuma Y, Terada T, et al. Postmortem survey of bile duct necrosis and biloma in hepatocellular carcinoma after transcatheter arterial chemoembolization therapy: relevance to microvascular damages of peribiliary capillary plexus. Am J Gastroenterol 1993;88:1410–5. [PubMed] [Google Scholar]
- [22].Kemeny M, Battifora H, Blayney D, et al. Sclerosing cholangitis after continuous hepatic artery infusion of FUDR. Ann Surg 1985;202:176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim HK, Chung YH, Song BC, et al. Ischemic bile duct injury as a serious complication after transarterial chemoembolization in patients with hepatocellular carcinoma. J Clin Gastroenterol 2001;32:423–7. [DOI] [PubMed] [Google Scholar]
- [24].Sakamoto N, Monzawa S, Nagano H, et al. Acute tumor lysis syndrome caused by transcatheter oily chemoembolization in a patient with a large hepatocellular carcinoma. Cardiovasc Intervent Radiol 2007;30:508–11. [DOI] [PubMed] [Google Scholar]


