Abstract
The aim of this study was to evaluate the relationship between the hypertriglyceridemic waist (HTGW) phenotype and metabolic abnormalities in hypertensive adults.
A cross-sectional study, with a sample of 5919 hypertensive adults (2892 men and 3027 women) aged 35 years or older, was recruited from rural areas of China. The participants underwent anthropometric measurements and laboratory examinations. The self-reported information was collected by trained personnel. The HTGW phenotype was defined as elevated triglycerides and elevated waist circumference. The logistic regression analysis was used to evaluate the associations of interest.
Hypertensive adults with the HTGW phenotype had significantly higher prevalences of all cardiometabolic risk factors than those without the HTGW phenotype (P < 0.001). Compared with the normal waist normal triglyceride (NWNT) group, hypertensive adults with the HTGW phenotype had much higher possibilities to have all cardiometabolic risk factors, especially for 8.35 times more likely of having ≥3 cardiometabolic risk factors [95% confidence interval (95% CI) 5.92–11.79], 6.14 times more likely of having low HDL cholesterol (95% CI 4.98–7.58), 5.49 times more likely of having hyperuricemia (95% CI 4.40–6.86), and 4.32 times more likely of having 1 to 2 cardiometabolic risk factors (95% CI 3.68–5.07) (P < 0.001). Multivariate analysis indicated that the HTGW phenotype was positively associated with metabolic abnormalities (P < 0.05).
This study concluded that the HTGW phenotype was positively associated with metabolic abnormalities in hypertensive adults. The HTGW phenotype showed to be an important tool for monitoring of hypertensive adults with metabolic abnormalities, which is low cost, simple, and useful in clinical practice, especially in primary health care in the rural area of China.
Keywords: hypertension, hypertriglyceridemia, hypertriglyceridemic waist phenotype, waist circumference
1. Introduction
Cardiovascular disease (CVD) is one of the major causes of death worldwide,[1] and even in China.[2] During recent decades, the prevalence of CVD in China has increased as a result of the enormous population, rapid economic development, lifestyle changes, and aging of the population.[3] Obesity is considered as a major risk factor for CVD.[4,5] Although body mass index (BMI) is most commonly used to assess general obesity, waist circumference (WC), as the measure of abdominal obesity, has been proved to be a better predictor of CVD than BMI.[6–8] However, WC alone is not sufficient to distinguish between intra-abdominal and subcutaneous abdominal adiposity. Several studies presented that visceral adiposity has positive associations with metabolic abnormalities, including hypertension, type 2 diabetes, dyslipidemia, and hyperinsulinemia.[9,10] Measurements of visceral adiposity require imaging techniques such as computed tomography (CT) or magnetic resonance imaging (MRI), which are not practical means for the general population due to the high cost and radiation exposure. Therefore, the hypertriglyceridemic waist (HTGW) phenotype (defined as hypertriglyceridemia and elevated WC) was firstly proposed by Lemieux et al[11] and suggested to be a simple and inexpensive tool to predict cardiometabolic abnormalities in individuals with excess visceral adipose.[12,13]
Some investigators have reported that hypertensive patients have a greater risk for having HTGW phenotype.[14] However, data regarding the relationship between HTGW phenotype and metabolic abnormalities are scanty, especially in Asian populations that have a higher prevalence of central obesity than other populations. Until now, only 1 Brazilian study[15] found that the HTGW phenotype was significantly associated with metabolic abnormalities in hypertensive women, but not in hypertensive men. Thus, we conducted this cross-sectional study to evaluate the association between HTGW phenotype and metabolic abnormalities in hypertensive adults.
2. Methods
2.1. Study population
In 2012 and 2013, we recruited a representative sample of general population aged ≥35 years to describe the prevalence, incidence, and natural history of cardiovascular risk factors in rural areas of Liaoning Province, which was named Northeast China Rural Cardiovascular Health Study (NCRCHS). Participants were selected using a multistage-stratified random cluster sampling method. Step 1, 3 counties (Dawa, Zhangwu, and Liaoyang County) were randomly selected from Liaoning province; step 2, 1 town was randomly selected from each county (a total of 3 towns); step 3, 8 to 10 rural villages from each town were randomly selected (a total of 26 rural villages). Finally, a total of 11,956 participants from all the 14,016 eligible permanent residents aged ≥35 years agreed and completed the present study, with a response rate of 85.3%. The study was approved by the Ethics Committee of China Medical University (Shenyang, China) (AF-SDP-07-1, 0-01). All procedures were performed in accordance with the ethical standards. Written consent was obtained in all participants after they had been informed of the objectives, benefits, medical items, and confidentiality agreement of personal information. If the participants were illiterate, we obtained the written informed consents from their proxies. After conducting the survey, we analyzed the data of this general population and found that the prevalence of hypertension was significantly high. Then, we selected the participants with hypertension and studied the data of them. In this report, we used data of baseline and only participants with hypertension and a complete set of data regarding the variables analyzed in the study were included, making a final sample size of 5919 people (2892 men and 3027 women).
2.2. Data collection
Data were collected during a single clinic visit by cardiologists and trained nurses using a standard questionnaire by face-to-face interview. Before the survey was performed, we invited all eligible investigators to attend the organized training. The training contents included the purpose of this study, how to administer the questionnaire, the standard method of measurement, the importance of standardization, and the study procedures. A strict test was evaluated after this training; only those who scored perfectly on the test could become investigators. During data collection, our inspectors had further instructions and support.
Data on demographic characteristics, lifestyle risk factors, and medical history were obtained by interview with a standardized questionnaire. The questionnaire was designed by statistical experts and clinical specialists. There was a central steering committee with a subcommittee for quality control. The project management office of Liaoning Province will check randomly for 5% questionnaires, the unqualified questionnaires will be re-investigated again, and if the investigator made the fake questionnaire, we will cancel the qualification of this investigator and abandon all of his or her questionnaires. People who ever smoked at least 1 cigarette per day for over 6 months and smoke now were defined as current smokers. People who ever taken alcohol at least twice a week for over a year and take alcohol now were defined as current drinkers. Information about physical activity was obtained by questionnaire. Physical activity, including occupational and leisure-time physical activity, were categorized into low, moderate, and high groups according to the self-reported questionnaire.[16] Educational degree was also investigated and classified into 3 levels: primary school or below, middle school, and high school or above.
2.3. Blood pressure measurements
Blood pressure was measured 3 times by an automatic electronic sphygmomanometer (HEM-741C; Omron, Tokyo, Japan), according to American Heart Association protocol. Every month, 2 doctors calibrated the Omron sphygmomanometer using a standard mercury one under protocol of the British Hypertension Society.[16,17] Blood pressure measurements were performed after at least 30 minutes rest in a sitting position. During the measurement, the participants were seated with the arm supported at the level of the heart. We calculated the mean of 3 measurements as the participant's blood pressure.
2.4. Anthropometric assessment
Weight and height were measured to the nearest 0.5 kg and 0.1 cm, respectively, with the subjects standing and wearing only underwear. Waist circumstance (WC) was measured with a tape (to the nearest 0.1 cm), at the level midway between the lower rib margin and the iliac crest.
2.5. Biochemical measurements
A venous blood sample was drawn from all of the participants after 12-hour overnight fasting and obtained into EDTA vacutainer tubes. As the blood was obtained in the rural areas, we prepared many thermo containers in advance to transport the blood samples immediately. After about 1 hour, the blood samples were transported to the clinical laboratory of the First Affiliated Hospital of China Medical University by refrigerated truck. Serum was subsequently isolated and frozen at –20°C for further testing. Serum concentrations of total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting plasma glucose (FPG), serum uric acid, and other routine blood biochemical indexes were determined using enzymatic kits by an Olympus AU640 auto analyzer (Olympus, Kobe, Japan).
2.6. Definitions
In agreement with JNC-7 report,[18] hypertension was defined as being under antihypertensive treatment and/or having a systolic blood pressure (SBP) ≥140 mm Hg and/or having a diastolic blood pressure (DBP) ≥90 mm Hg. The BMI was calculated using the formula weight (kg)/height2 (m2). According to the WHO criteria,[19] participants were classified into 3 groups: having normal weight when BMI <25 kg/m2, overweight when 25 ≤BMI<30 kg/m2, and obesity when BMI ≥30 kg/m2. Diabetes was diagnosed with the criteria of WHO[20]: FPG ≥7 mmol/L (126 mg/dL) and/or being on treatment for diabetes. The reference values for high TC, LDL, and low HDL were those recommended by the NCEP: TC ≥6.21 mmol/L (240 mg/dL), LDL-C ≥4.16 mmol/L (160 mg/dL), and HDL-C <1.03 mmol/L (40 mg/dL).[5] Serum uric acid ≥416 μmol/L in men and ≥357 μmol/L in women were considered as hyperuricemia.[21] In this study, participants were categorized into 4 phenotype groups based on the following cut-off points[22,23]: (1) NWNT (normal waist normal TG: WC <90 cm for men and <80 cm for women; TG <1.7 mmol/L); (2) EWNT (enlarged waist-normal TGs: WC ≥90 cm for men and ≥80 cm for women; TG <1.7 mmol/L); (3) NWET (normal waist-elevated TGs: WC <90 cm for men and <80 cm for women; TG ≥1.7 mmol/L); (4) HTGW (hypertriglyceridemic-waist: WC ≥90 cm for men and ≥80 cm for women; TG ≥1.7 mmol/L).
2.7. Statistical analysis
Continuous variables were shown as mean ± standard deviation (SD), whereas categorical variables were expressed as percentages. Among the phenotype groups, analysis of variance (ANOVA) test was used for the continuous variables, and χ2-test analyses for the categorical variables. Logistic regression analyses with odds ratios (ORs) and 95% confidence intervals (CIs) were performed to estimate the association between HTGW phenotype and metabolic abnormalities. All statistical analyses were performed using SPSS version 19.0 software (SPSS Inc, Chicago, IL), and P < 0.05 was considered statistically significant.
3. Results
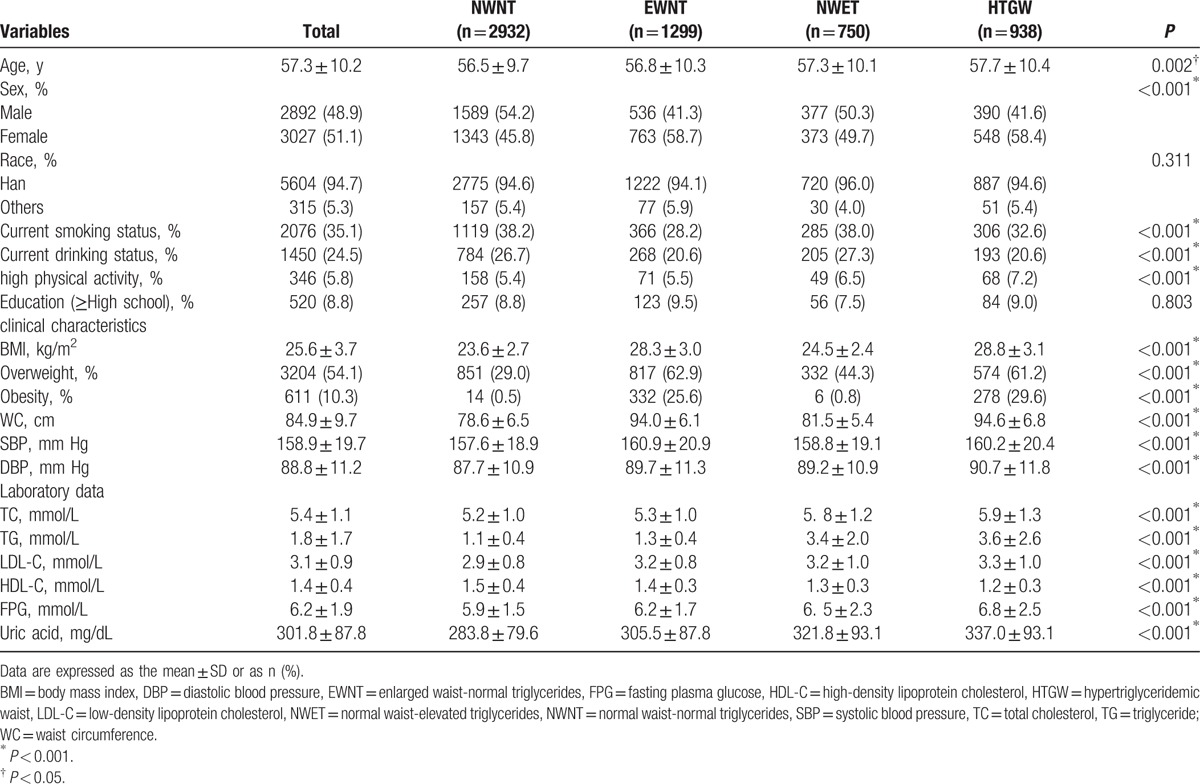
Participants in this study were 5919 hypertensive adults, 2932 (49.5%) were NWNT, 1299 (21.9%) were EWNT, 750 (12.7%) were NWET, and 938 (15.8%) were HTGW phenotype. The baseline characteristics according to phenotype groups are presented in Table 1. Subjects with the HTGW phenotype were more likely to be females, with a mean age of 57.7 (10.4) years. The HTGW phenotype group had lower frequencies of both current smoking status (P < 0.001) and current drinking status (P < 0.001), whereas a higher frequency of high-intensity physical activity (P < 0.001). For the clinical characteristics and laboratory data, categorized by the 4 phenotype groups, participants in the groups of EWNT, NWET, and HTGW phenotype presented averages significantly higher levels of BMI, WC, SBP, DBP, FPG, uric acid, TC, TG, LDL-C, and lower levels of HDL-C (P < 0.001). In the HTGW phenotype, the prevalence of obesity (29.6%) was quite high and was significantly higher than other groups (P < 0.001). No significant differences were found in race and educational level between the 4 phenotype groups.
Table 1.
Baseline characteristics of the hypertensive participants across HTGW phenotype groups (N = 5919).
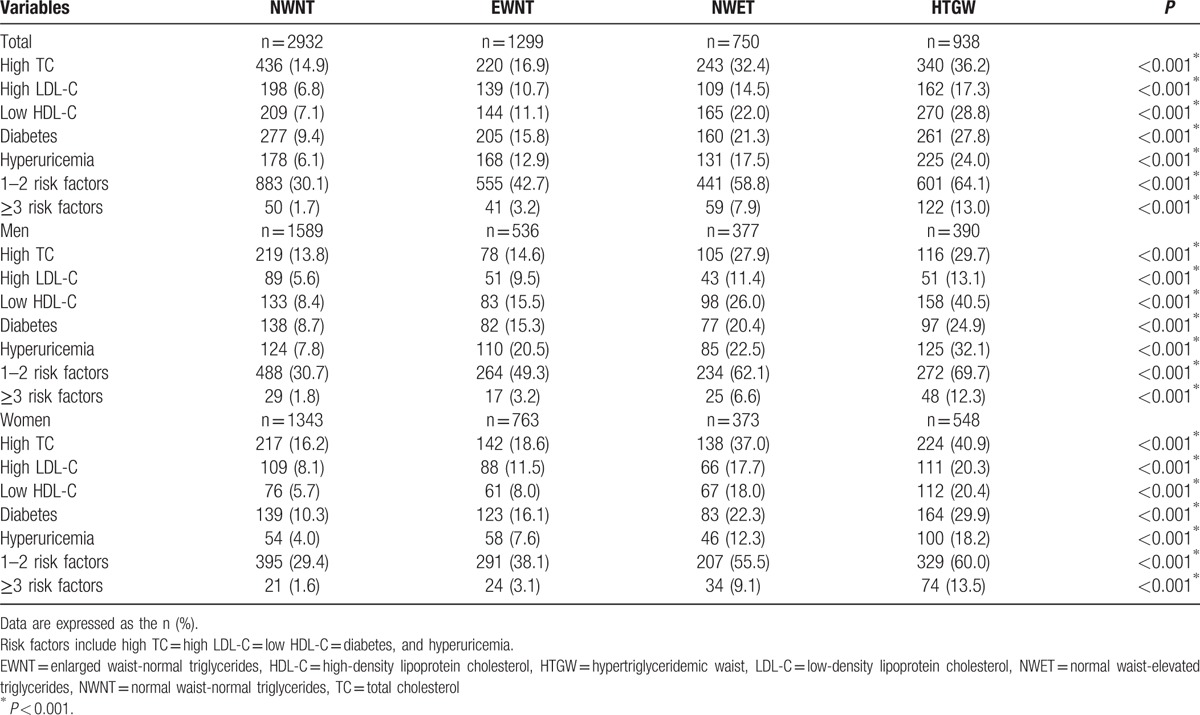
The prevalence of metabolic abnormalities in the hypertensive participants in each phenotype group is summarized in Table 2. Individuals with HTGW phenotype, 64.1% had 1 to 2 and 13.0% had 3 or more metabolic abnormalities. Subjects with the HTGW phenotype had significantly higher prevalences of all metabolic abnormities than the other 2 phenotypes for both sexes (P < 0.001). Among men with the HTGW phenotype, 29.7% had hypercholesterolemia, 13.1% high LDL-C, 40.5% low HDL-C, 24.9% diabetes, and 32.1% hyperuricemia (P < 0.001). Among women with the HTGW phenotype, 40.9% had hypercholesterolemia, 20.3% high LDL-C, 20.4% low HDL-C, 29.9% diabetes, and 18.2% hyperuricemia (P < 0.001).
Table 2.
Prevalence of metabolic abnormalities in the hypertensive participants across HTGW phenotype groups (N = 5919).
The cardiovascular risk factors frequency was presented among individuals of the 4 phenotype groups in Fig. 1. It was observed that among individuals with HTGW phenotype, 13.0% had 3 or more cardiovascular risk factors and 64.1% had 1 to 2 risk factors. While from individuals with the NWNT phenotype, 1.7% had 3 or more risk factors, and 30.1% had 1 to 2 risk factors, with statistically significant difference between groups (P < 0.001).
Figure 1.
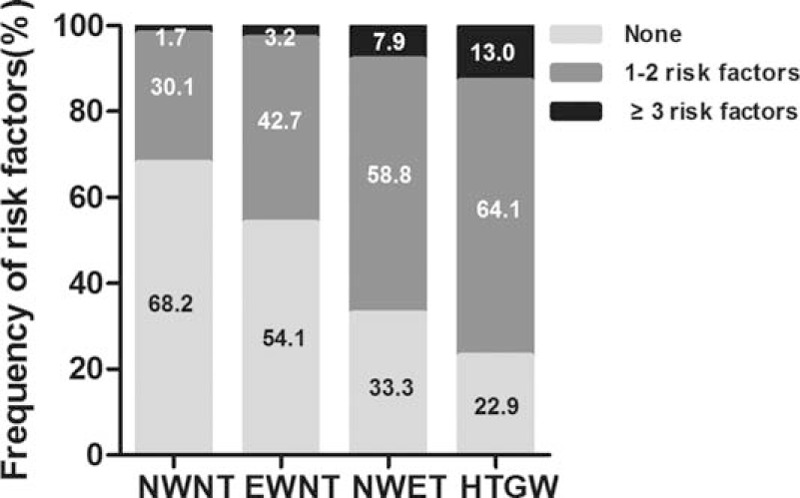
Frequency of cardiovascular risk factors among individuals of the 4 phenotype groups. EWNT = enlarged waist-normal triglycerides, HTGW = hypertriglyceridemic waist, NWET = normal waist-elevated triglycerides, NWNT = normal waist-normal triglycerides.
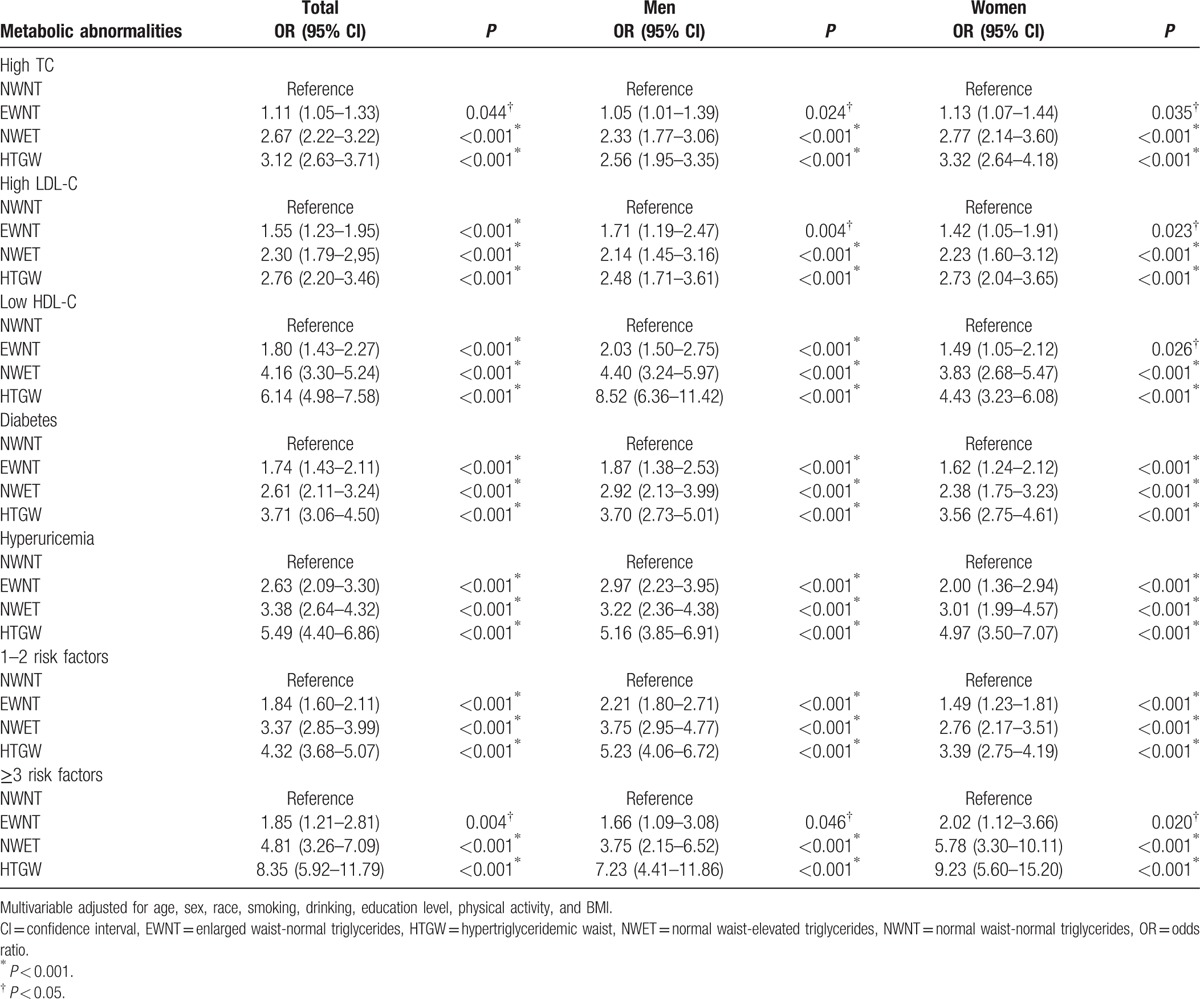
Then, multivariate analysis by logistic regression showed the relationship between the HTGW phenotype and metabolic abnormalities (Table 3). After adjusting for age, sex, race, smoking, drinking, education level, physical activity, SBP, and DBP, subjects with the HTGW phenotype had (OR 8.35; 95% CI 5.92–11.79) in total population (OR 7.23; 95% CI 4.41–11.86) in men and (OR 9.23; 95% CI 5.60–15.20) in women for ≥3 risk factors, respectively, compared with those subjects with NWNT phenotype. The results presented HTGW phenotype had a positive relationship with high TC, high LDL-C, low HDL-C, diabetes, and hyperuricemia (P < 0.001). The data indicated that compared with the NWNT phenotype, the HTGW phenotype had 3.12 times more likely (95% CI 2.63–3.70) of having high TC, 2.76 times (95% CI 2.20–3.46) of having high LDL-C, 6.14 times (95% CI 4.98–7.58) of having low HDL-C, 3.71 times (95% CI 3.06–4.50) of having diabetes, and finally 5.49 times (95% CI 4.40–6.86) of having hyperuricemia. Multivariate analysis for both genders indicated separately that the HTGW phenotype was positively associated with metabolic abnormalities (P < 0.05). Among subjects with EWNT or NWET, the strength of the association for metabolic abnormalities was attenuated but remained statistically significant (P < 0.05).
Table 3.
Multivariate-adjusted odds ratios (and 95% CIs) for metabolic risk factors across HTGW phenotype groups.
3.1. Discussion
The major finding of this present study was that the HTGW phenotype was a strong marker of metabolic abnormalities for both genders in this rural Chinese hypertensive population. It is the first study assessing the association of HTGW phenotype with metabolic abnormalities in hypertensive adults of China. The prevalence of HTGW phenotype in the studied hypertensive adults was 15.8% for total individuals, 13.5% for men and 18.1% for women, respectively. After adjusting for the effect of covariates, the HTGW phenotype was associated with hypercholesterolemia, high LDL-C, low HDL-C, diabetes, and hyperuricemia.
In our present study, the prevalence of HTGW phenotype was similar to that observed by Mendes,[24] but was much lower than a Brazilian study which also found that the HTGW phenotype was significantly associated with metabolic abnormalities in hypertensive women.[15] Other studies that evaluated HTGW phenotype in nonhypertensive participants found a prevalence of 10.8% in Spanish adults[25] and 23.6% in Iranian adults.[26] Note that variations in prevalence may be due to different cutoff points for WC and serum TGs, as well as ethnic differences.[26] Moreover, we previously evaluated HTGW phenotype in nonhypertensive patients and found it not associated with metabolic abnormalities. In contrast, it must be considered that subjects of the present study were hypertensive patients, with older mean age, high prevalence of diabetes and dyslipidemia, and high percentage of smoking and drinking, which represented a greater chance of developing HTGW phenotype and CVDs.
This study indicated a positive and significant relationship between the HTGW phenotype and abnormal lipid profiles. In accordance with the study from Mendes,[24] the HTGW phenotype was positively associated with high TC, high LDL-C, and low HDL-C. Another study conducted in Iran showed that in female participants, there were significant associations between HTGW phenotype and levels of TC and HDL-C.[26] The study by Blackburn et al[27] also indicated that white men had an abnormal lipid profile associated with the HTGW phenotype. Similarly, the HTGW phenotype was found positively associated with TC and HDL-C in diabetic patients from a developed country.[28] It is noteworthy that subjects with the HTGW phenotype tend to have high TC, high LDL-C, and low HDL-C, characterizing an abnormal lipid profile, contributing to an increased cardiovascular risk.[14]
In the same way that the present study did, other studies[26,29] demonstrated an association between the HTGW phenotype and diabetes, which significantly contributed to the risk of metabolic abnormalities and developing CVD.[12]
Another important metabolic variable analyzed was the uric acid. Our results indicated that there was a positive association between the HTGW phenotype and hyperuricemia in both sexes. However, until now, only 1 study in Brazilian adults had been conducted to assess this association, in which the significant association was showed only in women.[22]
Cunha de Oliveira et al[22] observed that 82% of individuals in the HTGW phenotype group had 3 or more cardiovascular risk factors, but different cut-off points were used for the analyzed biochemical variables. In our study, 77.1% of hypertensive participants with HTGW phenotype had at least 1 metabolic abnormality, which was nearly 2.5 times higher than the NWNT phenotype group. Some possible reasons might be responsible for this phenomenon. An elevated WC alone does not identify individuals with excess amount of visceral adipose tissue (VAT) or high cardiovascular risk, because the accumulation of adipose tissue can be subcutaneous.[12] Data presented here corroborated with scientific literature when describing the HTGW phenotype as a marker for a variety of metabolic abnormalities[11,30,31] strengthen the use of HTGW phenotype as a global cardiometabolic risk. The HTGW phenotype, as a practical and easily applied tool, is a useful approach to identify subjects with VAT. The low cost makes it an available and easily applicable indicator in clinical practice and research. The HTGW phenotype can be useful for health professionals to identify hypertensive adults with a variety of metabolic abnormalities who may benefit from early intervention. Data from the present study are particularly important in rural Chinese population who has a high absolute risk of CVDs. Therefore, further researches should be necessary to assess the HTGW phenotype before firm conclusions can be drawn.
The main strengths of our study are its population-based design, and the first assessment of the relationship between the HTGW phenotype and metabolic abnormalities. However, there are a number of limitations in the current study. First, the main limitation of this study is its cross-sectional nature, which may lead to a causal association between the HTGW phenotype and metabolic abnormalities. Second, some unmeasured confounders may explain part of the associations in our study.
In conclusion, the current study indicated that the HTGW phenotype was positively associated with metabolic abnormalities in hypertensive adults. Due to the simplicity and low cost of measurements of WC and TG levels, using the HTGW phenotype to identify hypertensive patients at a high risk of metabolic abnormalities may be useful for early intervention. The findings of this study play a vital role in public health issues of CVD. In recent years, with the rapid growth of obesity and CVD across the world, it is important and urgent to develop convenient and cheap indicators for the early recognition of individuals at a high risk of metabolic abnormalities and CVD.
Acknowledgment
The authors thank YZ, LX, and GP for their assistance.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, CVD = cardiovascular disease, DBP = diastolic blood pressure, EWNT = enlarged waist-normal triglycerides, FPG = fasting plasma glucose, HDL-C = high-density lipoprotein cholesterol, HTGW = hypertriglyceridemic waist, LDL-C = low-density lipoprotein cholesterol, NWET = normal waist-elevated triglycerides, NWNT = normal waist-normal triglycerides, OR = odds ratio, SBP = systolic blood pressure, TC = total cholesterol, TG = triglyceride, WC = waist circumstance.
Authorship: SC analyzed data and drafted the manuscript. XG and SY gave guidance on writing this paper. HY, ZL, and GS performed the research. YS designed the research. All authors have read and approved the final manuscript.
Funding/support: This study was supported by grants from the “Twelfth Five-Year” project funds (National Science and Technology Support Program of China, Grant # 2012BAJ18B02) that YS is responsible for the project completion.
The authors have no potential conflict of interest to declare.
References
- [1].WHO. World Health Statistics 2011. Geneva: World Health Organization; 2011. Available at: http://www.who.int/gho/publications/ Accessed February 7, 2012. [Google Scholar]
- [2].He J, Gu D, Wu X, et al. Major causes of death among men and women in China. N Engl J Med 2005;353:1124–34. [DOI] [PubMed] [Google Scholar]
- [3].Gao B, Zhang L, Wang H. Clustering of major cardiovascular risk factors and the association with unhealthy lifestyles in the Chinese adult population. PLoS One 2013;8:e66780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i–10. 1-253. [PubMed] [Google Scholar]
- [5].Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- [6].Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 2004;79:379–84. [DOI] [PubMed] [Google Scholar]
- [7].Larsson B, Svardsudd K, Welin L, et al. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Brit Med J (Clinical research ed) 1984;288:1401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oppert JM, Charles MA, Thibult N, et al. Anthropometric estimates of muscle and fat mass in relation to cardiac and cancer mortality in men: the Paris Prospective Study. Am J Clin Nutr 2002;75:1107–13. [DOI] [PubMed] [Google Scholar]
- [9].Arsenault BJ, Lachance D, Lemieux I, et al. Visceral adipose tissue accumulation, cardiorespiratory fitness, and features of the metabolic syndrome. Arch Intern Med 2007;167:1518–25. [DOI] [PubMed] [Google Scholar]
- [10].Zhang C, Rexrode KM, van Dam RM, et al. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation 2008;117:1658–67. [DOI] [PubMed] [Google Scholar]
- [11].Lemieux I, Pascot A, Couillard C, et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation 2000;102:179–84. [DOI] [PubMed] [Google Scholar]
- [12].Sam S, Haffner S, Davidson MH, et al. Hypertriglyceridemic waist phenotype predicts increased visceral fat in subjects with type 2 diabetes. Diabetes Care 2009;32:1916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arsenault BJ, Lemieux I, Despres JP, et al. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. CMAJ 2010;182:1427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Esmaillzadeh A, Mirmiran P, Azizi F. Clustering of metabolic abnormalities in adolescents with the hypertriglyceridemic waist phenotype. Am J Clin Nutr 2006;83:36–46. quiz 183-184. [DOI] [PubMed] [Google Scholar]
- [15].Cabral NA, Ribeiro VS, Franca AK, et al. Hypertriglyceridemic waist and cardiometabolic risk in hypertensive women. Rev Assoc Med Bras 1992;58:568–73. [DOI] [PubMed] [Google Scholar]
- [16].Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Medicine and science in sports and exercise 2000;32(9 Suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- [17].O’Brien E, Petrie J, Littler W, et al. An outline of the revised British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens 1993;11:677–9. [DOI] [PubMed] [Google Scholar]
- [18].Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- [19].Hypertension control. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1996;862:1–83. [PubMed] [Google Scholar]
- [20].Alberti KG, Zimmet PZ. Definition diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- [21].Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension 2003;42:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cunha de Oliveira C, Carneiro Roriz AK, Eickemberg M, et al. Hypertriglyceridemic waist phenotype: association with metabolic disorders and visceral fat in adults. Nutr Hosp 2014;30:25–31. [DOI] [PubMed] [Google Scholar]
- [23].Chen S, Guo X, Yu S, et al. Association between the hypertriglyceridemic waist phenotype, prediabetes, and diabetes mellitus in rural Chinese population: a cross-sectional study. Int J Environ Res Public Health 2016;13:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mendes MSF MJ. Cintura hipertrigliceridêmica e sua associação com fatores de risco metabólicos [MSc dissertation]. Belo Horizonte, Brasil, Univer-sidade Federal de Minas Gerais; 2009. [Google Scholar]
- [25].Gomez-Huelgas R, Bernal-Lopez MR, Villalobos A, et al. Hypertriglyceridemic waist: an alternative to the metabolic syndrome? Results of the IMAP Study (multidisciplinary intervention in primary care). Int J Obes 2005;35:292–9. [DOI] [PubMed] [Google Scholar]
- [26].Amini M, Esmaillzadeh A, Sadeghi M, et al. The association of hypertriglyceridemic waist phenotype with type 2 diabetes mellitus among individuals with first relative history of diabetes. J Res Med Sci 2011;16:156–64. [PMC free article] [PubMed] [Google Scholar]
- [27].Blackburn P, Lemieux I, Almeras N, et al. The hypertriglyceridemic waist phenotype versus the National Cholesterol Education Program-Adult Treatment Panel III and International Diabetes Federation clinical criteria to identify high-risk men with an altered cardiometabolic risk profile. Metabolism 2009;58:1123–30. [DOI] [PubMed] [Google Scholar]
- [28].de Graaf FR, Schuijf JD, Scholte AJ, et al. Usefulness of hypertriglyceridemic waist phenotype in type 2 diabetes mellitus to predict the presence of coronary artery disease as assessed by computed tomographic coronary angiography. Am J Cardiol 2010;106:1747–53. [DOI] [PubMed] [Google Scholar]
- [29].Egeland GM, Cao Z, Young TK. Hypertriglyceridemic-waist phenotype and glucose intolerance among Canadian Inuit: the International Polar Year Inuit Health Survey for Adults 2007-2008. CMAJ 2011;183:E553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Okosun ISBJ. Abdominal obesity, hypertriglyceridemia, hypertriglyceridemic waist phenotype and risk of type 2 diabetes in American adults. Diabetes Metab Syndr 2008;2:273–81. [Google Scholar]
- [31].Despres JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008;28:1039–49. [DOI] [PubMed] [Google Scholar]


