Abstract
Staphylococcus aureus is a leading cause of infective endocarditis in people who inject drugs (PWID). The management of S aureus endocarditis (SAE) in PWID can be problematic. The objective of this retrospective observational study was to assess the epidemiology, clinical characteristics, and mortality of S aureus endocarditis (SAE) in PWID in Stockholm, Sweden.
The Department of Infectious Diseases at the Karolinska University Hospital serves as a regional referral center for drug users with severe infections. Patients with active intravenous drug use treated for SAE at the department between January 2004 and December 2013 were retrospectively identified. Clinical and microbiological data were obtained from medical records and the diagnosis verified according to the modified Duke criteria.
In total, 120 SAE episodes related to intravenous drug use were identified. Its incidence in Stockholm was 0.76/100,000 adult person-years for the entire period, increasing from 0.52/100,000 person-years in 2004 to 2008 to 0.99 in 2009 to 2013 (P = 0.02). The SAE incidence among PWID specifically was 249 (range 153–649) /100,000 person-years. Forty-two (35%) episodes were left-sided, and multiple valves were involved in 26 (22%). Cardiac valve surgery was performed in 10 (8%) episodes, all left-sided. The in-hospital and 1-year mortality rates were 2.5% (3 deaths) and 8.0% (9 deaths), respectively.
We noted a high and increasing incidence over time of SAE related to intravenous drug use in Stockholm. The increased incidence partly reflects a rising number of PWID during the study period. The low mortality noted, despite a substantial proportion with left-sided endocarditis, probably in part reflects the quality of care obtained at a large and specialized referral center for drug users with severe infections.
Keywords: cardiac surgery, incidence, infective endocarditis, intravenous drug abuse, mortality, referral centre, Staphylococcus aureus
1. Introduction
Intravenous drug use is a global health problem associated with a wide range of medical complications, including infections. Infective endocarditis (IE) is an important and serious reason for hospitalization among people who inject drugs (PWID).[1]Staphylococcus aureus is a leading cause of IE in PWID, accounting for approximately two-thirds of cases.[2–4]S aureus endocarditis (SAE) affecting the left-sided heart valves is often associated with a high morbidity and mortality, whereas right-sided endocarditis usually is milder and more exclusively associated with intravenous drug use.[3,5,6] Treatment of SAE in PWID generally involves at least 2 weeks of intravenous antibiotic therapy, but can be associated with challenges in compliance. Cardiac valve surgery may also be necessary.[7,8] Hence, providing care with specialized healthcare personnel experienced in treating PWID could lead to better compliance and improved outcome. Despite the potential seriousness of SAE in PWID, recent studies are few and have often included only a small number of cases.[6,9–15]
Our objective was to study the incidence, clinical characteristics, management, and mortality of SAE in PWID at a Swedish referral center specialized in treating drug users, and to compare the characteristics in PWID with those in non-drug users.
2. Materials and methods
2.1. Study population and protocol
Stockholm County has some 2.2 million inhabitants. The number of PWID in the region has been estimated to 1850 to 7800 individuals, with an average of 4800 persons[16,17] (National Board of Health and Welfare, unpublished data, December 2014; M Kåberg, MD, personal communication). The Karolinska University Hospital is the largest hospital in the county, serving as a tertiary referral center for the entire population and providing secondary healthcare to part of it. In Stockholm patients with suspected IE are usually admitted to specialized infectious diseases departments, and those who need valvular heart surgery are generally admitted to an infectious diseases department before and after surgery. Approximately two-thirds of all infectious diseases in-patient beds in Stockholm County are located at the Karolinska University Hospital, including a specific ward serving as a referral center for drug users with severe infections. The only thoracic surgery department in the region is located at the hospital. Hence, nearly all PWID with suspected IE are treated at the Karolinska University Hospital.
PWID treated for SAE at the Department of Infectious Diseases at the Karolinska University Hospital during January 2004 to December 2013 were included in the study. Retrospectively, a search was done in the records of the department for diagnostic codes representing IE according to the 10th revision of International Classification of Diseases (ICD-10). The medical records were reviewed and microbiological data obtained to identify cases with IE caused by S aureus. Clinical data, including information on intravenous drug use, and echocardiography reports were reviewed and the diagnosis of SAE was verified according to the modified Duke criteria.[18] Information on population statistics was retrieved from Statistics Sweden. The Regional Ethical Review Board in Stockholm approved the study, and did not require informed consent from the individual patients because of the retrospective nature of the study.
2.2. Definitions
An episode of IE was defined as definite or possible according to the modified Duke criteria.[18] IE was defined as right-sided if it only involved structures on the heart's right side (tricuspid valve, pulmonary valve, pacemaker, or implantable cardioverter defibrillator [ICD] leads). IE was called left-sided if the aortic or mitral valves were engaged. SAE episodes involving both the right and left sides were classified as left-sided. A new SAE episode within 90 days after completing treatment for an initial SAE was considered to be a relapse. Hence, it was not counted as a separate episode. The blood culture systems used at the hospital were BACTEC (Becton Dickinson and Company, Sparks, MD) during 2004 to 2007 and BacT/ALERT (bioMérieux, Marcy l’Etoile, France) during 2004 to 2013.
Intravenous drug use was said to be active if according to the person's medical records there was a history of intravenous drug use within 3 years before the SAE episode, or if there was evidence of drug use in the following year. Infection was considered nosocomial if signs or symptoms of IE presented more than 48 hours after admission. Infection was also defined as nosocomial if it was related to hemodialysis. Other cases were considered to be community-onset episodes. A healthcare-associated community-onset infection was defined as previously described.[19] In-hospital mortality was defined as death from all causes while still admitted at an acute care hospital.
2.3. Statistical analysis
Description of data is given by number of observations, medians, quartiles, and ranges. The Pearson χ2 test, or Fisher exact test when needed, was used for comparing categorical data. The Mann–Whitney U test was used to compare continuous data between groups. Time trend in incidence rates was tested by the χ2 trend test. Survival data is displayed by Kaplan–Meier curves and groups were compared by the log-rank test. Level of significance was set at 0.05. For analyzing the data the JMP 8.0.2 statistical software from SAS Institute Inc (Cary, NC) was used.
3. Results
3.1. Incidence
A total of 120 SAE episodes associated with active intravenous drug use were identified in 101 individuals during 2004 to 2013, of 673 screened medical records with an IE diagnosis during the period. Eleven (9%) patients had 2 SAE episodes and 4 (3%) had 3. Definite SAE cases were 117 (97.5%) and possible 3 (2.5%). Polymicrobial etiology was present in 5 (4%) episodes. Echocardiography was done in 119 (99%) cases, trans-esophageal in 101 (84%), and solely trans-thoracic in 18 (15%) (the patient who did not have an echocardiogram nevertheless had a definite endocarditis according to the modified Duke criteria). Additional 125 SAE episodes (110 definite, 15 possible) were seen in non-drug users during the study period, of which 6 were noted in individuals with prior but not currently active intravenous drug use (all with documented more than 10 years [10–30 years] since the last injection, without any evidence of relapses).[19]
In the first half of the study period 39 SAE episodes associated with active intravenous drug use were seen and in the second half 81. The incidence of SAE associated with intravenous drug use among adults (≥18 years) in Stockholm County was 0.76/100,000 person-years for the entire period, increasing from 0.52/100,000 person-years during 2004 to 2008 to 0.99/100,000 person-years during 2009 to 2013 (P = 0.02). By focusing specifically on PWID in Stockholm (4800 persons [range 1850–7800]) the incidence of SAE in this group was estimated to be 249 (range 153–649) /100,000 person-years. The proportion of patients referred to our hospital from other Stockholm hospitals was 56/81 (69%) in 2009 to 2013 compared with 24/39 (62%) in 2004 to 2008 (P = 0.41).
3.2. Characteristics
The characteristics of the 120 SAE episodes in PWID are depicted in Table 1, with a comparison to SAE in nonaddicts. The median age of the patients was 39.3 years (interquartile range [IQR] 30–47 years), and 77 (64%) were male (Table 1). One (0.8%) SAE episode was acquired nosocomially, and 2 (1.7%) were healthcare associated with a community onset. Two (1.7%) methicillin-resistant S aureus (MRSA) isolates were identified, both community acquired, and both patients surviving. Left-sided episodes were 42 (35%) and solely right-sided 77 (65%) (1 with unknown location), compared with 110 (89%) left-sided and 14 (11%) right-sided episodes in non-drug users (1 with unknown location) (P <0.0001). A history of previous IE was present in 34 (28%) cases compared with 9 (7%) in the non-drug users (P <0.0001), while 11 (9%) had another predisposing heart disease compared with 51 (41%) of the non-drug users (P <0.0001).
Table 1.
Demographics of S aureus endocarditis in people who inject drugs, and a comparison with non-drug users.
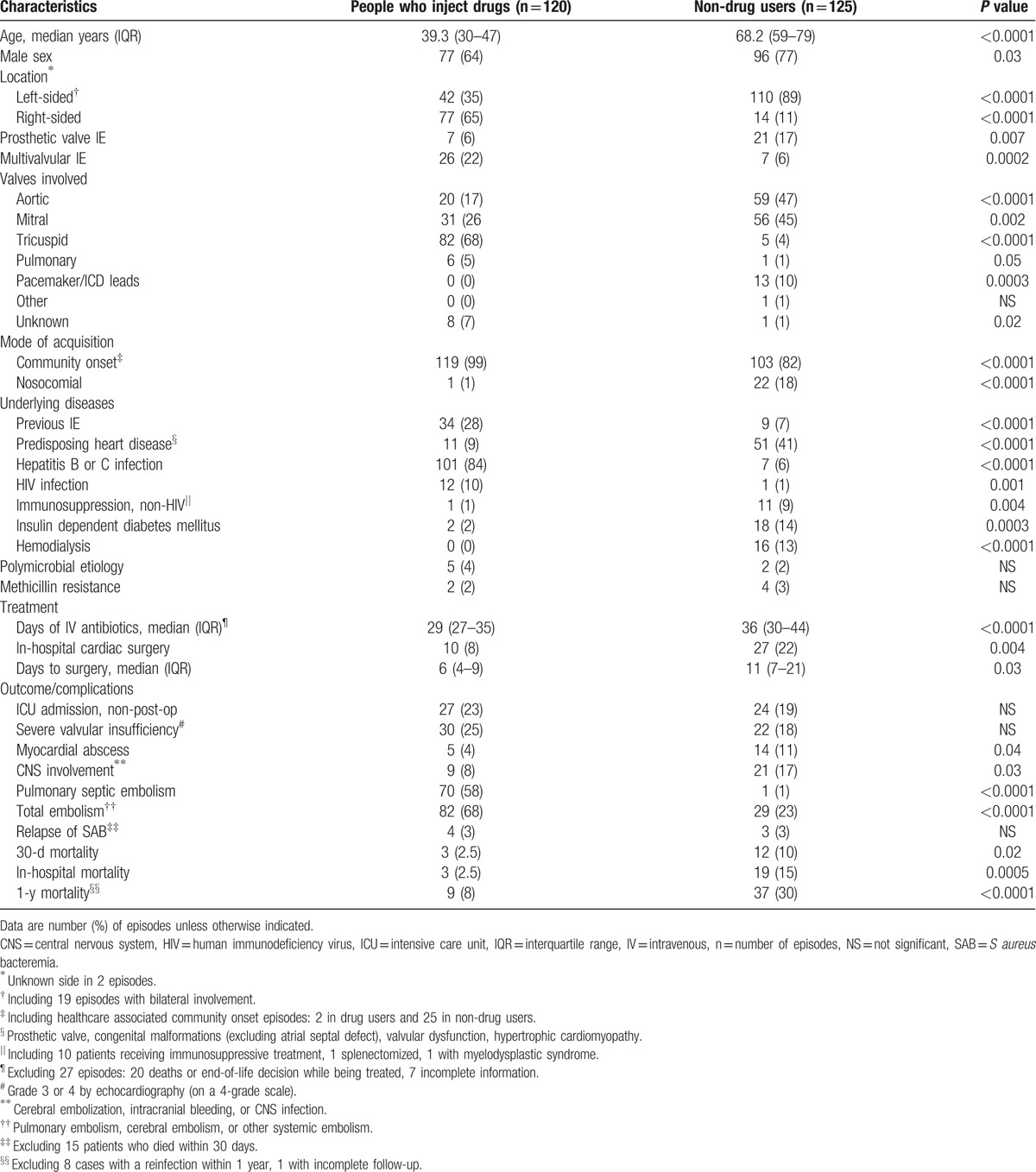
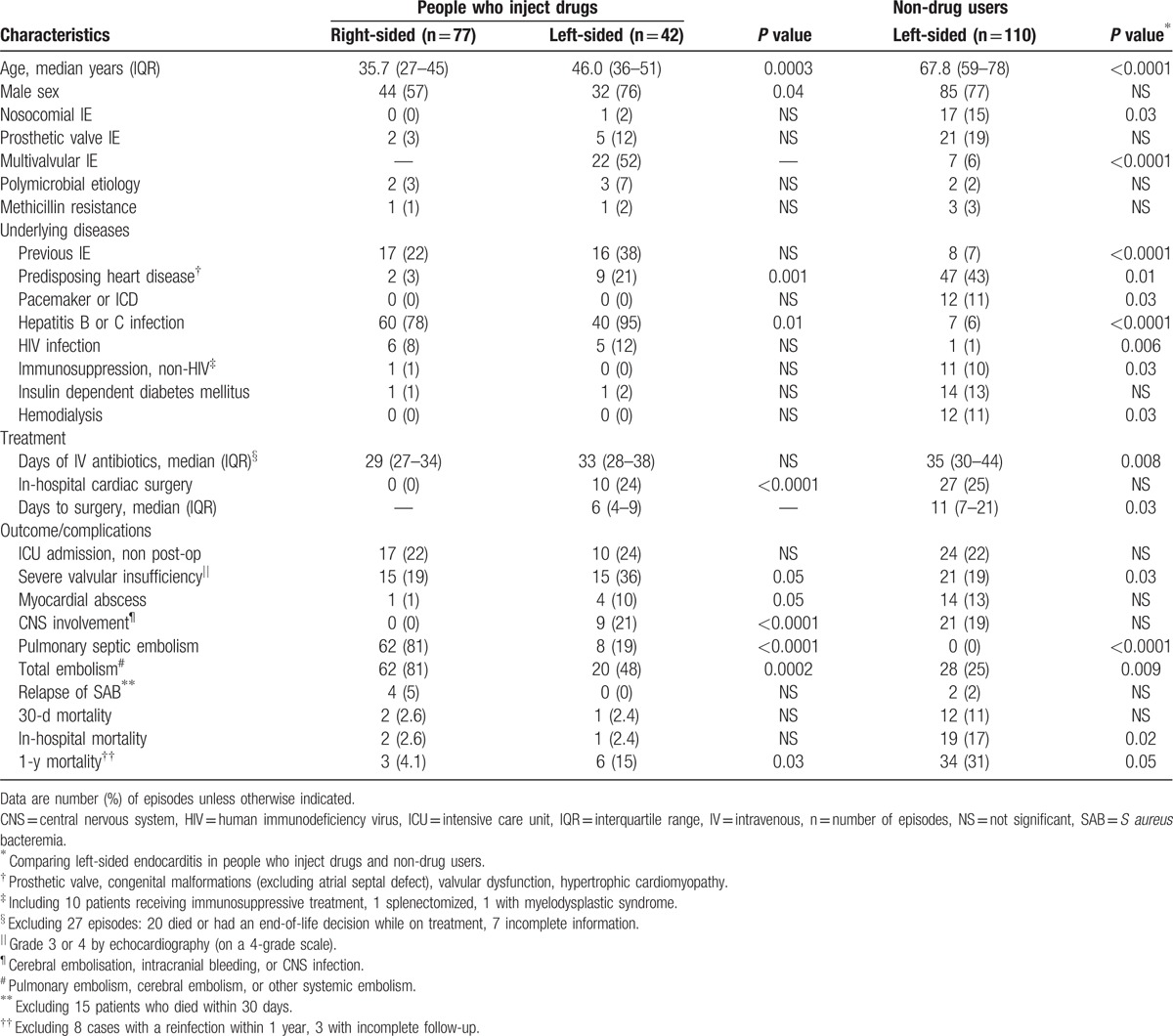
A comparison between left- and right-sided SAE episodes in PWID is depicted in Table 2. This table further compares the characteristics of left-sided SAE episodes in PWID with episodes in non-drug users. The median age of PWID with right-sided endocarditis was 35.7 years (IQR 27–45 years) compared with 46.0 years (IQR 36–51 years) in those with left-sided involvement (P = 0.0003). Further, the median age of non-drug users with left-sided SAE was 67.8 years (IQR 59–78 years) (P <0.0001, compared with PWID). Multivalvular involvement was seen in 22 (52%) of left-sided episodes in PWID compared with 7 (6%) in non-drug users (P <0.0001). Severe valvular insufficiency was seen in 15 (36%) of the PWID with left-sided SAE compared with 21 (19%) of the non-drug users (P = 0.03), but there was no significant difference in the frequency of intensive care unit (ICU) admissions, myocardial abscesses, and central nervous system (CNS) complications between these groups (Table 2).
Table 2.
Findings in people who inject drugs with right- and left-sided S aureus endocarditis, and a comparison with findings in non-drug users.
Of 120 intravenous drug use associated cases, 117 had documented history of injecting in the past 12 months, while 3 reported approximately 2 years since their last injection. In 56 (47%) cases amphetamine was the main drug used for injections, in 51 (43%) heroine, in 6 (5%) equal use of amphetamine and heroin was reported, and in 7 (6%) other opioids. None reported cocaine as the main drug used. No correlation was seen between the type of drug used and age, sex, valve involvement, surgical treatment or outcome, and no change was seen in the proportion of different types of drugs with time (data not shown). Twelve (10%) patients had HIV, 3 with CD4 counts <200 cells/mm3. Hepatitis C virus (HCV) infection was present in 98 (82%) of the patients, 28 (23%) had both HCV and hepatitis B virus (HBV), and 3 (2.5%) HBV only.
3.3. Treatment
Ten (8%) patients, all with left-sided endocarditis (4 with bilateral involvement), underwent cardiac surgery as a part of the in-hospital treatment, compared with 22% (27/125) of the non-drug users (P = 0.004) (Table 1). The principal reasons for surgery were heart failure (4 patients), risk for embolism (2 patients), uncontrolled infection with an abscess or pseudoaneurysm (2 patients), and a combination of heart failure and risk for embolism (2 patients). The median time to valvular surgery was 6 days from admission (IQR 4–9 days; range 2–35 days), compared with 11 days in the non-drug users (IQR 7–21 days; range 3–48 days) (P = 0.03). Seven patients received a biological valve, 2 a mechanical, and 1 got both a biological and a mechanical valve. None of the 10 patients who had surgery died within 30 days from surgery. Two (20%) died within 1 year (both of causes unrelated to the SAE) compared with 7 of 103 (7%) patients who did not have surgery (P = 0.14). In 100 (83%) patients the principal antibiotic used was cloxacillin, while 15 (12.5%) mainly received cephalosporins (cefuroxime, cefotaxime, ceftriaxone), 3 (2.5%) vancomycin, and 1 each daptomycin and clindamycin (0.8%).
The median treatment duration with intravenous antibiotics was 29 days (IQR 27–35 days; range 7–64 days). Among 40 nonimmunocompromised patients with uncomplicated right-sided SAE the median intravenous treatment duration was also 29 days (IQR 26–32 days; range 7–45 days). These patients had no septic emboli outside the lungs, and lacked other deep foci or complications requiring prolonged antibiotic treatment. The most common reason for a very short course of intravenous antibiotic treatment was poor compliance and self-discharge. Four patients (5%) with right-sided endocarditis received <13 days (7–11 days) of intravenous treatment. All 4 had problems with compliance. Two of them had a follow-up treatment with intramuscular ceftriaxone for 15 to 38 days, while 2 received oral antibiotics for 20 to 30 days. Three patients (7%) with left-sided endocarditis received <25 days (18–21 days) intravenous treatment. Two patients self-discharged, while the third was discharged after 20 days treatment after having received 2 weeks of intravenous antibiotic treatment following a successful valve replacement.
3.4. Mortality
The case fatality ratio at 30 days was 2.5% (3/120), and at 1 year 8.0% (9/113, excluding 6 reinfections and 1 patient with incomplete follow-up). Three (2.5%) patients died during hospital admission, compared with 19 (15%) among non-drug users (P = 0.0005) (Table 1). One was a 46-year-old HIV positive female with a large tricuspid vegetation (47 mm vegetation with moderate valvular insufficiency [grade 2 by echocardiography on a 4 grade scale]) and pericardial effusion who died from multiple organ failure 26 days after admission. The second patient was a 57-year-old HIV positive woman with tricuspid SAE who died 16 days after admission from pneumothorax complicating a ventilator-associated pneumonia. The third was a 45-year-old female with a mitral valve vegetation and growth of S aureus and Bacteroides fragilis in the blood who died 6 days after admission due to a hemothorax complicating a central venous catheter insertion. A survival curve for our PWID with left-sided SAE is depicted in Figure 1, with a comparison to the non-drug users with left-sided SAE.
Figure 1.
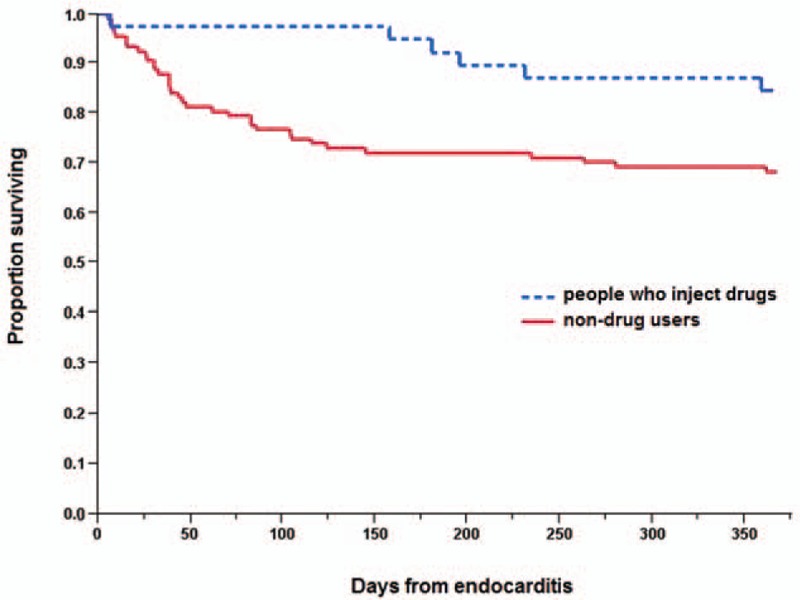
Survival after left-sided S aureus endocarditis in people who inject drugs and non-drug users (P = 0.04, log-rank test).
4. Discussion
This study is one of the largest reporting on SAE associated with intravenous drug use. Furthermore, most of our earlier knowledge is based on studies from the last century.[6,9–15] Our study highlights the epidemiology and characteristics of SAE related to intravenous drug use in Stockholm, Sweden. It further provides information on the management and outcome of SAE in PWID at a large referral hospital with a specialized inpatient ward for drug users with severe infections.
4.1. Incidence
We noted a high incidence of SAE related to intravenous drug use in Stockholm County (0.76/100,000 adult person-years). A slightly lower incidence has recently been reported from Australia,[20] while previous population-based studies on SAE have found significantly lower rates.[21–24] The high incidence noted in the study is probably in part related to our mainly urban population. In part it might also be caused by a high awareness and a liberal use of diagnostic procedures, as a consequence of the concentration of PWID to our tertiary referral hospital.
The reason for the large increase in incidence of SAE related to intravenous drug use noted over time is likely to be multifactorial. An increasing number of PWID probably plays a role. In Sweden a constant rise in the number of people who use intravenous drugs has been noted on a national basis from 1979 to 2007, with a 13% increase between 1998 and 2007.[16] Although precise data on the prevalence of intravenous drug use are lacking specifically for Stockholm County, it is likely to also have increased. A marked increase in hospitalizations for IE related to intravenous drug use has been reported also from the United States but without an increase of the at-risk population.[25] A similar increase has been seen in Australia.[20] Certain drugs have been claimed to be associated with a higher risk for IE than others.[25,26] No change, however, was noted in the type of narcotic drugs used during the study period. Injection frequency probably plays a role, but could not be assessed. Change in referral practice is an unlikely explanation since our hospital has treated almost all IE in PWID in Stockholm before, during, and after the study period. Finally, an increased awareness of IE among PWID and better and more frequent utilization of diagnostic procedures may have influenced the number of SAE cases observed.
We estimated the incidence of SAE to be 249 (range 153–649) /100,000 PWID per year. This high incidence underlines the importance of SAE in this specific risk group. The incidence range is wide since it is difficult to calculate the exact number of PWID in the region. Only a few other reports exist specifically concerning the incidence of IE in PWID. Two previous studies have reported a similar IE incidence among HIV negative PWID, with higher rates in PWID infected with HIV.[27,28] Others have presented somewhat lower estimates.[24,29,30] The proportion of different narcotic drugs injected, the male-to-female ratio, and the percentage of individuals infected with HCV, HBV, and HIV in our study was similar to that reported from the county and noted in the Stockholm needle exchange program.[17,31] Our SAE patient group therefore seems to be well representative for the PWID in general seen in Stockholm.
4.2. Mortality
The 2.5% in-hospital case fatality ratio noted in our hospital is lower than the 8% to 12% found in most previous reports on SAE in PWID.[5,6,10,12,14,15] The low mortality might in part reflect the quality of care at the study location. At our referral center infectious diseases specialists work closely together with specialists in addiction medicine. The infectious diseases specialists are responsible for the patients’ antiinfective therapy, while the addiction specialists focus on treatment of withdrawal syndromes, substitution therapy, pain management, and treatment of other psychiatric conditions. The priority is on enabling a full and optimal treatment course for the infection without unplanned interruptions. The department also has social workers who provide help and assist with social support. Concentrating the care of PWID with serious infections to a specialized hospital ward with physicians, nurses, social workers, and other healthcare personnel skilled and experienced in treating drug users has certain advantages. This seems to contribute to high patient satisfaction and generally good compliance, which in turn can lead to improved outcome. Another explanation for the low mortality noted could be a high awareness of IE in PWID among medical doctors in the region, leading to inclusion of a large number of early and mild cases. Furthermore, a low rate of methicillin resistance in the area might also have contributed,[32] and possibly bacterial or host genetic factors.[33,34]
The in-hospital and 1-year case fatality ratios in PWID were considerably lower than those seen in non-drug users at our hospital. Although the overall mortality was low, almost 1 quarter of the PWID needed intensive care treatment, and severe valvular insufficiency, myocardial abscesses, and CNS complications were at least as common in our PWID with left-sided SAE as in non-drug users. The lower mortality in PWID can in part be explained by their lower age, the lower frequency of nosocomial infections, and the lack of comorbidities besides the intravenous drug use. It has also been reported that PWID with S aureus bacteremia have a more vigorous antibody response to many S aureus antigens compared with non-drug users, probably due to previous exposure to the infecting strains. This might possibly offer some protection and contribute to the more favorable outcome.[35] Finally, transmission of S aureus strains can occur within drug-use networks,[36] and PWID with SAE might thus be colonized and infected with less pathogenic S aureus strains associated with a better outcome. The proportion of severe infections, as well as our 35% left-sided cases, however, appears to belie this. Furthermore, 1 study looking at microbiological factors in PWID with SAE did not find such an association.[37]
4.3. Left versus right-sided SAE
IE related to intravenous drug use has historically most often been right-sided. We found that left-sided SAE comprised 35%, a finding consistent with that in a few other,[6,9,10] but not all, recent reports.[5] PWID with left-sided SAE were older and had predisposing heart diseases more often than those with right-sided SAE. In turn non-drug users with left-sided SAE were generally older and more often had other comorbidities than PWID with left-sided disease. Multivalvular involvement and a history of previous IE were, on the other hand, much more common in PWID. A long duration of antibiotic therapy was noted in patients with uncomplicated right-sided SAE. Infectious diseases specialists in our institution thus do not seem to shorten the duration of antibiotic treatment in these cases, despite the fact that existing guidelines propose this.[7,8,38]
4.4. Cardiac surgery
The valvular heart surgery rate in PWID of 8% during the hospitalization (24% of the left-sided, no right-sided) is lower than the 35% observed in a multicenter cohort,[6] but similar to findings in older studies on PWID mostly from the 1980s.[10,11,15] This rate is also lower than that reported in association with SAE in general.[3,5,6] The low operation frequency does not, however, seem to have influenced the mortality rate in a negative way. We have recently shown that the presence of active intravenous drug use is an independent predictor of not having valvular heart surgery in patients with SAE.[19] A reluctance to operate PWID could reflect that active intravenous drug use is a risk to acquire a new IE, and that these patients are often regarded as less compliant to treatments.[39] Interestingly, PWID who underwent cardiac surgery had the procedure performed earlier than non-drug users. This implies that PWID who were selected for surgery had more advanced disease than non-drug users who had cardiac surgery.
5. Conclusions
The study has several limitations. First the retrospective nature of the study renders it inherited shortcomings. Relevant data might thus have been missed since they are not always documented in the medical records. Second, the study is based on an experience in a single referral center rather than in a population-based setting. However, since almost all PWID with IE in the region are referred to our hospital the likelihood of missed SAE cases is small. Hence, the study provides a fairly accurate overview of SAE related to intravenous drug use also on a population basis. A strength of our study is the unique setting with a single referral center for PWID with serious infections in a defined geographical area, leading to inclusion of a large number of patients, and a high availability and quality of the medical records.
In conclusion, a low mortality of SAE associated with intravenous drug use was noted in Stockholm as well as an incidence that increased over time. The increase in incidence probably in part reflects an increasing number of PWID during the study period. The in-hospital and 1-year mortality was low although a substantial proportion of the PWID had left-sided endocarditis. The study results implicate that it could be advantageous to concentrate the care of drug users with severe acute infections to specialized referral centers.
Acknowledgments
The authors thank Martin Kåberg, MD, at Capio Maria Addiction Centre Stockholm and Department of Infectious Diseases, Karolinska University Hospital, for his help and information regarding data on the addiction problem in Stockholm County.
Footnotes
Abbreviations: CNS = central nervous system, HBV = hepatitis B virus, HCV = hepatitis C virus, HIV = human immunodeficiency virus, ICD = implantable cardioverter defibrillator, ICU = intensive care unit, IE = infective endocarditis, IQR = interquartile range, IV = intravenous, MRSA = methicillin-resistant Staphylococcus aureus, n = number of episodes, NS = not significant, PWID = people who inject drugs, SAB = Staphylococcus aureus bacteremia, SAE = Staphylococcus aureus endocarditis.
The authors had no writing assistance. The study was approved by the Regional Ethical Review Board in Stockholm, not requiring an informed consent on an individual basis due to the retrospective nature of the study.
This work was supported by a grant from the Department of Infectious Diseases, Karolinska University Hospital, Stockholm, Sweden.
The authors have no conflicts of interest to disclose.
References
- [1].Cherubin CE, Sapira JD. The medical complications of drug addiction and the medical assessment of the intravenous drug user: 25 years later. Ann Intern Med 1993;119:1017–28. [DOI] [PubMed] [Google Scholar]
- [2].Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Miro JM, Anguera I, Cabell CH, et al. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis 2005;41:507–14. [DOI] [PubMed] [Google Scholar]
- [4].Thalme A, Westling K, Julander I. In-hospital and long-term mortality in infective endocarditis in injecting drug users compared to non-drug users: a retrospective study of 192 episodes. Scand J Infect Dis 2007;39:197–204. [DOI] [PubMed] [Google Scholar]
- [5].Fernández Guerrero ML, González López JJ, Goyenechea A, et al. Endocarditis caused by Staphylococcus aureus: a reappraisal of the epidemiologic, clinical, and pathologic manifestations with analysis of factors determining outcome. Medicine (Baltimore) 2009;88:1–22. [DOI] [PubMed] [Google Scholar]
- [6].Fowler VG, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005;293:3012–21. [DOI] [PubMed] [Google Scholar]
- [7].Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–128. [DOI] [PubMed] [Google Scholar]
- [8].Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394–434. [DOI] [PubMed] [Google Scholar]
- [9].Ruotsalainen E, Sammalkorpi K, Laine J, et al. Clinical manifestations and outcome in Staphylococcus aureus endocarditis among injection drug users and nonaddicts: a prospective study of 74 patients. BMC Infect Dis 2006;6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Faber M, Frimodt-Møller N, Espersen F, et al. Staphylococcus aureus endocarditis in Danish intravenous drug users: high proportion of left-sided endocarditis. Scand J Infect Dis 1995;27:483–7. [DOI] [PubMed] [Google Scholar]
- [11].Levine DP, Crane LR, Zervos MJ. Bacteremia in narcotic addicts at the Detroit Medical Center. II. Infectious endocarditis: a prospective comparative study. Rev Infect Dis 1986;8:374–96. [DOI] [PubMed] [Google Scholar]
- [12].Julander I. Unfavourable prognostic factors in Staphylococcus aureus septicemia and endocarditis. Scand J Infect Dis 1985;17:179–87. [DOI] [PubMed] [Google Scholar]
- [13].Chambers HF, Korzeniowski OM, Sande MA. Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine (Baltimore) 1983;62:170–7. [PubMed] [Google Scholar]
- [14].Tuazon CU, Cardella TA, Sheagren JN. Staphylococcal endocarditis in drug users. Clinical and microbiologic aspects. Arch Intern Med 1975;135:1555–61. [PubMed] [Google Scholar]
- [15].Miró JM, del Río A, Mestres CA. Infective endocarditis and cardiac surgery in intravenous drug abusers and HIV-1 infected patients. Cardiol Clin 2003;21:167–84. [DOI] [PubMed] [Google Scholar]
- [16].Statens offentliga utredningar (SOU) 2011:35. Bättre insatser vid missbruk och beroende. Stockholm: Statens offentliga utredningar; 2011. [Google Scholar]
- [17].Britton S, Hillgren K, Marosi K, et al. Baslinjestudie om blodburen smitta bland injektionsnarkomaner i Stockholms län 1 juli 2007 – 31 augusti 2008. Stockholm: Karolinska Institutet and Maria Addiction Centre Stockholm; 2009. [Google Scholar]
- [18].Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- [19].Asgeirsson H, Thalme A, Kristjansson M, et al. Incidence and outcome of Staphylococcus aureus endocarditis-a 10-year single-centre northern European experience. Clin Microbiol Infect 2015;21:772–8. [DOI] [PubMed] [Google Scholar]
- [20].Tung MK, Light M, Giri R, et al. Evolving epidemiology of injecting drug use-associated infective endocarditis: a regional centre experience. Drug Alcohol Rev 2015;34:412–7. [DOI] [PubMed] [Google Scholar]
- [21].Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest 2007;132:1025–35. [DOI] [PubMed] [Google Scholar]
- [22].Scudeller L, Badano L, Crapis M, et al. Population-based surveillance of infectious endocarditis in an Italian region. Arch Intern Med 2009;169:1720–3. [DOI] [PubMed] [Google Scholar]
- [23].Selton-Suty C, Célard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 2012;54:1230–9. [DOI] [PubMed] [Google Scholar]
- [24].Hogevik H, Olaison L, Andersson R, et al. Epidemiologic aspects of infective endocarditis in an urban population. A 5-year prospective study. Medicine (Baltimore) 1995;74:324–39. [DOI] [PubMed] [Google Scholar]
- [25].Cooper HL, Brady JE, Ciccarone D, et al. Nationwide increase in the number of hospitalizations for illicit injection drug use-related infective endocarditis. Clin Infect Dis 2007;45:1200–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chai LY, Khare CB, Chua A, et al. Buprenorphine diversion: a possible reason for increased incidence of infective endocarditis among injection drug users? The Singapore experience. Clin Infect Dis 2008;46:953–5. [DOI] [PubMed] [Google Scholar]
- [27].Wilson LE, Thomas DL, Astemborski J, et al. Prospective study of infective endocarditis among injection drug users. J Infect Dis 2002;185:1761–6. [DOI] [PubMed] [Google Scholar]
- [28].Spijkerman IJ, van Ameijden EJ, Mientjes GH, et al. Human immunodeficiency virus infection and other risk factors for skin abscesses and endocarditis among injection drug users. J Clin Epidemiol 1996;49:1149–54. [DOI] [PubMed] [Google Scholar]
- [29].Cherubin CE, Baden M, Kavaler F, et al. Infective endocarditis in narcotic addicts. Ann Intern Med 1968;69:1091–8. [DOI] [PubMed] [Google Scholar]
- [30].Banks T, Fletcher R, Ali N. Infective endocarditis in heroin addicts. Am J Med 1973;55:444–51. [DOI] [PubMed] [Google Scholar]
- [31].Kåberg M, Lindström F. Sprututbytet i Stockholm. Verksamhetsberättelse 2013. Stockholm: Karolinska University Hospital; 2014. [Google Scholar]
- [32].Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53–9. [DOI] [PubMed] [Google Scholar]
- [33].Aamot HV, Blomfeldt A, Eskesen AN. Genotyping of 353 Staphylococcus aureus bloodstream isolates collected between 2004 and 2009 at a Norwegian university hospital and potential associations with clinical parameters. J Clin Microbiol 2012;50:3111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Giannitsioti E, Damoraki G, Rokkas C, et al. Impact of haplotypes of TNF in the natural course of infective endocarditis. Clin Microbiol Infect 2014;20:459–64. [DOI] [PubMed] [Google Scholar]
- [35].Grumann D, Ruotsalainen E, Kolata J, et al. Characterization of infecting strains and superantigen-neutralizing antibodies in Staphylococcus aureus bacteremia. Clin Vaccine Immunol 2011;18:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Quagliarello B, Cespedes C, Miller M, et al. Strains of Staphylococcus aureus obtained from drug-use networks are closely linked. Clin Infect Dis 2002;35:671–7. [DOI] [PubMed] [Google Scholar]
- [37].Ruotsalainen E, Kardén-Lilja M, Kuusela P, et al. Methicillin-sensitive Staphylococcus aureus bacteraemia and endocarditis among injection drug users and nonaddicts: host factors, microbiological and serological characteristics. J Infect 2008;56:249–56. [DOI] [PubMed] [Google Scholar]
- [38].Ribera E, Gómez-Jimenez J, Cortes E, et al. Effectiveness of cloxacillin with and without gentamicin in short-term therapy for right-sided Staphylococcus aureus endocarditis. A randomized, controlled trial. Ann Intern Med 1996;125:969–74. [DOI] [PubMed] [Google Scholar]
- [39].Rabkin DG, Mokadam NA, Miller DW, et al. Long-term outcome for the surgical treatment of infective endocarditis with a focus on intravenous drug users. Ann Thorac Surg 2012;93:51–7. [DOI] [PubMed] [Google Scholar]


