Supplemental Digital Content is available in the text
Keywords: escape mutant, HBsAg-negative, hepatitis B vaccination, nucleic acid testing, occult HBV infection, seronegative
Abstract
The presence of anti-hepatitis B virus (HBV) core antibody (anti-HBc) is considered a sensitive lifetime marker of HBV infection. Here, we examined this dogma by investigating the prevalence of hepatitis B viremia in anti-HBc negative complete vaccines in Taiwan.
A total of 795 participants (1.7–20.0 years old) had completed HBV vaccination in infancy and were anti-HBc negative. Serum samples were available for 460 individuals with isolated anti-HBV surface antibodies (anti-HBs) (HBsAg-negative and anti-HBc negative) and for 245 individuals who tested negative for all 3 markers (triple seronegative). All samples were submitted for polymerase chain reaction (PCR) targeting both the preS/S and X/pre-C gene regions.
Of the 460 participants with isolated anti-HBs, 26 (5.65%) were positive for HBV by 2-target PCR. Of the 245 triple seronegative samples, 12 (4.90%) were positive for HBV DNA. In the former group, the prevalence of viremia was significantly higher in individuals aged 6 to 10 years than in all other ages combined (11.82% vs 3.7%, P = 0.001). The anti-HBs titers were significantly lower in participants 6 to 10 years old than in all other ages combined (72.06 vs 99.64 mIU/mL, P = 0.038). In total, 7 (0.99%) subjects had quantifiable HBV DNA levels (280–18,820 IU/mL). Sequence analysis of the S gene revealed vaccine escape like mutations.
Hepatitis B viremia can occur in completely vaccinated individuals who are negative for anti-HBc.
1. Introduction
Documentation of hepatitis B virus (HBV) infection often relies on serological assays. The presence of hepatitis B surface antigen (HBsAg) and positive anti-HBV core antibody (anti-HBc) indicates persistent active HBV infection, whereas the presence of anti-HBc in the absence of HBsAg is classically termed a resolved infection, although it may reflect low-level persistent infection. Following the advent of nucleic acid testing, recognition of an HBsAg-negative, HBV DNA positive status, termed “occult” HBV infection (OBI), has gained attention. An expert meeting held in Taormina in 2008 defined OBI as the presence of HBV DNA in the liver (with detectable or undetectable HBV DNA in the serum) of individuals testing HBsAg negative.[1] In real-world practice such as blood bank screening or epidemiological surveys, HBV DNA detected in plasma or serum of HBsAg-negative individuals is considered as evidence of OBI despite the fact that intrahepatic HBV DNA is not confirmed. The clinical significance of OBI is highlighted in cases of hepatitis B reactivation among immunocompromised hosts and seronegative patients with hepatocellular carcinoma.[2–5] Although the concentration of HBV in patients with OBI is usually very low, that amount of virus retains the ability to replicate rapidly and cause fulminant hepatic failure or transform hepatocytes into cancer cells.[6,7] OBI may be accompanied by anti-HBV surface antibody (anti-HBs) and/or anti-HBc, but dual seronegativity has not been reported in many patients. HBsAg seroclearance can occur in chronic hepatitis B patients who have remained viremic.[8] Furthermore, mutations in the S gene immunoreactive region may result in a false-negative diagnosis, which, if they are paired with high viral loads, are considered “false OBI.”[1] In community- or hospital-based studies, OBI has been documented in about 8% to 18% of serum or liver samples.[3,9] Less understood is the prevalence of OBI in vaccinated populations.
In people who have been completely vaccinated against HBV, a serological status of anti-HBs-positive, HBsAg-negative, and anti-HBc-negative (termed “isolated anti-HBs”) is considered successfully immunized and protected from HBV infection. It is believed that these vaccines will not develop chronic HBV infection. Previous epidemiological studies on the efficacy of HBV vaccines in Taiwan demonstrated a progressive decrease with age in the prevalence of isolated anti-HBs, followed by a progressive increase in the prevalence of anti-HBs with or without anti-HBc.[10,11] To identify cases of OBI, only those who were positive for anti-HBc were selected for molecular testing.[11,12] The increased prevalence of isolated anti-HBs could be caused by boosting from either vaccines or natural encounters with HBV. The latter could result in OBI. In this study, we investigated the prevalence of hepatitis B viremia in complete vaccines who either had isolated anti-HBs or were negative for HBsAg, anti-HBs, and anti-HBc (“triple seronegative”).
2. Methods
2.1. Universal vaccination program
The HBV vaccination program was launched in Taiwan on July 1, 1984. Newborns of HBsAg-positive mothers were vaccinated in the first 2 years of the program. In July 1986, the program was expanded to all newborns. A plasma-derived vaccine with a 4-dose schedule (0, 1, 6, 12 months) was given until November 1992. Then, recombinant vaccines H-B-Vax II (Merck Sharp & Dohme Corp., NJ) or Engerix-B (SmithKline Beecham Biologicals, Rixensart, Belgium) were administered in a 3-dose schedule (0, 1, 6 months). Hepatitis B immunoglobulin was given to newborns of mothers with HBV who were considered highly infectious (positive for hepatitis B e antigen or high titers of HBsAg). Complete vaccination is defined by ≥3 doses of either the plasma vaccine or recombinant vaccine given in infancy.
2.2. Study population
This study was conducted with approval by the Institutional Review Board of Chang-Gung Memorial Hospital, Linkou, Taiwan. Among the 1214 participants recruited from northern, middle, southern, and eastern Taiwan for an epidemiological study of vaccine-preventable diseases in 2007, 800 were HBsAg-negative, born after July 1986 and had completed ≥3 doses of HBV vaccines in infancy. Among these participants, 481 were HBsAg-negative, anti-HBc-negative and anti-HBs-positive (i.e., isolated anti-HBs), and 314 were negative for HBsAg, anti-HBc, and anti-HBs (i.e., triple seronegative). Four hundred sixty cases (M: F = 225: 235; mean age, 8.2 ± 5.2 years, range 3.3–21.0 years) in the former group and 245 cases (M: F = 121: 124; mean age, 10.3 ± 4.0 years, range 1.7–19.8 years) in the latter had serum samples available for further analysis. Figure 1 shows the serological characteristics of participants in the previous and current study. Blood samples were collected and stored in aliquots in −80°C freezers until analysis.
Figure 1.
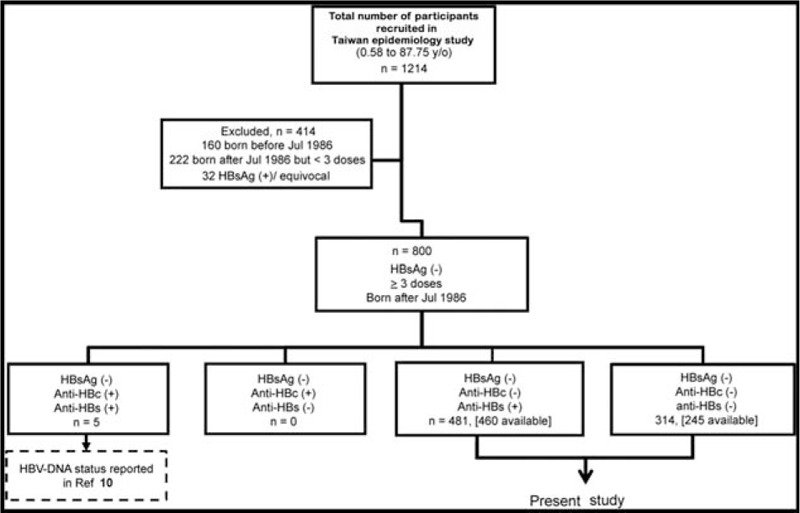
Flow chart of the inclusion of participants in this study.
2.3. Serology of HBV
An aliquot (500 μL) of serum sample from each participant was sent to the CGMH Department of Laboratory Medicine for HBV serology, which was executed separately from other clinical samples. Hepatitis B seromarkers were checked by electrochemiluminescent immunoassay (ECLIA) on the Modular Analytics E170 (Roche Diagnostics, Mannheim, Germany). A sample was considered HBsAg positive if the cutoff index (COI) was >10, equivocal if it was between 1 and 10, and negative if it was <1; anti-HBs positive if the level ≥10 mIU/mL; and anti-HBc positive if the COI was <0.95 and negative if it was >0.95.
2.4. DNA extraction, polymerase chain reaction (PCR), and Southern blot analysis
To isolate HBV DNA, an aliquot of serum (100 μL) was mixed with 300 μL of buffer (13.3 mmol/L Tris-HCl, pH 8.0; 6.7 mmol/L ethylenediaminetetraacetic acid; 0.67% sodium dodecyl sulfate; 133 μg/μL proteinase K) and incubated at 55°C for 4 hours. Extractions using equal volumes of phenol, phenol-chloroform, and chloroform were performed, and DNA was precipitated with cold ethanol and suspended in TE buffer. Nested PCR was used to detect 2 HBV genomic regions (preS/S and X/pre-C). The primers used are listed in Supplementary Table 1. The primers for the first round amplification and the second round amplification of the preS/S region were S-os/ S-oa and S-is/ S-ia, respectively. The primers for the first round amplification and the second round of X/ preCore region were PreC-os/ PreC-oa and PreC-is/ PreC-ia, respectively. PCR was performed for 30 cycles in a DNA thermal cycler. To avoid PCR-generated mutation, TaKaRa Ex Tag polymerase (Takara Shuzo Co., Shiga, Japan), which was capable of proofreading, was used in the PCR assay. An HBsAg-negative serum sample and an aliquot of double distilled water were included as negative controls.
To confirm the PCR results, a Southern blot analysis was performed with digoxigenin-labeled probes flanked by primer pairs S-is/ S-ia and PreC-is/ PreC-ia (Supplementary Table 1) using a plasmid containing a greater-than-unit-length HBV genome as the template. The detailed methods for probe labeling and Southern blotting have been described previously.[13] Surface gene sequencing was completed on samples that were positive for both preS/S and X/pre-C regions. Amino acid sequences were imputed by in silico translation of DNA sequences and genotypes were determined by a genotyping tool by NCBI (www.ncbi.nlm.nih.gov/projects/genotyping/). An aliquot of serum was sent for HBV DNA quantification by COBAS TaqMan HBV test (Roche, Branchburg, NJ). Because of the pre-test dilution, the qualitative lower limit of detection was 60 IU/mL and the linear quantitative range was 290 to 109 IU/mL.
2.5. Statistical analysis and phylogenetic analysis
Differences in frequencies between groups were compared by conventional Chi-square test or Fisher exact test when appropriate. Differences in continuous variables were compared by Student t test. A 2-sided P value of less than 0.05 was considered significant. A 2-way analysis of variance (ANOVA) was performed to clarify the interaction between age group and viremia status or anti-HBs levels with post hoc analysis by Scheffe test. The statistical analysis was performed using SPSS Statistics for Windows, Version 17.0. (SPSS Inc., Chicago, IL). To clarify the variation of the surface gene sequences, phylogenetic and molecular evolutionary analyses were conducted using MEGA version 6.[14]
3. Results
3.1. Prevalence of hepatitis B viremia in completely immunized, isolated anti-HBs, or triple-seronegative participants
In 460 isolated anti-HBs participants, 26 (5.65%) were determined hepatitis B viremic by 2-target PCR detection. The results were confirmed by Southern blot analysis for the HBV preS/S and X/pre-C gene regions (Table 1). The peak prevalence of viremia was observed in the 6 to 10-year-old group and the prevalence in this group was significantly higher than that in all other age groups combined [13/110 (11.82%) vs 13/350 (3.7%), X2 test, P = 0.001]. Overall, a progressive drop in the anti-HBs geometric mean titers (GMTs) was seen in the <6-year-old, to the 6 to 10-year-old, and 10 to 14-year-old groups, followed by a progressive increase in the anti-HBs GMT thereafter (Fig. 2). The GMTs of anti-HBs in the 6 to 10-year-old group were significantly lower than that of all other age groups combined (72.06 vs 99.64 mIU/mL, t test, P = 0.038). There was a significant decline in anti-HBs GMT in the 6 to 10-year-old group (vs <6-year-old, mean difference ratio 1.35; vs 10 to 14-year-old, mean difference ratio 1.31, respectively) correlated with the peak prevalence of viremia in this age group.
Table 1.
Prevalence of HBV preS/S and X/pre-C genes in 460 isolated anti-HBs positive, completely vaccinated individuals born after July 1986.
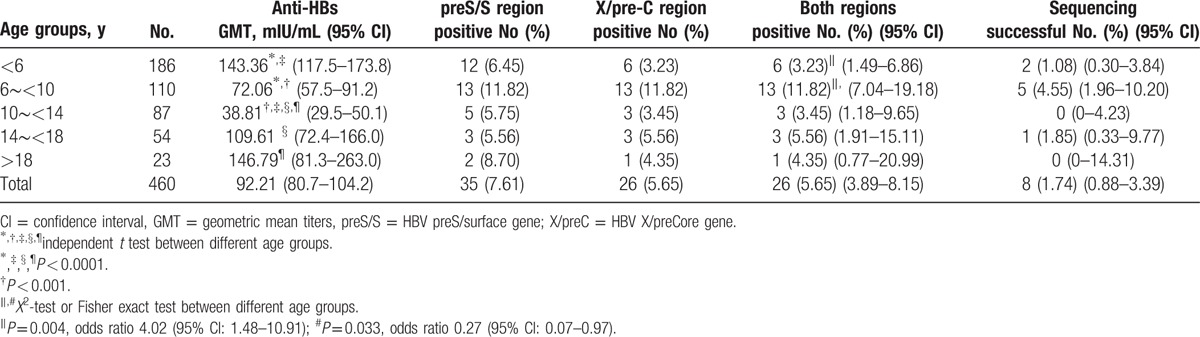
Figure 2.
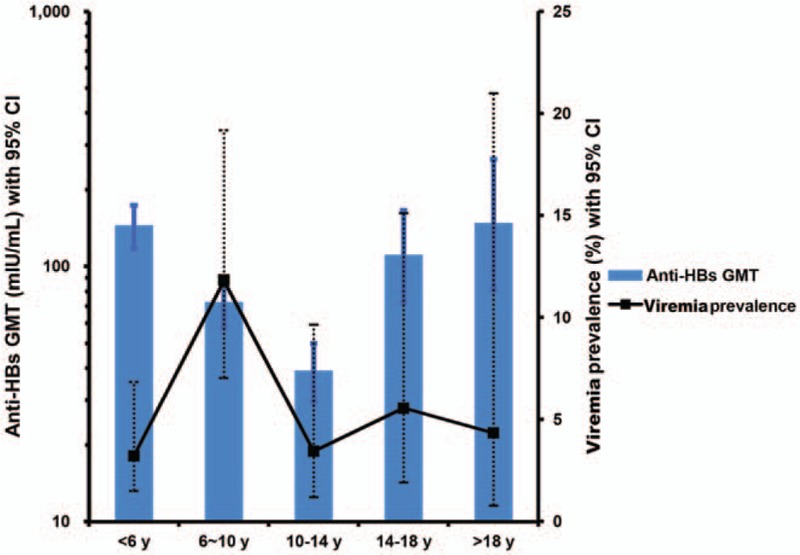
Distribution of the prevalence of viremia and anti-HBs geometric mean titers (GMTs) in different age groups. Solid square, prevalence of viremia; dotted lines, 95% CI (confidence interval) of prevalence; solid bar, anti-HBs GMT; thin lines, 95% CI of GMT.
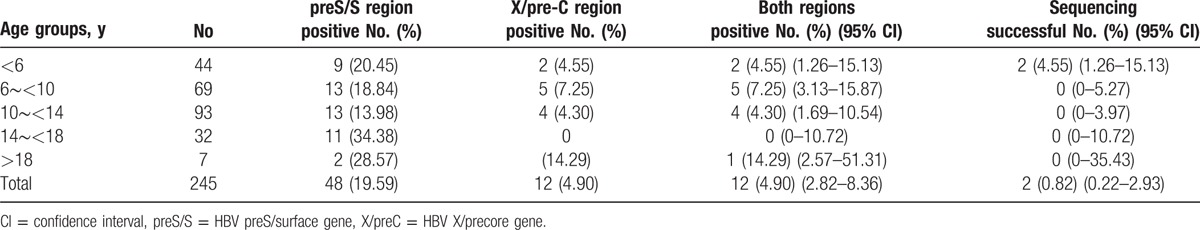
In the 245 triple-seronegative participants, 12 (4.90%) tested positive for HBV DNA and were considered viremic (Table 2).
Table 2.
Prevalence of HBV preS/S and X/pre-C gene in 245 triple-seronegative, completely vaccinated cases born after July 1986.
Of all 705 completely immunized anti-HBc negative participants, including triple seronegative and isolated anti-HBs individuals, 38 (5.39%) were found to be viremic. The highest prevalence was also found in 6 to 10-year-old group compared with that in all other age groups combined [18/179 (10.06%) vs 20/526 (3.80%), X2 test, P = 0.001].
3.2. Analysis of risk factors in completely immunized, viremic individuals
Neither gender nor age was associated with the risk of viremia, either in the isolated anti-HBs or in the triple-seronegative individuals (Table 3). When stratified by age, in the <6 years old group, anti-HBs GMT were significantly lower in viremic than in the nonviremic group. This trend was reversed in participants >14 years old, that is, anti-HBs GMT were significantly higher in the viremic than those in nonviremic group (Table 3 and Fig. 3). Anti-HBs levels were affected by age and by the interaction of viremia status with age in 2-way ANOVA (Fig. 3).
Table 3.
The demographic and anti-HBs titer analysis between viremic versus nonviremic cases.
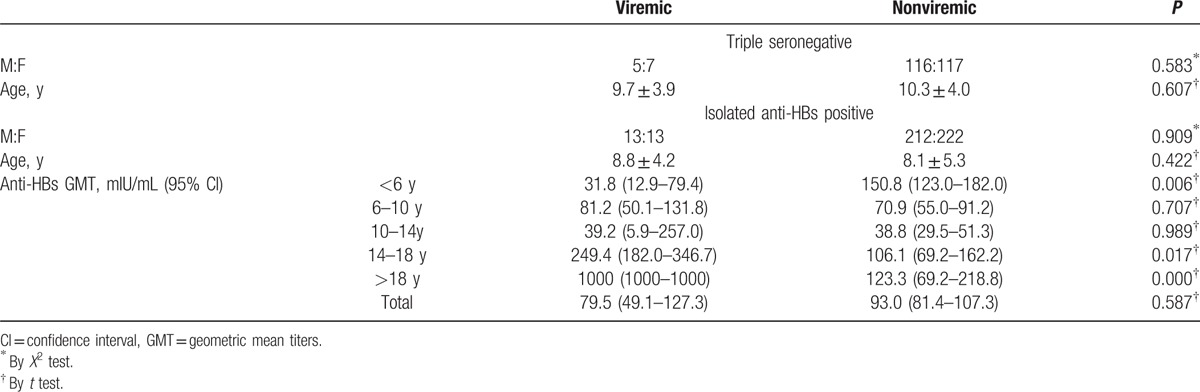
Figure 3.
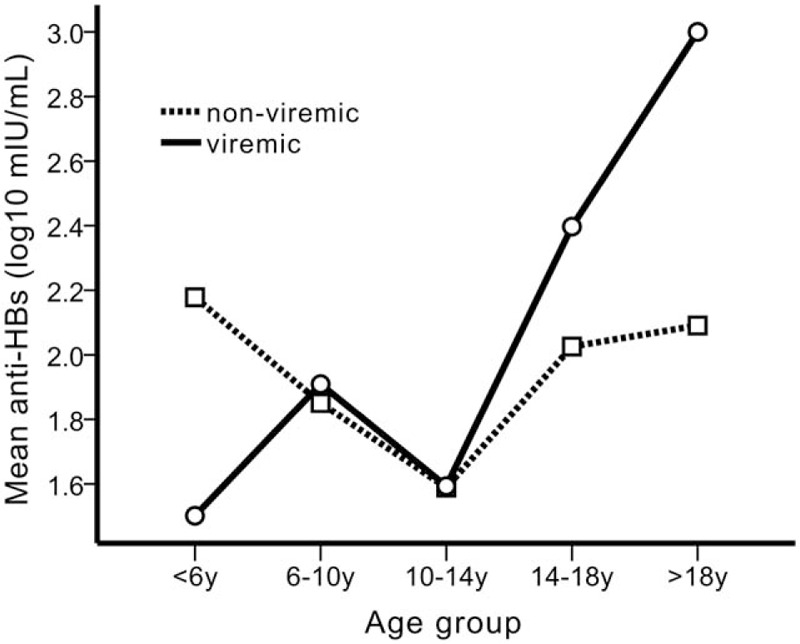
The effect of the interaction between viremia status and age groups on the anti-HBs titers in the different age groups.
3.3. HBV DNA loads, sequence analysis, and biochemistry in viremic cases
Of the 26 viremic cases among the 460 isolated anti-HBs complete vaccines, only 4 samples contained viral loads >60 IU/mL, ranging from 2485 to 18,820 IU/mL. HBV DNA was successfully sequenced from 8 samples from viremic individuals. HBV in 7 of these samples was genotype A and in 1, genotype B. Because genotype A is a rare genotype in Taiwan, a phylogenetic tree was constructed to determine the genotype distribution (Supplementary Figure. 1). HBV from 4 individuals harbored surface mutations, with sP120T in 3 cases, sV180A in 2 cases, and sT189I in 3 cases. One case also presented with A1762T/G1764A double mutations in the basal core promoter region. Aspartate aminotransferase (AST) levels were 32.0 ± 13.5 IU/L, with 11 of them above the upper limit of normal. Bilirubin levels were all within normal range.
In the 12 triple-seronegative individuals who were viremic, only 3 contained HBV DNA loads >60 IU/mL, ranging from 280 to 440 IU/mL. DNA was successfully sequenced from 2 cases. One was genotype A with multiple mutations, including an sN131T substitution, and the other, also genotype A, had a frame shift mutation at the 68th amino acid, which resulted in a distant branching from the genotype A clusters in the phylogenetic tree (Supplementary Figure 1). AST levels were 41.2 ± 1.81 IU/mL, with 6 of them above the upper limit of normal. Bilirubin levels were all within normal range. All viremic cases were asymptomatic during the epidemiological survey. Supplementary Table 2 lists genotypes, DNA loads, and surface gene sequences of the anti-HBc-negative viremic cases. Among the viremic cases, only one who was born before November 1992 received plasma-derived vaccine and harbored surface mutations. The others received recombinant vaccines.
4. Discussion
Previous attempts to assess the prevalence of OBI in a large number of healthy subjects have been mostly limited to individuals who tested positive for anti-HBc. However, our recent study in hepatitis B vaccines found an increasing prevalence of isolated anti-HBs beyond adolescence, which was the opposite of the trend expected with natural waning of immunity.[15] This reversion in anti-HBs prevalence (waning before 14 years of age followed by waxing beyond) has been observed in several epidemiological surveys.[10,11,16] The waning and waxing of anti-HBs was reported to be especially prominent in those with lower peak anti-HBs (10–99 mIU/mL) after neonatal vaccination in Gambia.[16,17] These findings led to speculation that isolated anti-HBs could be the only seromarker of HBV infection in some vaccines. Viremia without anti-HBc has been sporadically reported in vaccinated children (some positive for anti-HBs), vaccinated lymphoma patients with reactivated HBV infection following anti-CD20 therapy, and vaccinated blood donors.[2,18,19] In this study, we showed that about 5% of anti-HBc negative complete vaccines were viremic, defined by detection of HBV DNA by 2-region PCR. Using the strictest criteria (quantifiable HBV DNA levels of >60 IU/mL in this study), viremia was still detected in 7 of 705 (1.0%) vaccines. As it is generally believed that HBV DNA levels in OBI patients are extremely low, the true prevalence of viremia could be much greater than 1.0%.
In a completely vaccinated individual, HBV infection should be rare, unless the virus is transmitted perinatally from carrier mothers.[20] In this study, lower anti-HBs titers were significantly associated with viremia in isolated anti-HBs hosts <6 years of age, suggesting transmission from household contacts and possible mother-to-child transmission. We speculated that HBV infection could be suppressed by higher anti-HBs titers in most vaccines <6 years of age, which would explain the significantly lower anti-HBs titers in viremic cases in this age group. After 6 years of age, the prevalence of viremia increased (peak prevalence in the 6–10 years old), owing to the waning of anti-HBs. However, the anti-HBs titer could be boosted in those with OBI, and thus, the antibody titer was not the lowest in the 6 to 10-year-old group. It is unclear why both the prevalence of viremia and anti-HBs titers reached their lowest levels at 10 to 14 years of age. A possible explanation might be the maturation of cell-mediated immunity at this age, which would compensate for the insufficient immunity resulting from low anti-HBs titers. After 14 years of age, the anti-HBs titers were boosted progressively higher, both in the viremic and nonviremic groups, indicating repeated exposure to HBV. Human and chimpanzee studies have both indicated that HBV vaccination provides nonsterilizing protection.[21,22] Pande et al[23] also reported that neonatal vaccination may prevent overt (HBsAg-positive) but not occult infections in infants born to carrier mothers. If this view is correct, a vaccine booster given before antibody titers wane below a certain level and risk of exposure to HBV increases might prevent the emergence of OBI resulting from horizontal transmission.
Sequence analysis for the anti-HBc negative viremic cases revealed the presence of surface gene mutation in 6 of 10 (60%) sequencible cases, which is similar to the prevalence of S gene mutation (5 of 8, 63%) in HBsAg-positive and/or anti-HBc-positive vaccines in the same epidemiological study.[10] The mutation rate in our viremic cases is higher than that (44%) reported in vaccines with HBsAg-negative/anti-HBc positive occult infection,[24] higher than that (19–28%) in HBV DNA-positive vaccines who were selectively tested for HBV DNA based on HBsAg positivity or anti-HBc positivity in previous seroepidemiological surveys in Taiwan, and significantly higher than that (8%) in the pre-vaccination (born before 1984) generations.[25] Several mutation hot spots were identified inside or outside the “a” determinant. sP120T and sN131T were reported to be vaccine-escape mutations,[26] while sV180A and sT189I might result in conformational changes in putative B-cell epitopes (amino acid 160–207).[27,28] Phylogenetic analysis of the surface gene showed that genotype A, a less prevalent genotype (about 1%) in Taiwan, was predominant in this study. Genotype A is more prevalent in Europe (A2/Ae) and Africa (A1/Aa). However, an increasing prevalence of genotype A2, especially in acute hepatitis B cases, has been noted in Japan since 1991,[29] and the investigation suggested a horizontal transmission pattern.[30] Moreover, genotype A infection has a tendency toward chronicity.[31] The 9 genotype A cases were all recruited from southern Taiwan, with 6 of them from Pingtung County, a region where the number of immigrant brides from Philippines is the highest. Increased immigrant brides or foreign workers/housekeepers from Philippines, where genotype A is prevalent,[32] might be a source of horizontal transmission in some parts of Taiwan. Therefore, we speculated that occult infection with less prevalent genotypes (via horizontal transmission) resulting from anti-HBs decay might be occurring.
OBI may not present itself until a patient becomes immune-compromised. Transmission from an OBI donor is possible if the donation is only screened negative for HBsAg, especially in highly endemic regions where anti-HBc/anti-HBs single or dual positive donors are often allowed. A high prevalence of OBI in hematopoietic stem cell transplant donors was reported in Hong Kong.[3] The global prevalence of OBI in blood donors was found to be about 0% to 4.6% in anti-HBc positive cases.[33] In this study, about half of the viremic cases had an AST level above the upper limit of normal. If these patients were excluded, the prevalence of viremia was comparable to that in anti-HBc positive blood donors. A previous study also indicated that vaccinated donors who were negative for HBsAg and anti-HBc did not guarantee safe donations.[19] An immunized recipient might still develop post-transfusion or post-transplantation viremia and subclinical infection with normal or suppressed immunity, manifested clinical hepatitis years after immune reconstitution.[34,35] These cases argue for nucleic acid testing to identify OBI in potential donors or in immune-compromised hosts among vaccines.
This study has some limitations. Because it was a cross-sectional study, the results represent a snapshot only; longitudinal data from these subjects are lacking. Further, the HBV infection status of mothers was not determined in this study, which made determining the route of infection (mother-to-child transmission) speculative.
In conclusion, hepatitis B viremia can occur in 1% to 5% anti-HBc negative, completely vaccinated individuals, depending on whether quantifiable HBV DNA level is included in the definition. In individuals with isolated anti-HBs, the highest viremia prevalence was observed in participants 6 to 10 years of age, when anti-HBs titers dropped precipitously. Sequence analysis revealed S gene mutations that signified acquisition of vaccine-escape mutants.
Supplementary Material
Acknowledgment
We thank Miss Tzu-Chi Yu and Miss Yen-Ling Chuang for their diligent laboratory work and data collection.
Footnotes
Abbreviations: anti-HBc = antibody to hepatitis B core antigen, anti-HBs = antibody to hepatitis B surface antigen, AST = aspartate aminotransferase, COI = cutoff index, GMT = geometric mean titers, HBeAg = hepatitis B e antigen, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, OBI = occult hepatitis B infection, PCR = polymerase chain reaction.
Authorship: M-WL designed and conducted the study, analyzed and interpreted the data, and drafted the manuscript. T-YL was responsible for recruitment of participants and for medical oversight. W-RL and K-HL contributed to the data collection, statistical analysis, and interpretation. C-TY codesigned and supervised the conduct of the project and revised the manuscript critically.
Funding/Support: This work was supported by Chang-Gung Memorial Hospital Research Program Grant (CMRPG490063, CMRPG4A0153).
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Raimondo G, Allain J-P, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008;49:652–7. [DOI] [PubMed] [Google Scholar]
- [2].Awerkiew S, Däumer M, Reiser M, et al. Reactivation of an occult hepatitis B virus escape mutant in an anti-HBs positive, anti-HBc negative lymphoma patient. J Clin Virol 2007;38:83–6. [DOI] [PubMed] [Google Scholar]
- [3].Hui C-k, Sun J, Au W-y, et al. Occult hepatitis B virus infection in hematopoietic stem cell donors in a hepatitis B virus endemic area. J Hepatol 2005;42:813–9. [DOI] [PubMed] [Google Scholar]
- [4].Lai M-Y, Chen P-J, Yang P-M, et al. Identification and characterization of intrahepatic hepatitis B virus DNA in HBsAg-seronegative patients with chronic liver disease and hepatocellular carcinoma in Taiwan. Hepatology 1990;12(3 Pt 1):575–81. [DOI] [PubMed] [Google Scholar]
- [5].Chang I-C, Huang S-F, Chen P-J, et al. The hepatitis viral status in patients with hepatocellular carcinoma: a study of 3843 patients from Taiwan Liver Cancer Network. Medicine 2016;95:e3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hui C-K, Cheung WWW, Zhang H-Y, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg–negative patients undergoing cytotoxic chemotherapy. Gastroenterology 2006;131:59–68. [DOI] [PubMed] [Google Scholar]
- [7].Pollicino T, Squadrito G, Cerenzia G, et al. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology 2004;126:102–10. [DOI] [PubMed] [Google Scholar]
- [8].Chu C-M, Liaw Y-F. Hepatitis B surface antigen seroclearance during chronic HBV infection. Antivir Ther 2010;15:133–43. [DOI] [PubMed] [Google Scholar]
- [9].Minuk GY, Sun D-f, Uhanova J, et al. Occult hepatitis B virus infection in a North American community-based population. J Hepatol 2005;42:480–5. [DOI] [PubMed] [Google Scholar]
- [10].Lai M-W, Lin T-Y, Tsao K-C, et al. Increased seroprevalence of HBV DNA with mutations in the s gene among individuals greater than 18 years old after complete vaccination. Gastroenterology 2012;143:400–7. [DOI] [PubMed] [Google Scholar]
- [11].Ni Y-H, Chang M-H, Wu J-F, et al. Minimization of hepatitis B infection by a 25-year universal vaccination program. J Hepatol 2012;57:730–5. [DOI] [PubMed] [Google Scholar]
- [12].Xu L, Wei Y, Chen T, et al. Occult HBV infection in anti-HBs-positive young adults after neonatal HB vaccination. Vaccine 2010;28:5986–92. [DOI] [PubMed] [Google Scholar]
- [13].Yeh C-T, Chiu H-T, Chu C-M, et al. G1 phase dependent nuclear localization of relaxed-circular hepatitis B virus DNA and aphidicolin-induced accumulation of covalently closed circular DNA. J Med Virol 1998;55:42–50. [DOI] [PubMed] [Google Scholar]
- [14].Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013;30:2725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Poovorawan Y, Chongsrisawat V, Theamboonlers A, et al. Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J Viral Hepat 2011;18:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mendy M, Peterson I, Hossin S, et al. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PLoS One 2013;8:e58029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].van der Sande MA, Waight P, Mendy M, et al. Long-term protection against carriage of hepatitis B virus after infant vaccination. J Infect Dis 2006;193:1528–35. [DOI] [PubMed] [Google Scholar]
- [18].Mu S-C, Lin Y-M, Jow G-M, et al. Occult hepatitis B virus infection in hepatitis B vaccinated children in Taiwan. J Hepatol 2009;50:264–72. [DOI] [PubMed] [Google Scholar]
- [19].Stramer SL, Wend U, Candotti D, et al. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med 2011;364:236–47. [DOI] [PubMed] [Google Scholar]
- [20].Chen H-L, Lin L-H, Hu F-C, et al. Effects of maternal screening and universal immunization to prevent mother-to-infant transmission of HBV. Gastroenterology 2012;142:773–81. [DOI] [PubMed] [Google Scholar]
- [21].Kamili S, Sozzi V, Thompson G, et al. Efficacy of hepatitis B vaccine against antiviral drug-resistant hepatitis B virus mutants in the chimpanzee model. Hepatology 2009;49:1483–91. [DOI] [PubMed] [Google Scholar]
- [22].Werner JM, Abdalla A, Gara N, et al. The hepatitis B vaccine protects re-exposed health care workers, but does not provide sterilizing immunity. Gastroenterology 2013;145:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pande C, Sarin SK, Patra S, et al. Hepatitis B vaccination with or without hepatitis B immunoglobulin at birth to babies born of HBsAg-positive mothers prevents overt HBV transmission but may not prevent occult HBV infection in babies: a randomized controlled trial. J Viral Hepat 2013;20:801–10. [DOI] [PubMed] [Google Scholar]
- [24].Hsu H-Y, Chang M-H, Ni Y-H, et al. Universal infant immunization and occult hepatitis B virus infection in children and adolescents: a population-based study. Hepatology 2015;61:1183–91. [DOI] [PubMed] [Google Scholar]
- [25].Hsu H-Y, Chang M-H, Ni Y-H, et al. Survey of hepatitis B surface variant infection in children 15 years after a nationwide vaccination programme in Taiwan. Gut 2004;53:1499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Amini-Bavil-Olyaee S, Vucur M, Luedde T, et al. Differential impact of immune escape mutations G145R and P120T on the replication of Lamivudine-resistant hepatitis B virus e antigen-positive and -negative strains. J Virol 2010;84:1026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Scheiblauer H, El-Nageh M, Diaz S, et al. Performance evaluation of 70 hepatitis B virus (HBV) surface antigen (HBsAg) assays from around the world by a geographically diverse panel with an array of HBV genotypes and HBsAg subtypes. Vox Sang 2010;98:403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lin Y-M, Jow G-M, Mu S-C, et al. Naturally occurring hepatitis B virus B-cell and T-cell epitope mutants in hepatitis B vaccinated children. Sci World J 2013;2013:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kobayashi M, Suzuki F, Arase Y, et al. Infection with hepatitis B virus genotype A in Tokyo, Japan during 1976 through 2001. J Gastroenterol 2004;39:844–50. [DOI] [PubMed] [Google Scholar]
- [30].Kobayashi M, Arase Y, Ikeda K, et al. Clinical features of hepatitis B virus genotype A in Japanese patients. J Gastroenterol 2003;38:656–62. [DOI] [PubMed] [Google Scholar]
- [31].Suzuki Y, Kobayashi M, Ikeda K, et al. Persistence of acute infection with hepatitis B virus genotype A and treatment in Japan. J Med Virol 2005;76:33–9. [DOI] [PubMed] [Google Scholar]
- [32].Shi W, Zhang Z, Ling C, et al. Hepatitis B virus subgenotyping: history, effects of recombination, misclassifications, and corrections. Infect Genet Evol 2013;16:355–61. [DOI] [PubMed] [Google Scholar]
- [33].Liu C-J, Chen D-S, Chen P-J. Epidemiology of HBV infection in Asian blood donors: emphasis on occult HBV infection and the role of NAT. J Clin Virol 2006;36suppl 1:S33–44. [DOI] [PubMed] [Google Scholar]
- [34].Liu C-J, Lo S-C, Kao J-H, et al. Transmission of occult hepatitis B virus by transfusion to adult and pediatric recipients in Taiwan. J Hepatol 2006;44:39–46. [DOI] [PubMed] [Google Scholar]
- [35].Onozawa M, Hashino S, Izumiyama K, et al. Progressive disappearance of anti-hepatitis B surface antigen antibody and reverse seroconversion after allogeneic hematopoietic stem cell transplantation in patients with previous hepatitis B virus infection. Transplantation 2005;79:616–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


