Abstract
The effects of the dietary polyunsaturated fatty acids (PUFA) n-6:n-3 ratio and vitamin E (vE) on the levels of pro-inflammatory eicosanoids, the incorporation of docosahexaenoic acid (DHA) and arachidonic acid (AA) into immune tissues, and changes in leukocyte population after phytohemagglutinin (PHA) challenge were investigated in broiler chickens of different ages. One-day-old female broilers (48 per treatment) were fed 4 different wheat-soybean-corn-based diets containing corn oil with a high PUFA n-6:n-3 ratio (HR) or a mixture of linseed and fish oils with a low PUFA n-6:n-3 ratio (LR). Diets contained either 50 mg vE kg−1 of diet (basal vE) or 300 mg vE kg−1 of diet (increased vE). At d 14 and d 34, 8 chickens per treatment were challenged with PHA, and wing web swelling (WWS) was measured. The blood concentration of leukotriene (LTB4), prostaglandin (PGE2), and thromboxane (TBX2) in 17-day-old and 43-day-old chickens was determined. The pattern of AA and DHA incorporation into bursa, spleen, and brain lipids reflected the level of their precursors in the diet. WWS was the highest in chickens fed a LR diet and in 14-day-old chickens (P < 0.01). Leukocyte proportions varied with dietary PUFA n-6:n-3 ratio and with age. The heterophil:lymphocyte ratio was the highest at 6 h post PHA challenge, and was higher in 34-day-old chickens (P < 0.001). TBX2 and PGE2 concentrations were higher in chickens fed HR diet, whereas TBX2 and LTB4 concentrations were lower at high vE level. Lower PGE2 and LTB4, but higher TBX2 concentrations were measured in younger birds (P < 0.001). The results indicated that LR increased the phagocytic cell proportion in the blood; HR promoted the incorporation of AA into the immune tissues, which increased the levels of more pro-inflammatory eicosanoids in the blood; and vE counteracts these effects to some extent. Owing to the immaturity of the immune system, dietary interventions might be promising at the early stage of chicken growth.
Keywords: broiler, polyunsaturated fatty acid n-6:n-3 ratio, vitamin E, eicosanoids, immune system
INTRODUCTION
The production of broiler chicken meat has substantially and constantly increased worldwide. An 80% increase in the consumption of poultry meat over the last 3 decades (Puvača et al., 2014) has presented a great challenge for the poultry industry to meet this demand while maintaining high production standards. Thus, modern commercial broiler chickens are selected for early and fast growth, which results in a predetermined weight gain within 5 to 6 weeks. The consequence of such a rapid growth rate and the environmental conditions on chicken farms is immunosuppression of birds that are exposed to pathogens and other stressors (Cheema et al., 2003; Shira et al., 2005). Since the use of antibiotic feed additives has been banned in the European Union, several strategies for the improvement of chicken health and performance have been investigated, including the modulation of the chicken immune system by dietary intervention (Calder, 2001; Klasing, 2007).
Modern lines of broiler chickens require high-energy diets to achieve their full genetic potential; therefore, supplementation with fats is necessary to increase the caloric density of feed. Apart from energy, dietary lipids provide essential n-6 and n-3 fatty acids (FA), which are involved in most biological processes, including the development and function of the immune system.
Polyunsaturated fatty acids (PUFA), incorporated as phospholipids, are the major components of cell membranes with important immunological functions. Arachidonic acid (AA; C20:4n-6), eicosapentaenoic acid (EPA; C20:5n-3), and docosahexaenoic acid (DHA; C22:6n-3) are the precursors of lipid mediators of inflammation (eicosanoids), such as prostaglandins, thromboxanes, and leukotrienes (Friedman and Sklan, 1995). Therefore, the modification of the dietary PUFA n-6 and n-3 composition can potentially affect eicosanoids production, thus improving chicken health and welfare.
The immunomodulatory effect of dietary PUFA on broiler chickens has been extensively studied, but the results are inconsistent (Swiatkiewicz et al., 2015). Some studies indicated that the dietary administration of fish oil decreases the production of a more pro-inflammatory series of leukotrienes (Hall et al., 2007) and improves the indices of specific immune responses in growing chickens (Korver and Klasing, 1997), whereas others showed either adverse effects of fish oil on antibody production and lymphocyte proliferation (Fritsche et al., 1991) or no effects (Puthpongsiriporn and Scheideler, 2005).
The effects of vitamin E (vE) on chicken immunity are also well documented (Boa-Amponsem et al., 2000; Leshchinsky and Klasing, 2001; Konjufca et al., 2004; Khan et al., 2012), but a predictable immune response to dietary vE still remains to be elucidated, since it depends on dietary levels and chicken age, sex, or strain (Khan et al., 2012). Moreover, the general recommendation for vE supplementation for chickens (NRC, 1994), formulated to ensure optimal growth, may be insufficient to maintain chicken immunocompetence. Several limitations must be taken into account to understand the contradictory results found in the reported studies. The avian immune system is complex and composed of organs and cellular and soluble elements that cooperate to generate the immune response. Thus, experimental conditions, including nutritional factors, experimental protocols, and animal handling may affect immune responses in different studies. Additionally, PUFA, and particularly PUFA n-3 (Konieczka et al., 2014), are prone to lipid peroxidation and may lead to the oxidative destruction of cell membranes and the formation of free radicals (Rhee et al., 1996). Consequently, the immunomodulatory effect of PUFA may depend on the dose and form of antioxidants (i.e., tocopherols) used in chicken diets. Ultimately, specific immune system responses vary with chicken growth conditions. The duration of early feed restriction not only affects chicken performance, but also can lead to impaired systemic immune competence (Panda et al., 2015). Therefore, early post-hatch events may have a marked effect on immune response in the long term.
A consistent study that addresses the above issues may provide a new pathway to the understanding and improvement of chicken performance and welfare. Therefore, the objectives of this study were to investigate the effects of the dietary PUFA n-6:n-3 ratio, vE level and age on the pro-inflammatory eicosanoids level in the blood, the concentration of their precursor (AA) in selected immune organs, the response to phytohemagglutinin (PHA) challenge, and the growth performance of broiler chickens.
MATERIALS AND METHODS
Animal Ethics
Experimental procedures were approved by the Local Animal Care and Ethics Committee in Warsaw (Poland) and were in accordance with the principles of the European Union and Polish Law on Animal Protection.
Birds, Housing, Diets, and Experimental Design.
Birds and Housing.
One-day-old Ross 308 broiler females, purchased from a local commercial hatchery, were used in this study, with four dietary treatments of 48 chicks each. Chicks were kept in electrically heated battery brooders with wire-mesh floor and fed from d one of age with the appropriate diets for 8 d (mash-like diets for the first 3 d and pelleted thereafter). At 9 d of age, the broilers were weighed after 4 h of feed deprivation; this period of feed deprivation was used before each weighing in this study. Eight birds, with a body weight close to the group average, were sacrificed by cervical dislocation for organ sampling. The remaining 40 chickens were allocated to individual cages (30 × 50 cm wire-mesh floor area). Each chick was considered a replication, and feed intake was measured individually. The temperature of the room was maintained at 22°C with an 18 h light:6 h dark cycle throughout the experimental period.
Diets.
Four isoprotein and isoenergetic starter, grower, and finisher diets based on wheat, soybean meal, and corn were prepared (Table 1): two high PUFA n-6:n-3 ratio (HR) diets and two low PUFA n-6:n-3 ratio (LR) diets. The HR diets were fortified with corn oil, and the LR diets with a mixture of linseed oil and fish oil. Crude fat content was 49, 61, and 70 g kg−1 in starter, grower, and finisher diets, respectively. The PUFA n-6:n-3 ratio in the oils and diets are listed in Table 2. Diets contained either 50 mg vE kg−1 of feed (basal) or 300 mg vE kg−1 of feed (increased). Diets were cold-pelleted by a Laboratory Pellet Mill (model CL-2, CPM Inc., San Francisco, CA). Chickens were given starter diets from d one to d 14, grower diets from d 15 to d 35, and finisher diets from d 36 until slaughter at 43 d of age. Chickens had ad libitum access to feed and water.
Table 1.
Composition and calculated nutrients content of starter, grower, and finisher diets with high (HR) and low (LR) PUFA n-6:n-3 ratio, g kg−1 of diet.
| Starter, 1 to 14 d | Grower, 15 to 35 d | Finisher, 36 d to slaughter | ||||
|---|---|---|---|---|---|---|
| Ingredients | HR | LR | HR | LR | HR | LR |
| Wheat | 280.3 | 280.3 | 280.15 | 280.15 | 302.8 | 302.8 |
| Soybean meal | 359.0 | 359.0 | 346.0 | 346.0 | 318.0 | 318.0 |
| Corn | 300.0 | 300.0 | 300.0 | 300.0 | 300.0 | 300.0 |
| Fish oil | – | 0.5 | – | 0.5 | – | 0.5 |
| Corn oil | 23.0 | – | 36.0 | – | 45.0 | – |
| Linseed oil | – | 22.5 | – | 35.5 | – | 44.5 |
| Limestone | 13.5 | 13.5 | 13.52 | 13.52 | 13.61 | 13.61 |
| Mono-Ca-phosphate | 13.6 | 13.6 | 12.85 | 12.85 | 11.7 | 11.7 |
| NaCl | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| NaHCO3 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| l-Lys (78%) | 1.0 | 1.0 | 1.48 | 1.48 | – | – |
| dl-Met (98%) | 1.59 | 1.59 | 1.76 | 1.76 | 0.89 | 0.89 |
| l-Thr (98%) | 0.01 | 0.01 | 0.24 | 0.24 | – | – |
| Vitamin-mineral premix1,2 | 5.01 | 5.01 | 5.01 | 5.01 | 5.02 | 5.02 |
| Calculated | ||||||
| Crude protein (N x 6.25) | 215.7 | 215.7 | 210.0 | 210.0 | 200.4 | 200.4 |
| ME, (MJ/kg) | 11.88 | 11.88 | 12.25 | 12.25 | 12.63 | 12.63 |
| Crude fat | 48.87 | 48.87 | 61.40 | 61.40 | 70.15 | 70.15 |
1,2Provided per kilogram diet 1/2: (IU) vit. A (trans-retinyl acetate), 12,500/10,000; vit. D3 (cholecalciferol), 5,000/4,000; (mg) vit. B1, 3/2; vit. B2, 8/5; biotin, 0.2/0.1; vit. B6, 5/3; vit. B12, 0.02/0.01; vit. K3, 3/2; nicotinic acid, 55/35; folic acid, 2/1.5; pantothenic acid, 13/13; choline, 350/225; Mn, 120/120; Zn, 100/100; Se, 0.3/0.3; Cu, 16/16; Fe, 50/50; J, 1.3/1.3; Co, 0.3/0.3; Ca, 1.1/1.4 g; endo-1,4-β-xylanase (EC 3.2.1.8), −/70 U; coccidiostat (narasin and nicarbazin), 80/− mg. Premixes for HR and LR diets of basal or increased vitamin E level provided either 50 or 300 mg l-α-tocopheryl acetate kg−1 of diet, respectively.
Table 2.
Fatty acid composition of dietary oils, and starter, grower, and finisher diets with high (HR) and low (LR) dietary PUFA n-6:n-3 ratio, % of total fatty acids.
| Diet1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dietary oils | Starter, 1 to 14 d | Grower, 15 to 35 d | Finisher, 36 d to slaughter | ||||||
| Fatty acids | Fish oil | Linseed oil | Corn oil | HR | LR | HR | LR | HR | LR |
| C16:0 | 10.86 | 4.97 | 13.57 | 14.34 | 10.98 | 16.12 | 10.82 | 11.68 | 8.26 |
| C18:0 | 1.70 | 3.62 | 1.45 | 0.95 | 2.06 | 1.26 | 2.31 | 0.74 | 2.20 |
| C18:1 | 45.21 | 22.11 | 32.82 | 24.64 | 22.48 | 25.80 | 22.36 | 24.89 | 20.33 |
| C18:2n-6 | 10.22 | 13.33 | 50.68 | 58.51 | 32.32 | 55.92 | 25.55 | 61.11 | 23.60 |
| C18:3n-3 | 4.97 | 55.75 | 0.85 | 1.39 | 32.16 | 0.91 | 38.96 | 1.20 | 45.61 |
| C20:4n-6 | 0.16 | nd8 | nd | 0.08 | nd | 0.12 | nd | 0.15 | nd |
| C20:5n-3 | trace7 | nd | nd | nd | nd | nd | nd | nd | nd |
| C22:5n-3 | 0.65 | nd | nd | nd | trace | nd | trace | nd | trace |
| C22:6n-3 | 3.03 | nd | nd | nd | trace | nd | trace | nd | trace |
| SFA2 | 15.82 | 8.71 | 15.43 | 15.29 | 13.04 | 17.38 | 13.13 | 12.80 | 10.46 |
| MUFA3 | 64.29 | 22.21 | 33.03 | 24.64 | 22.48 | 25.80 | 22.36 | 24.89 | 20.33 |
| PUFA4 | 19.90 | 69.07 | 51.54 | 60.07 | 64.48 | 56.82 | 64.52 | 62.31 | 69.21 |
| PUFA n-65 | 10.96 | 13.33 | 50.68 | 58.68 | 32.32 | 55.92 | 25.55 | 61.11 | 23.60 |
| PUFA n-36 | 8.88 | 55.75 | 0.85 | 1.39 | 32.16 | 0.91 | 38.96 | 1.20 | 45.61 |
| PUFA n-6:n-3 | 1.23 | 0.24 | 59.48 | 43.13 | 1.00 | 62.19 | 0.66 | 51.10 | 0.52 |
1HR - diets with corn oil with high PUFA n-6:n-3, LR - diets with a mixture of linseed and fish oils with low PUFA n-6:n-3 ratio;
2SFA – saturated fatty acids (C14:0 + C16:0 + C18:0 + C20:0 + C22:0 + C24:0);
3MUFA – monounsaturated fatty acids (C16:1 + C17:1 + C18:1 + C20:1 + C21:1 + C22:1);
4PUFA – polyunsaturated fatty acids (C18:2n-6 + C18:3n-3 + C18:3n-6 + C20:2n-6 + C20:3n-6 + C20:4n-6 + C20:5n-3 + C22:4n-6 + C22:5n-3 + C22:6n-3);
5PUFA n-6 (C18:2n-6 + C18:3n-6 + C20:2n-6 + C20:3n-6 + C20:4n-6 + C22:4n-6);
6PUFA n-3 (C18:3n-3 + C20:5n-3 + C22:5n-3 + C22:6n-3);
7less than 0.002% of total fatty acids;
8nd = not detected.
Measurement of Response to PHA Challenge.
At d 14 and d 34 of age, eight chickens per treatment were injected in the right wing with a PHA (Sigma-Aldrich, St. Louis, MO) solution (100 μg in 100 μl PBS) and immediately in the left wing with 100 μl PBS. The thickness of the right and left wing web was measured prior to the injection and at 6, 12, 24, 48, and 72 h post PHA injection using a digital caliper (Mitutoyo Inc., Kawasaki, Japan); then, chickens were sacrificed by cervical dislocation. The wing web swelling response (WWS) was expressed as a swelling index, calculated as follows: Swelling index = [(thickness of right wing web post PHA injection − initial thickness of right wing web) − (thickness of left wing web post PBS injection − initial thickness of left wing web)]. Blood samples also were collected from the same chickens by venipuncture prior to injection and at 6, 24, and 48 h post PHA injection and prepared for white blood cell (WBC) differential count measurements. Briefly, one drop of blood was placed on a glass slide, and the edge of a second glass slide was used to smear a bullet-shaped film along the slide by moving it forward in a single quick movement. After air-drying, slides were placed in 99.8% methanol for 5 min, dried, placed on a staining rack, and flooded with 2 mL of Wright-Giemsa Stain (Sigma-Aldrich) for 2 min. After 1 min of staining, 2 mL of deionized water was added, and the solution was mixed gently by moving the staining rack. Smears were thoroughly rinsed with deionized water and left to air-dry. Slides were covered with a cover glass using a mounting medium (Roti®-Histol 6640; Carl Roth, Karlsruhe, Germany). Duplicate slides were prepared for each blood collection. One hundred cells per slide were counted for differential leukocyte analysis. Measurements were conducted in 2 independent replications by 2 different researchers. Slides were examined using an Olympus BX51 microscope (Olympus Corp., Tokyo, Japan), and leukocytes were counted using the CellD Imaging Software (Olympus Soft Imaging Solutions, Münster, Germany).
Eicosanoid Assay
Blood samples were collected at d 17 from eight PHA-challenged chickens and eight unchallenged chickens at d 43 in each treatment. Chickens were sacrificed, 5 mL of blood was collected in a tube, placed on ice for 30 min, centrifuged (4,629 g, 10 min at 4°C), and the serum was stored at −80°C until analysis. The concentration of leukotriene (LTB4), prostaglandin (PGE2), and thromboxane (TBX2) in the serum was determined by enzyme-linked immunosorbent assay (ELISA) using a specific quantitation kit for each eicosanoid (Cayman Chemical Company, Ann Arbor, MI), according to the manufacturer's protocols. Two replications of each sample were averaged, and a standard curve was prepared for each ELISA plate.
Sampling Procedure, Tissue Collection, and Fatty Acid Analysis
Feed intake and body weight were measured, and performance was calculated for each chicken from d 9 to the d of slaughter. At d 9, 16, 36, and 43, eight unchallenged chickens from each treatment were sacrificed, and the bursa of Fabricius, spleen, and brain were immediately excised and weighed. Tissues collected at d 16, d 36, and d 43 were vacuum-packed and stored at −30°C until FA analysis.
Prior to FA analysis, feed samples were finely ground using a tissue homogenizer (IKA T18 basic Ultra-Turrax; IKA, Haufen, Germany), and tissue samples were thawed at 4°C for 12 h and homogenized. FA content of the feed, immune tissue, and brain was extracted, saponified, and methylated as described by Czauderna et al. (2007). Briefly, analyses of fatty acid methyl ester composition were performed using a Shimadzu GC-MS-QP2010 Plus EI gas chromatograph equipped with a BPX70 fused silica capillary column (120 m × 0.25 mm i.d. × 0.25 μm film thickness), a quadrupole mass selective detector (Model 5973 N), and an injection port (Shimadzu, Kyoto, Japan). Helium was the carrier gas and operated at a constant pressure and a flow rate of 1 mL min−1.
Statistical Analysis
This experiment was run as a completely randomized design (CRD) with four treatments. Organ weight was calculated relative to the body weight prior to sacrificing. Chicken performance was analyzed as a 2 × 2 factorial CRD, considering the dietary PUFA n-6:n-3 ratio and vE level as the main effects, according to the following model:
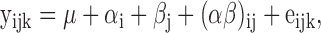 |
where: yijk – individual observation; μ – overall mean; αi – effect of PUFA n-6:n-3 ratio (i = 1, 2); βj – effect of vE level (j = 1, 2); (αβ)ij – effect of interaction; and eijk – residual error.
Other examined results were analyzed as a 2 × 2 × 2 factorial CRD, considering the dietary PUFA n-6:n-3 ratio, vE level, and age of chickens as the main effects, according to the following model:
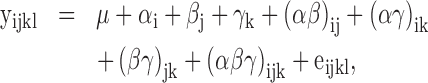 |
where: μ – overall mean; αi – effect of PUFA n-6:n-3 ratio (i = 1, 2); βj – effect of vE level (j = 1, 2); γk – effect of chickens’ age (k = 1, 2); (αβ)ij, (αγ)ik, (βγ)jk, (αβγ)ijk – effects of interactions; and eijkl – residual error.
Non-significant interactions were removed from the statistical model. Multifactorial analysis of variance (MANOVA) was applied to analyze the effects using STATGRAPHICS® Centurion XVI 16.1.03 (StatPoint Technologies, Inc., Warrenton, VA). Additionally, repeated-measures ANOVA was applied to analyze the WWS response using the General Linear Model function. Significant differences were identified by the least significant difference (LSD) test and considered significant at P ≤ 0.05.
RESULTS
Birds Performance and Tissue Weight
The effects of the dietary PUFA n-6:n-3 ratio and vE level on chicken performance are shown in Table 3. Dietary treatments did not affect the body weight gain and feed conversion ratio (FCR) in unchallenged chickens throughout the experimental period or in any earlier periods of feeding (data not shown). The relative weights of the bursa, spleen, and brain are shown in Table 4. The dietary PUFA n-6:n-3 ratio did not affect the weight of the immune tissues. Chickens sacrificed at d 16 had higher relative weight of bursa (P < 0.001) and lower relative weight of spleen (P < 0.001) than those sacrificed at 9 d, 36 d, and 43 d of age.
Table 3.
Main effects of dietary factors on the performance of unchallenged chickens by phytohemagglutinin (PHA) calculated between d 9 and d 42.
| Main | Final body | Feed intake, | FCR, kg feed · |
|---|---|---|---|
| effect | weight, g | g | kg−1 BWG |
| Dietary PUFA n-6:n-3 ratio (D)1 | |||
| High | 2,855 | 3,918 | 1.48 |
| Low | 2,884 | 4,038 | 1.51 |
| Vitamin E level (E)2 | |||
| Basal | 2,866 | 3,962 | 1.49 |
| Increased | 2,873 | 3,994 | 1.50 |
| Pooled SEM | 22.5 | 36.2 | 0.007 |
| Probability (P-value) | |||
| D | 0.54 | 0.11 | 0.13 |
| E | 0.89 | 0.66 | 0.58 |
| D × E | 0.66 | 0.88 | 0.23 |
1High, diets with corn oil; Low, diets with a mixture of linseed and fish oils.
2 Basal, 50 mg l-α-tocopheryl acetate kg−1 of diet; Increased, 300 mg l-α-tocopheryl acetate kg−1 of diet.
Table 4.
Main effects of dietary treatments on the relative weight of immune tissues and brains of broiler chickens at different ages, mg 100 g−1 live body weight.
| Effect | Bursa | Spleen | Brain |
|---|---|---|---|
| Dietary PUFA n-6:n-3 ratio (D)1 | |||
| High | 174.3 | 82.6 | 308.4a |
| Low | 180.6 | 81.0 | 319.2b |
| Vitamin E level (E)2 | |||
| Basal | 170.4a | 82.8 | 320.6b |
| Increased | 184.5b | 80.7 | 307.1a |
| Age of chickens (A)3 | |||
| d 9 | 165.8a | 84.3b | 714.9d |
| d 16 | 234.8b | 68.6a | 326.8c |
| d 36 | 162.3a | 87.8b | 116.8b |
| d 43 | 146.8a | 86.4b | 96.9a |
| Pooled SEM | 4.69 | 1.90 | 22.45 |
| Probability (P-value) | |||
| D | 0.39 | 0.66 | 0.040 |
| E | 0.050 | 0.55 | 0.010 |
| A | 0.001 | 0.001 | 0.001 |
1High, diets with corn oil; Low, diets with a mixture of linseed and fish oils.
2Basal, 50 mg l-α-tocopheryl acetate kg−1 of diet; Increased, 300 mg l-α-tocopheryl acetate kg−1 of diet.
3Tissues were sampled at 9, 16, 36, and 43 d of age.
a–dDifferent letters indicate significant differences within a column at P < 0.05; all interactions are not significant.
Chickens fed diets with increased vE level had higher bursa weight (P < 0.05) than those fed diets with basal vE level, whereas the spleen weight was not affected by the vE level. The relative weight of the brain was higher in chickens fed LR diets (P < 0.04), decreased gradually with the increasing age of chickens (P < 0.001), and was higher in chickens fed diets with basal vE level than those fed diets with increased vE level (P < 0.01).
Immune Tissue and Brain AA and DHA Incorporation Level: Effect of Dietary PUFA n-6:n-3 Ratio, vE Level, and Age
Differences in the dietary PUFA n-6 and n-3 ratio were clearly reflected in the level of AA and DHA incorporation in the bursa, spleen, and brain lipids of chickens (Table 5). HR diets led to higher levels of AA incorporation in the bursa (P < 0.001), spleen (P < 0.001), and brain (P < 0.001) lipids. The level of DHA incorporation in the bursa (P < 0.02), spleen (P < 0.001), and brain (P < 0.001) lipids of chickens fed LR diets was approximately 3-fold, 67-fold, and 2-fold higher, respectively, than in the respective organs of chickens fed HR diets.
Table 5.
Main effects of dietary PUFA n-6:n-3 ratio, vitamin E level, and age of chickens on the level of arachidonic acid (AA) and docosahexaenoic acid (DHA) in the lipids of bursa, spleen, and brain, % of total fatty acids.
| Bursa | Spleen | Brain | |||||
|---|---|---|---|---|---|---|---|
| Dietary PUFA n-6:n-3 ratio1 | Vitamin E level2 | AA | DHA | AA | DHA | AA | DHA |
| 16-day-old chickens | |||||||
| High | Basal | 2.11 | 0.54 | 3.69 | 0.06 | 3.77 | 3.12 |
| Increased | 1.38 | 0.01 | 2.09 | <0.01 | 3.67 | 2.15 | |
| Low | Basal | 0.73 | 0.31 | 2.91 | 1.77 | 2.85 | 3.29 |
| Increased | 0.51 | 0.37 | 2.79 | 1.73 | 2.77 | 3.13 | |
| 36-day-old chickens | |||||||
| High | Basal | 1.30 | <0.01 | 3.60 | <0.01 | 4.24 | 2.15 |
| Increased | 1.39 | <0.01 | 4.63 | 0.03 | 4.07 | 2.21 | |
| Low | Basal | 0.65 | 0.49 | 2.34 | 0.58 | 2.85 | 5.18 |
| Increased | 0.56 | 0.58 | 2.10 | 1.23 | 3.85 | 4.67 | |
| Pooled SEM | 0.101 | 0.064 | 0.172 | 0.126 | 0.156 | 0.169 | |
| Probability (P-value) | |||||||
| Dietary PUFA n-6:n-3 ratio (D) | 0.001 | 0.020 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Age of chickens (A) | 0.20 | 0.73 | 0.30 | 0.020 | 0.11 | 0.001 | |
| Vitamin E level (E) | 0.15 | 0.43 | 0.30 | 0.42 | 0.58 | 0.070 | |
| D × A | 0.23 | 0.060 | 0.002 | 0.020 | 0.85 | 0.001 | |
| D × E | 0.62 | 0.17 | 0.85 | 0.37 | 0.32 | 0.77 | |
| A × E | 0.14 | 0.26 | 0.030 | 0.27 | 0.41 | 0.44 | |
| D × A × E | 0.28 | 0.30 | 0.020 | 0.94 | 0.34 | 0.11 | |
1High, diets with corn oil; Low, diets with a mixture of linseed and fish oils.
2Basal, 50 mg l-α-tocopheryl acetate kg−1 of feed; Increased, 300 mg l-α-tocopheryl acetate kg−1 of feed.
The level of DHA incorporation in older chickens was lower in spleen lipids (P < 0.02), but higher in brain lipids (P < 0.001) than that in younger chickens. An interaction between the dietary PUFA n-6:n-3 ratio and age was observed in the level of DHA incorporation in spleen (P < 0.02) and brain (P < 0.001) lipids. Feeding LR diets resulted in 3-fold higher levels of incorporation of DHA in the spleen lipids in younger chickens, whereas this increase was only 2-fold higher in older birds. A higher level of DHA incorporation in brain lipids was observed only in 36-day-old chickens when fed LR diets.
The levels of AA and DHA incorporation in the lipids of the examined tissues were not affected by dietary vE level.
Blood Eicosanoid Response: Effect of Dietary PUFA n-6:n-3 Ratio, vE Level, and Age of Chickens
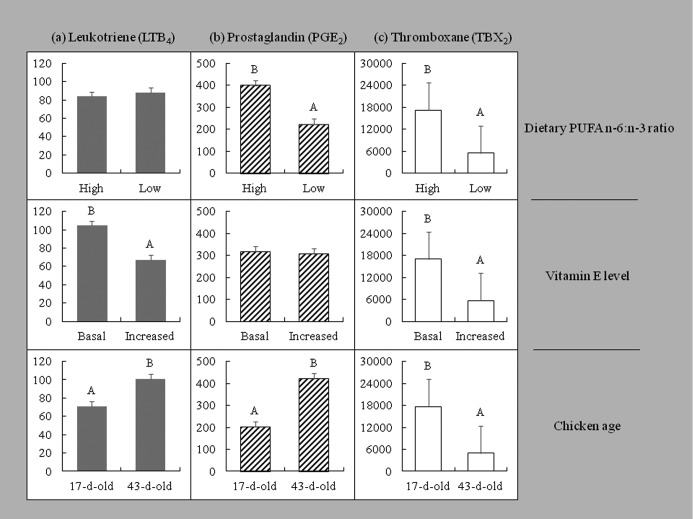
The blood concentration of LTB4, PGE2, and TBX2 was affected by the dietary PUFA n-6:n-3 ratio and vE level in 17-day-old PHA-challenged and 43-day-old unchallenged chickens (Figure 1). TBX2 and PGE2 concentrations were higher in chickens fed HR diets (P < 0.001). LTB4 and PGE2 concentrations were lower, and TBX2 concentration was higher in 17-day-old than in 43-day-old chickens (P < 0.001). TBX2 and LTB4 concentrations decreased (P < 0.001) in chickens fed diets with increased vE level than those fed diets with basal vE level, whereas PGE2 concentration was not affected by the vE level. PGE2 and TBX2 concentrations were negatively correlated (r = −0.3, P < 0.04).
Figure 1.
Main effects of dietary PUFA n-6:n-3 ratio, vitamin E level, and age of chickens on the concentration (pg/mL) of leukotriene (LTB4), prostaglandin (PGE2), and thromboxane (TBX2) in blood. Each bar represents mean ± SEM; A,BWithin main effects means with different letters indicate significant differences at P < 0.001.
Interaction effects among the dietary PUFA n-6:n-3 ratio, vE level, and age were observed on TBX2 concentration (P < 0.001). TBX2 concentration increased in 17-day-old chickens, but decreased in 43-day-old chickens fed HR diets and diets with increased vE level. The interaction between the dietary PUFA n-6:n-3 ratio and age-influenced PGE2 concentration was significant (P < 0.001). PGE2 concentration was approximately 3-fold higher in older chickens fed HR diets and approximately 2-fold higher in older chickens fed LR diets compared with younger chickens fed the respective diets. The interaction between the dietary vE level and age-influenced LTB4 concentration (P < 0.001) was also significant; the increased vE level decreased LTB4 concentration by 9% in 17-day-old chickens and by 50% in 43-day-old chickens.
PHA-induced WWS Response and Changes in Blood WBC Differential Count: Effect of Dietary PUFA n-6:n-3 Ratio, vE Level, and Age
The effects of the dietary PUFA n-6:n-3 ratio, vE level, age of chickens, and time post PHA challenge on the WWS response are shown in Table 6. PHA challenge led to a higher increase in wing web thickness in chickens fed LR diets than in those fed HR diets (P < 0.009). WWS was higher in PHA-challenged chickens injected at d 14 than in those injected at d 34 (P < 0.001). WWS was the highest at 6 h and 12 h post PHA injection, declined significantly at 24 h, and was the lowest at 48 h and 72 h post PHA injection (P < 0.001).
Table 6.
Main effects of dietary PUFA n-6:n-3 ratio, vitamin E level, age of chickens, and post-phytohemagglutinin (PHA) injection time on the wing web swelling response in chickens challenged with PHA.
| Main effect | Swelling index |
|---|---|
| Dietary PUFA n-6:n-3 ratio (D)1 | |
| High | 0.41a ± 0.067 |
| Low | 0.71b ± 0.052 |
| Vitamin E level (E)2 | |
| Basal | 0.68 ± 0.058 |
| Increased | 0.64 ± 0.062 |
| Age of chickens (A)3 | |
| d 14 | 0.76b ± 0.049 |
| d 34 | 0.35a ± 0.068 |
| Time post PHA-challenge, h (T)4 | |
| 6 | 0.76c ± 0.045 |
| 12 | 0.87c ± 0.046 |
| 24 | 0.54b ± 0.047 |
| 48 | 0.32a ± 0.051 |
| 72 | 0.29a ± 0.057 |
1High, diets with corn oil; Low, diets with a mixture of linseed and fish oils.
2Basal, 50 mg l-α-tocopheryl acetate kg−1 of diet; Increased, 300 mg l-α-tocopheryl acetate kg−1 of diet.
3Chickens were injected at d 14 and d 34 of age in the right wing web with PHA (100 μg in 100 μl PBS) and in the left wing with PBS.
4Swelling response was measured at 6 h, 12 h, 24 h, 48 h, and 72 h post PHA injection.
a–cDifferent letters indicate significant differences at: P < 0.009 for D; P < 0.001 for A; P < 0.001 for T; and P = 0.93 for E; All interactions are not significant.
The proportion of leukocytes was affected by the experimental factors (Table 7). The percentage of lymphocytes was higher in PHA-challenged chickens injected at d 14 than those injected at d 34 (P < 0.001). It was the highest prior the injection, declined thereafter, and subsequently increased over time (P < 0.001). The percentage of macrophages in the blood was higher in younger chickens (P < 0.001) than older chickens (P < 0.001) fed LR diets at 2 h, 6 h, and 48 h post PHA injection (P < 0.001). The percentage of heterophils in the blood was higher in older chickens than in younger chickens (P < 0.004). It was the highest at 6 h post PHA injection, but declined thereafter (P < 0.001). Similarly, the percentage of eosinophils also was affected by the age of chickens and post-PHA injection time; it was higher in older chickens than in younger chickens (P < 0.001) and at 24 h and 48 h than at 6 h post PHA injection (P < 0.001). The percentage of basophils was affected only by the dietary PUFA n-6:n-3 ratio and was the highest in chickens fed LR diets (P < 0.02). The effect of the interaction between age and post-PHA injection time on the percentage of lymphocytes (P < 0.01), macrophages (P < 0.05), heterophils (P < 0.03), eosinophils (P < 0.02), and basophils (P < 0.003) demonstrated that changes in leukocyte proportions, as a result of PHA challenge, were higher in younger chickens than in older chickens.
Table 7.
Changes in leukocyte population in the blood in chickens challenged by phytohemagglutinin (PHA).
| Effect | % lymphocytes | % macrophages | % heterophils | % eosinophils | % basophils |
|---|---|---|---|---|---|
| Dietary PUFA n-6:n-3 ratio (D)1 | |||||
| High | 61.72 | 2.24a | 29.16 | 4.75 | 2.42a |
| Low | 63.30 | 3.13b | 26.66 | 4.17 | 3.27b |
| Vitamin E level (E)2 | |||||
| Basal | 62.69 | 2.12 | 27.53 | 4.43 | 2.79 |
| Increased | 62.33 | 2.27 | 28.29 | 4.49 | 2.89 |
| Age of chickens (A)3 | |||||
| d 14 | 63.94b | 3.31b | 27.19a | 3.02a | 2.59 |
| d 34 | 57.79a | 2.06a | 32.03b | 5.32b | 3.09 |
| Time post PHA challenge, h (T)4 | |||||
| 0 | 67.11d | 1.76a | 24.88a | 3.95a,b | 2.50 |
| 6 | 55.81a | 2.91b | 34.88d | 3.53a | 2.22 |
| 24 | 61.98c | 3.14b | 27.22b | 4.55b,c | 2.61 |
| 48 | 58.55b | 2.93b | 31.46c | 4.67c | 2.49 |
| Pooled SEM | 0.684 | 0.108 | 0.598 | 0.163 | 0.103 |
| P-value (repeat measures ANOVA) | |||||
| D | 0.42 | 0.001 | 0.76 | 0.77 | 0.020 |
| A | 0.001 | 0.001 | 0.004 | 0.001 | 0.28 |
| E | 0.69 | 0.28 | 0.74 | 0.44 | 0.91 |
| T | 0.001 | 0.001 | 0.001 | 0.001 | 0.48 |
| A × T | 0.010 | 0.050 | 0.030 | 0.020 | 0.003 |
1High, diets with corn oil; Low, diets with a mixture of linseed and fish oils.
2Basal, 50 mg l-α-tocopheryl acetate kg−1 of feed; Increased, 300 mg l-α-tocopheryl acetate kg−1 of feed.
3At d 14 and d 34 of age, 8 chickens per treatment were injected in the right wing web with PHA (100 μg in 100 μl PBS).
4Blood samples were collected for leukocyte population determination prior the injection and at 6 h, 24 h, and 48 h post PHA injection.
a–dDifferent letters indicate significant differences within a column at P < 0.05. Only significant interactions between experimental factors are shown.
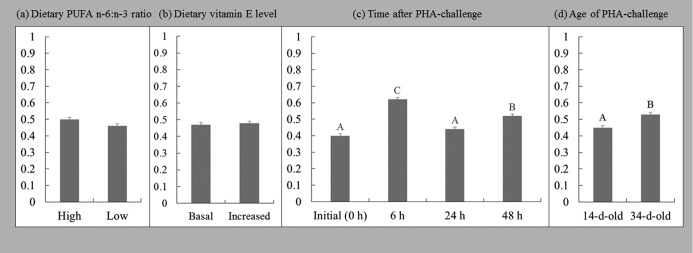
The heterophil:lymphocyte ratio (H:L) varied with age (Figure 2). The H:L ratio in the blood of 34-day-old chickens was higher than in the blood of 14-day-old chickens (P < 0.003). The H:L ratio measured at 6 h post PHA injection was higher than that prior to injection and at 24 h and 48 h post PHA injection (P < 0.001).
Figure 2.
Main effects of dietary PUFA n-6:n-3 ratio (a), dietary vitamin E level (b), post-phytohemagglutinin (PHA)-injection time (c), and age of chickens (d) on the heterophil:lymphocyte ratio (H:L) in the blood. Each bar represents mean ± SEM; A–CDifferent letters indicate significant differences at (c) P < 0.001 and (d) P < 0.003.
DISCUSSION
This study assessed the response of broiler chickens to diets with high or low PUFA n-6:n-3 ratios and vE level. Lipids are known to provide FA substrates necessary to maintain the function of the immune system (Gonzalez et al., 2011). The dietary ratio of PUFA n-6:n-3, vE level, and age of chickens are important for the optimal synthesis and inclusion of long-chain (LC) PUFA in the membrane phospholipids of immune cells (Cherian, 2011). Therefore, it was hypothesized that feeding, immediately after hatch, diets rich in PUFA n-3 protected against oxidative damage by increased level of antioxidants (300 mg kg−1 of diet vE), resulting in accumulation of PUFA n-3 in the immune tissues, and, thus, perhaps modulating the immune system in chickens.
In the current study, diets with different PUFA n-6:n-3 ratio were equally well utilized by the chickens, and in all groups performance was better than that expected for female Ross 308 at the respective ages (Aviagen, 2014). These results are in agreement with previous studies that reported either no effect or a positive effect of different dietary PUFA n-6:n-3 ratios on chicken performance (Cortinas et al., 2004; Rymer and Givens, 2006; Qi et al., 2010). Additionally, the dietary vE level did not affect performance indices, as reported previously for dietary vE doses of 5 to 400 mg kg−1 of feed (Kim et al., 2006).
The relative weight of brain in chickens decreased with age, but it was higher in chickens fed LR diets than those fed HR diets and lower in chickens fed diets with increased vE level than those fed diets with basal vE level throughout the experimental period. The relatively higher weight of brain accompanied with higher DHA incorporation might be beneficial for the further growth and function of the nervous tissues, which are particularly important in newborns (Innis, 2007) and may have positive long-term implications in later growth stages (Innis, 2008). However, the modulating effect of vE on brain weight remains unclear. Although the tissue-specific responses to dietary vE are known in chickens (Surai and Sparks, 2000), the underlying mechanism needs to be elucidated. Nevertheless, the analysis of fatty acid profile of brain lipids revealed that the DHA level in the brains of chickens fed LR diets was higher than in the brains of those fed HR diets and increased with age. A diet with low PUFA n-6:n-3 ratio, even if it contained a low level of DHA, was more effective in supplying the brain with DHA than a diet with high PUFA n-6:n-3 ratio. The brain requires a constant dietary supply of FA (particularly DHA) to maintain its functions and development, and the selective uptake and preferable incorporation of FA are necessary to maintain brain functions. Salem et al. (2015) have shown that brain in rats has an apparent selectivity in uptake of FA, in which DHA is predominate. A significant interaction also was documented in the case of DHA level in the brain lipids. LR diets resulted in a little lower level of DHA in younger chickens, while the prolonged feeding of LR diets resulted in substantially higher levels of DHA in brains of older birds. It may indicate a less efficient synthesis of DHA in younger than older chickens.
It is commonly accepted that the relative weight of immune tissues, including the bursa and spleen, reflect immune system functionality (Al-Khalifa et al., 2012). The thymus, bursa of Fabricius, and spleen are the major primary and secondary lymphoid organs for the maturation of lymphocytes and other immune cells. In the current study, the dietary PUFA n-6:n-3 ratio did not affect the weight of the bursa and spleen, whereas bursa weight was higher and spleen weight was lower in 16-day-old chickens than in 9-day-, 36-day, and 43-day-old chickens, which could be attributed to physiological processes associated with the maturation of the immune system in chickens. Al-Khalifa et al. (2012) reported that the weight of the bursa declines at 4 to 8 wk of age, and its immune function is lost as growth and development proceed. Thus, this immune function may be controlled by the spleen, and its relative weight increases in this period. Our results showed that the weight of the bursa increased with the dietary vE level. According to Niu et al. (2009), a dietary vE level up to 200 mg kg−1 of feed does not affect the weight of the bursa or other lymphoid organs in broiler chickens. We hypothesized that the increased vE level used in our study might have a pro-oxidative effect on the bursa, leading to the enlargement of this organ when compared to that of chickens fed diets with basal vE level. Marsh et al. (1986) suggested that the effect of the dietary deficiency of vE is exerted largely on the primary lymphoid organs. Thus, the pro-oxidative effect of excessive vE supplementation might result in the observed effects on bursa weight. The response of the bursa to the pro-oxidative effect of vE might be reflected in the hyperactivity of this organ with respect to the higher proliferation of immune cells, leading to bursal enlargement.
FA profile in the spleen and bursa indicated that AA and DHA incorporation in these tissues reflects the level of their precursors (LA and ALA) in the diet. HR diets resulted in higher incorporation of AA and smaller DHA, whereas LR diets resulted in higher incorporation of DHA and smaller AA in the lipids. Interestingly, the level of DHA in spleen lipids was significantly higher in 16-day-old chickens than in 36-day-old chickens. A similar trend was observed in studies on the incorporation of LC-PUFA in different tissues of chickens (Hall et al., 2007; Cherian, 2011; Al-Khalifa et al., 2012; Konieczka et al., 2015). The DHA level in spleen lipids of younger chickens was higher than that of older chickens, probably due to the maturation process of the spleen, which controls the immune function after bursa atrophy.
The level of AA or DHA deposited in the cells may have considerable consequences for immune system function. Long chain PUFA n-3 competes with AA for incorporation into cell membrane phospholipids (Surai and Sparks, 2000). Both AA and DHA are mobilized by phospholipase A2, become available as substrates for cyclooxygenase and lipooxygenase, and are precursors of eicosanoids. Eicosanoids derived from AA, including PGE2, TBX2, and LTB4, are more pro-inflammatory than those derived from DHA (Calder, 2006). The results of our study confirmed this pathway of eicosanoid formation. Chickens fed LR diets had significantly lower levels of PGE2 and TBX2 in the blood than those fed HR diets. These results are in agreement with those of Cherian et al. (2009), who showed that the level of incorporation of n-3 FA was higher, but that of AA in cardiac ventricle lipids was lower, and the levels of PGE2 and TBX2 in the blood of progeny (7-, 14-, 28- and 42-day-old chickens) were lower after feeding HR diets to broiler hens. Liu et al. (2014) reported significantly lower levels of pro-inflammatory eicosanoids (PGE2 and TBX2) in the plasma due to inhibition of phospholipase A2 activity in chickens fed diets rich in PUFA n-3 than in chickens fed diets rich in PUFA n-6. In the present study, no significant differences were observed in LTB4 concentration due to dietary treatments, even when the level of its precursor (AA) in HR diet and the level of AA in the examined immune tissues were high, probably due to the inhibitory effect of leukotriene B5 (LTB5) on LTB4 via competition for receptors (Kragballe et al., 1987). Hall et al. (2007) reported that hens fed HR diets have significantly higher production of LTB5 by thrombocytes in the progeny and that the ratio of LTB5 to LTB4 is a good indicator of the effect of PUFA on the synthesis of pro-inflammatory leukotriene series. The negative correlation between PGE2 and TBX2 might indicate that some groups of eicosanoids compete for their precursor and suppress their synthesis. The suppressive effect of increased vE on the synthesis of pro-inflammatory eicosanoids was evident in the present study, since LTB4 and TBX2 concentrations were significantly lower in chickens fed diets with increased vE level compared with those fed diets with basal vE level. According to Gore and Qureshi (1997), vE is an antagonist of AA peroxidation and thus limits its availability. However, the effect of vE on the eicosanoid-related immune response depends on the level and the age of chickens (Khan et al., 2012), since no regulatory effect on PGE2 concentration was observed in chickens fed diets with high or basal vE levels.
Our findings indicated that the dietary PUFA n-6:n-3 ratio could not only affect the PHA-induced swelling response at different stages of chicken growth, but might also induce alterations in the leukocyte profile in response to PHA challenge. Chickens at the age of 14 d fed LR diets had a higher swelling response than those at the age of 35 days. The peak of WWS was observed between 6 h and 12 h post PHA injection. The PHA-induced WWS is a good indicator of acquired immunity and allows the assessment of leukocyte interactions during the immune response. However, it must be noted that since we were unable to count an absolute number of WBC, precise interpretation of the results is difficult. Based on previous studies, it can be assumed that changes in leukocyte population, occurring during the swelling response, are a common delayed-type hypersensitivity reaction in birds. Martin et al. (2006) showed (in wild birds) that leukocytes, except for heterophils and lymphocytes, increased over time after the PHA challenge, whereas heterophils appeared early and then disappeared; lymphocytes remained present throughout much of the swelling response. A typical swelling response in chickens is regulated in 2 phases. The first is driven by the local innate cell populations (largely basophils and macrophages), and occurs between 6 h and 12 h post PHA injection. During this phase, plasma exudation accrues from the surrounding vascular tissue into the injected region. The second phase starts at 24 h post injection and involves the infiltration of additional PHA-sensitive T-lymphocytes (McCorkle et al., 1980; Elgert, 1996). Lymphocytes are the main immunocytes in the body involved in the immune response, whereas heterophils (granular leukocytes) are phagocytic cells whose main function is killing foreign cells. Thus, the number of heterophils increases dramatically in chickens during inflammation and stress (Wang et al., 2011). Changes in WBC differential counts were associated with significant interactions between age and post-PHA injection time, since the response was higher in younger than older chickens. These results could be attributed to the process of immune system maturation. According to Schat et al. (2014), the avian immune system at the early growth period is immature, undergoes rapid changes, and spontaneously responds to numerous factors. The H:L ratio has been used as an indicator of immunological condition, inflammation, and stress (particularly caused by dietary factors) in chickens. The H:L ratios of 0.2, 0.5, and 0.8 indicate low, medium, and high degrees of stress, respectively (Gross and Siegel, 1983). In our study, the dietary PUFA n-6:n-3 ratio and vE level caused low to medium level of stress in chickens, whereas the peak of the primary response of heterophils occurred at 6 h post PHA injection.
LR diets resulted in a stronger WWS, results that were in agreement with those reported by Korver and Klasing (1997) and demonstrated the anti-inflammatory properties of PUFA n-3. This effect may be explained by the fact that PUFA n-3 are precursors of less pro-inflammatory eicosanoids than is AA, consequently a high PUFA n-3 diet reduces the level of AA in the immune tissues and of AA-derived eicosanoids.
In summary, the present study demonstrated that the modulation of dietary PUFA n-6:n-3 ratio and vE level could positively affect chicken physiology. Direct evidence was provided that feeding HR diets increased AA incorporation in immune tissues and consequently promoted the synthesis of pro-inflammatory eicosanoids in the blood, whereas LR diets improved WWS in PHA-challenged chickens. The beneficial effects of LR diets, especially with increased vE level, on immune system responses were more important during the first few weeks of chicken life.
Acknowledgments
This research was financially supported by The National Science Centre, Poland, grant number DEC-2012/05/N/NZ9/01288.
REFERENCES
- Al-Khalifa H., Givens D. I., Rymer C., Yaqoob P. Effect of n-3 fatty acids on immune function in broiler chickens. Poult. Sci. 2012;91:74–88. doi: 10.3382/ps.2011-01693. [DOI] [PubMed] [Google Scholar]
- Aviagen . Ross 308 Broiler: Performance Objectives. 2014. Accessed Dec. 2015, http://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-308-Broiler-PO-2014-EN.pdf. [Google Scholar]
- Boa-Amponsem K., Price S. E., Picard M., Geraert P. A., Siegel P. B. Vitamin E and immune responses of broiler pureline chickens. Poult. Sci. 2000;79:466–470. doi: 10.1093/ps/79.4.466. [DOI] [PubMed] [Google Scholar]
- Calder P. C. Polyunsaturated fatty acids, inflammation and immunity. Lipids. 2001;369:1007–1024. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- Calder P. C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:1505–1519. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- Cheema M. A., Qureshi M. A., Havenstein G. B. A comparison of the immune response of a 2001 commercial broiler with a 1957 randombred broiler strain when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1519–1529. doi: 10.1093/ps/82.10.1519. [DOI] [PubMed] [Google Scholar]
- Cherian G. Essential fatty acids and early life programming in meat-type birds. World's Poult. Sci. J. 2011;67:599–614. [Google Scholar]
- Cherian G., Bautista-Ortega J., Goeger D. E. Maternal dietary n-3 fatty acids alter cardiac ventricle fatty acid composition, prostaglandin and thromboxane production in growing chicks. Prostaglandins Leukot. Essent. Fatty Acids. 2009;80:297–303. doi: 10.1016/j.plefa.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Cortinas L., Villaverde C., Galobart J., Baucells M. D., Codony R., Barroeta A. C. Fatty acid content in chicken thigh and breast as affected by dietary polyunsaturation level. Poult. Sci. 2004;83:1155–1164. doi: 10.1093/ps/83.7.1155. [DOI] [PubMed] [Google Scholar]
- Czauderna M., Kowalczyk J., Korniluk K., Wasowska I. Improved saponification then mild base and acid-catalyzed methylation is a useful method for quantifying fatty acids, with special emphasis on conjugated dienes. Acta Chromatographica. 2007;18:59–71. [Google Scholar]
- Elgert K. Immunology: Understanding the Immune System. Wiley & Liss; New York, USA: 1996. [Google Scholar]
- Friedman A., Sklan D. Effect of dietary fatty acids on antibody production and fatty acid composition of lymphoid organs in broiler chicks. Poult. Sci. 1995;74:1463–1469. doi: 10.3382/ps.0741463. [DOI] [PubMed] [Google Scholar]
- Fritsche K. L., Cassity N. A., Huang S. C. Effect of dietary fat source on antibody production and lymphocyte proliferation in chickens. Poult. Sci. 1991;70:611–617. doi: 10.3382/ps.0700611. [DOI] [PubMed] [Google Scholar]
- Gonzalez D., Mustacich D. J., Traber M. G., Cherian G. Early feeding and dietary lipids affect broiler tissue fatty acids, vitamin E status, and cyclooxygenase-2 protein expression upon lipopolysaccharide challenge. Poult. Sci. 2011;90:2790–2800. doi: 10.3382/ps.2011-01452. [DOI] [PubMed] [Google Scholar]
- Gore A. B., Qureshi M. A. Enhancement of humoral and cellular immunity by vitamin E after embryonic exposure. Poult. Sci. 1997;76:984–991. doi: 10.1093/ps/76.7.984. [DOI] [PubMed] [Google Scholar]
- Gross W. B., Siegel H. S. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. 1983:972–979. [PubMed] [Google Scholar]
- Hall J. A., Jha S., Skinner M. M., Cherian G. Maternal dietary n-3 fatty acids alter immune cell fatty acid composition and leukotriene production in growing chicks. Prostaglandins Leukot. Essent. Fatty Acids. 2007;76:19–28. doi: 10.1016/j.plefa.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Innis S. M. Dietary (n-3) fatty acids and brain development. J. Nutr. 2007;137:855–859. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- Innis S. M. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- Khan R. U., Rahman Z. U., Nikousefat Z., Javdani M., Tufarelli V., Dario C., Selvaggi M., Laudadio V. Immunomodulating effects of vitamin E in broilers. World's Poult. Sci. J. 2012;68:31–40. [Google Scholar]
- Kim B. C., Ryu Y. C., Cho Y. J., Rhee M. S. Influence of dietary α-tocopheryl acetate supplementation on cholesterol oxidation in retail packed chicken meat during refrigerated storage. Biosci. Biotechnol. Biochem. 2006;70:808–814. doi: 10.1271/bbb.70.808. [DOI] [PubMed] [Google Scholar]
- Klasing K. C. Nutrition and the immune system. Br. Poult. Sci. 2007;48:525–537. doi: 10.1080/00071660701671336. [DOI] [PubMed] [Google Scholar]
- Konieczka P., Rozbicka-Wieczorek A. J., Więsyk E., Smulikowska S., Czauderna M. Improved derivatization of malondialdehyde with 2-thiobarbituric acid for evaluation of oxidative stress in selected tissues of chickens. J. Anim. Feed Sci. 2014;23:190–197. [Google Scholar]
- Konieczka P., Czauderna M., Rozbicka-Wieczorek A., Smulikowska S. The effect of dietary fat, vitamin E and selenium concentrations on the fatty acid profile and oxidative stability of frozen stored broiler meat. J. Anim. Feed Sci. 2015;24:244–251. [Google Scholar]
- Konjufca V. K., Bottje W. G., Bersi T. K., Erf G. F. Influence of dietary vitamin E on phagocytic functions of macrophages in broilers. Poult. Sci. 2004;83:1530–1534. doi: 10.1093/ps/83.9.1530. [DOI] [PubMed] [Google Scholar]
- Korver D. R., Klasing K. C. Dietary fish oil alters specific and inflammatory immune responses in chicks. J. Nutr. 1997;127:2039–2046. doi: 10.1093/jn/127.10.2039. [DOI] [PubMed] [Google Scholar]
- Kragballe K., Voorhees J. J., Goetzl E. J. Inhibition by leukotriene B5 of leukotriene B4-induced activation of human keratinocytes and neutrophils. J. Invest. Dermatol. 1987;88:555–558. doi: 10.1111/1523-1747.ep12470151. [DOI] [PubMed] [Google Scholar]
- Leshchinsky T. V., Klasing K. C. Relationship between the level of dietary vitamin E and the immune response of broiler chickens. Poult. Sci. 2001;80:1590–1599. doi: 10.1093/ps/80.11.1590. [DOI] [PubMed] [Google Scholar]
- Liu G., An S., Yuan J., Guo Y., Liu D., Chen H., Huang R. Dietary fish oil and zinc reduced plasma prostaglandin E 2 content by inhibiting phospholipase A 2 production in broilers. J. Poult. Sci. 2014;51:66–70. [Google Scholar]
- Marsh J. A., Combs G. F., Whitacre M. E., Dietert R. R. Effect of selenium and vitamin E dietary deficiencies on chick lymphoid organ development. Proc. Soc. Exp. Biol. Med. 1986;182:425–436. doi: 10.3181/00379727-182-42361. [DOI] [PubMed] [Google Scholar]
- Martin L. B., Han P., Lewittes J., Kuhlman J. R., Klasing K. C., Wikelski M. Phytohemagglutinin‐induced skin swelling in birds: Histological support for a classic immunoecological technique. Funct. Ecol. 2006;20:290–299. [Google Scholar]
- McCorkle F., Olah I., Glick B. The morphology of the phytohemagglutinin-induced cell response in the chicken's wattle. Poult. Sci. 1980;59:616–623. doi: 10.3382/ps.0592151. [DOI] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Poultry. 9th rev. ed. National Academy Press; Washington, DC: 1994. [Google Scholar]
- Niu Z. Y., Liu F. Z., Yan Q. L., Li W. C. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult. Sci. 2009;88:2101–2107. doi: 10.3382/ps.2009-00220. [DOI] [PubMed] [Google Scholar]
- Panda A. K., Bhanja S. K., Shyam Sunder G. Early post hatch nutrition on immune system development and function in broiler chickens. World's Poult. Sci. J. 2015;71:285–296. [Google Scholar]
- Puthpongsiriporn U., Scheideler S. E. Effects of dietary ratio of linoleic to linolenic acid on performance, antibody production, and in vitro lymphocyte proliferation in two strains of leghorn pullet chicks. Poult. Sci. 2005;84:846–857. doi: 10.1093/ps/84.6.846. [DOI] [PubMed] [Google Scholar]
- Puvača N., Lukač D., Ljubojević D., Stanaćev V., Beuković M., Kostadinović L., Plavša N. Fatty acid composition and regression prediction of fatty acid concentration in edible chicken tissues. World's Poult. Sci. J. 2014;70:585–592. [Google Scholar]
- Qi K. K., Chen J. L., Zhao G. P., Zheng M. Q., Wen J. Effect of dietary ω6/ω3 on growth performance, carcass traits, meat quality and fatty acid profiles of Beijing‐you chicken. J. Anim. Physiol. Anim. Nutr. (Berl.) 2010;94:474–485. doi: 10.1111/j.1439-0396.2009.00932.x. [DOI] [PubMed] [Google Scholar]
- Rhee K. S., Anderson L. M, Sams A. R. Lipid oxidation potential of beef, chicken, and pork. J. Food Sci. 1996;61:8–12. [Google Scholar]
- Rymer C., Givens D. I. Effect of species and genotype on the efficiency of enrichment of poultry meat with n− 3 polyunsaturated fatty acids. Lipids. 2006;41:445–451. doi: 10.1007/s11745-006-5118-2. [DOI] [PubMed] [Google Scholar]
- Salem N. M., Lin Y. H., Moriguchi T., Lim S. Y., Salem N., Hibbeln J. R. Distribution of omega-6 and omega-3 polyunsaturated fatty acids in the whole rat body and 25 compartments. Prostaglandins Leukot. Essent. Fatty Acids. 2015;100:13–20. doi: 10.1016/j.plefa.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schat K. A., Kaspers B., Kaiser P. Avian Immunology. Elsevier; Philadelphia, USA: 2014. [Google Scholar]
- Shira E. B., Sklan D., Friedman A. Impaired immune responses in broiler hatchling hindgut following delayed access to feed. Vet. Immunol. Immunopathol. 2005;105:33–45. doi: 10.1016/j.vetimm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Surai P. F., Sparks N. H. C. Tissue-specific fatty acid and α-tocopherol profiles in male chickens depending on dietary tuna oil and vitamin E provision. Poult. Sci. 2000;79:1132–1142. doi: 10.1093/ps/79.8.1132. [DOI] [PubMed] [Google Scholar]
- Swiatkiewicz S., Arczewska-Wlosek A., Jozefiak D. The relationship between dietary fat sources and immune response in poultry and pigs: An updated review. Livest Sci. 2015;180:237–246. [Google Scholar]
- Wang M. Z., Ding L. Y., Wang J. F., Wang H. R., Yu L. H. Effects of n-6: n-3 polyunsaturated fatty acid ratio on heterophil: lymphocyte ratio and T lymphocyte subsets in the peripheral blood of the Yangzhou gosling. Poult. Sci. 2011;90:824–829. doi: 10.3382/ps.2010-01199. [DOI] [PubMed] [Google Scholar]