Abstract
Disease inflicted by avian pathogenic Escherichia coli (APEC) causes economic losses and burden to the poultry industry worldwide. In this study, the efficacy of chitosan nanoparticles loaded ΦKAZ14 (C-ΦKAZ14 NPs) as an oral biological therapy for Colibacillosis was evaluated. C-ΦKAZ14 NPs containing 107 PFU/ml of ΦKAZ14 (Myoviridae; T4-like coliphage) bacteriophage were used to treat experimentally APEC-infected COBB 500 broiler chicks. C-ΦKAZ14 NPs and ΦKAZ14 bacteriophage were administered orally in a single dose. The clinical symptoms, mortality, and pathology in the infected birds were recorded and compared with those of control birds that did not receive C-ΦKAZ14 NPs or naked ΦKAZ14 bacteriophage. The results showed that C-ΦKAZ14 NP intervention decreased mortality from 58.33 to 16.7% with an increase in the protection rate from 42.00 to 83.33%. The bacterial colonization of the intestines of infected birds was significantly higher in the untreated control than in the C-ΦKAZ14 NP-treated group (2.30×109 ± 0.02 and 0.79×103 ± 0.10 CFU/mL, respectively) (P ≤ 0.05). Similarly, a significant difference in the fecal shedding of Escherichia coli was observed on d 7 post challenge between the untreated control and the C-ΦKAZ14 NP-treated group (2.35×109 ± 0.05 and 1.58×103 ± 0.06 CFU/mL, respectively) (P ≤ 0.05). Similar trends were observed from d 14 until d 21 when the experiment was terminated. Treatment with C-ΦKAZ14 NPs improved the body weights of the infected chicks. A difference in body weight on d 7 post challenge was observed between the untreated control and the C-ΦKAZ14 NP-treated group (140 ± 20 g and 160 ± 20 g, respectively). The increase was significant (P ≤ 0.05) on d 21 between the 2 groups (240 ± 30 g and 600 ± 80 g, respectively). Consequently, the clinical signs and symptoms were ameliorated upon treatment with C-ΦKAZ14 NPs compared with infected untreated birds. In all, based on the results, it can be concluded that the encapsulation of bacteriophage could enhance bacteriophage therapy and is a valuable approach for controlling APEC infections in poultry.
Keywords: C-ΦKAZ14 NPs, Escherichia coli, ΦKAZ14, COBB 500 broiler chicks, chitosan
INTRODUCTION
Escherichia coli is a commensal bacterium of the gastrointestinal tract of birds. Not all strains cause diseases; however, some strains cause disease following exposure to certain conditions, which may include abnormal prevalence over other commensals, a weakened host immune system, or adverse environmental conditions (Barnes et al., 2008). In addition to Escherichia coli strains that reside within the intestinal tract where they cause disease, some strains cause disease outside the gastrointestinal tract. They are referred to as avian pathogenic Escherichia coli (APEC) and cause a disease called Colibacillosis (Zhao et al., 2005). Avian Colibacillosis is a complicated disease characterized by different signs and symptoms, including numerous organ lesions, typically pericarditis, airsacculitis, perihepatitis, and peritonitis. In its acute form, it causes septicaemia. The disease leads to high economic losses worldwide following high mortality rates, carcass rejection, and condemnation at slaughter (Delicato et al., 2003; Ewers et al., 2004). Since the disease leads to septicemia and airsacculitis in the affected birds, these lesions are a cause of condemnation at slaughter. It has been shown that these lesions alone represent a yearly average condemnation rate of up to 0.2% of almost 170 million broilers dressed every wk in the United States, although the condemnation is seasonally dependent, increasing during the winter period. However, when 0.2% standard downgrade is used, the United States broiler operations suffer huge economic losses of up to €38 million yearly due to infectious processes and lesions observed on dressed chickens at slaughter (Simon M. S, 2009). Fecal contamination has been identified as the source of infection through inhalation of Escherichia coli into the respiratory tract (Barnes and Gross, 1997). The invading Escherichia coli isolated from chickens are usually resistant to multiple drug therapy (Levy, 2001, 2002).
Bacteriophages are viruses that attack bacteria and cause bacterial lysis. They are specific to the host that they infect and kill. Therefore, they do not have any effects on other living organisms besides bacteria. Thus, they are an attractive alternative to antibiotics and could be used to overcome bacterial infection and antibiotic resistance (Huff et al., 2013). However, one constraint that could limit the application of phage by the oral route is that the effectiveness of the administered phage is rapidly reduced by acid, enzymes, and bile (Joerger, 2003). Thus, there is a need to protect the phage for oral therapy to control Colibacillosis (Chibani-Chennoufi et al., 2004). It has been hypothesized that loading phage into chitosan nanoparticles would improve protection from inactivation by enzymes and lead to enhanced effective delivery to the target site.
Chitosan and its derivatives are natural polycationic polysaccharides containing glucosamine and N-acetyl-glucosamine, which have been used in various applications and have been shown to be non-toxic, biocompatible, and biodegradable (Hirano et al., 1990; Chandy et al., 1990; Struszczyk et al., 1991). Although chitosan has low oral toxicity (Knapczyk et al., 1989; Hirano et al., 1990), its toxicity may depend on its degree of deacetylation, molecular weight, purity, and route of administration. The efficacy of chitosan nanoparticles loaded ΦKAZ14 bacteriophage in the biological control of colibacillosis in chickens was evaluated and discussed.
MATERIALS AND METHODS
Experimental design and treatment
To evaluate the efficacy of chitosan nanoparticle-encapsulated ΦKAZ14 (C-ΦKAZ14 NPs), a 3-week experiment was performed. A total of 36 one-day-old mixed sex COBB 500 broiler chicks were obtained from a commercial hatchery in Seri Kembangan, Malaysia (supplied by FAJIMA TRADING). The hatchery had no history of Colibacillosis. On arrival, chicks were allowed to acclimatize to the laboratory setting for one wk, with access to water and antibiotic-free feed ad libitum. The internal room temperature was controlled at 20°C. Following stabilization, the chicks were weighed using a digital portable hanging scale (BlueDot Trading LLC, Kuala Lumpur, Malaysia) and were randomly divided into 3 experimental groups of 12 (n = 12) chicks per group. In this case, water and antibiotic-free feed were provided ad libitum throughout the experimental period.
The experimental groupings were as follows:
Untreated control (n = 12) Infected; no treatment
Naked ΦKAZ14 phage (n = 12) Infected and treated with naked bacteriophage
C-ΦKAZ14 NPs (n = 12) Infected and treated with C-ΦKAZ14 NPs
Experimental Colibacillosis infections in COBB 500 broiler chicks were induced by intra tracheal instillation of chicks with 109 CFU/mL Escherichia coli O1:K1:H7. The treatment regime was as follows. The birds in the untreated control group were treated with a 0.2 mL placebo dose of phosphate buffered saline (PBS) (0.14 M NaCl, 0.0027 M KCl, 0.01 M Na2HPO4, 0.0018 M KH2PO4; pH 7.4). The birds in the naked ΦKAZ14 bacteriophage group received 0.2 mL naked ΦKAZ14 bacteriophage at a concentration of 107 PFU/mL in SM buffer. The birds in the C-ΦKAZ14 NP group received 0.2 mL C-ΦKAZ14 NPs containing 107 PFU/mL. All groups were challenged with 0.2 mL of a 5-h-old culture containing 109 CFU/mL Escherichia coli O1:K1:H7 (grown in LB broth at 37°C with shaking at 180 rpm). The treatments were instituted 2 h post challenge by oral administration of bacteriophage. The challenge was performed by direct intra tracheal instillation of challenge culture into the trachea of the 7-day-old COBB 500 chicks via a blunt ended cannula.
Preparation of ΦKAZ14 bacteriophage chitosan nanoparticle (C-ΦKAZ14 NP)
Preparation of chitosan nanoparticles was earlier reported (Kaikabo et al., 2016). A medium molecular weight chitosan with degree of deacetylation of 75% to 85% was purchased (Sigma-Aldrich, St. Louis, MO) and used to prepare chitosan nanoparticles. Briefly, 1% chitosan nanoparticles were prepared by dissolving chitosan (0.1 g) in distilled water (10 mL) containing 100 μL acetic acid (QRëCTM, Kuala Lumpur, Malaysia) under continuous magnetic stirring for one hour. The mixture was vortexed and sonicated for 5 and 30 min, respectively. The resulting solution was centrifuged at 10,000 g and adjusted to a pH of 5.5 by adding 0.1 M sodium hydroxide (Sigma-Aldrich) with gentle swirling. The final solution was filtered through a povidone membrane (filter pore size 0.45 μM). Furthermore, 107 PFU/mL ΦKAZ14 bacteriophage was encapsulated with chitosan nanoparticles as follows: The bacteriophage suspension (10 mL) containing 107 PFU/mL of ΦKAZ14 bacteriophage was suspended in 10 mL of 1% chitosan solution (v/v) and gently stirred with a magnetic bar. The detailed physico-chemical characterization of ΦKAZ14 bacteriophage nanoparticles was discussed (Kaikabo et al., 2016).
Clinical signs of Colibacillosis in COBB 500 broiler chicks
The clinical signs of Collibacillosis in COBB 500 broiler chicks infected with APEC and treated with C-ΦKAZ14 NPs were observed and recorded (Antao et al., 2008) throughout the period of the experiment.
Body weight changes in COBB 500 broiler chicks
The body weights of chicks were measured at weekly intervals and at the end of the study period. Their major organs, such as the lung, liver, heart, spleen, and gizzard, were aseptically excised and weighed as previously described (Huff et al., 2006a). All birds were sacrificed at the end of the study by cervical dislocation. Animal care and procedures were carried out based on the approved guidelines of the Institutional Animal Care and Use Committee (IACUC) University Putra, Malaysia, under approval number: UPM/IACUC/AUP-R089/2014.
Mortality in COBB 500 broiler chicks
The birds that died in the course of the experiments were collected for postmortem analysis to assess any gross pathological lesions. Tissue samples were taken for bacteriological culture (Piercy and West, 1976). The mortality and survivors were recorded, and the mortality rate was calculated as follows:
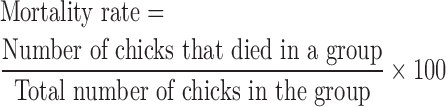 |
Intestinal colonization and shedding of E. coli in the feces of COBB 500 broiler chicks
The birds’ intestinal contents were aseptically scooped using a sterile spatula and transferred into sterile plastic bags and weighed. The contents were homogenized in 0.85% sodium chloride. The homogenates were serially diluted 10-fold, plated in triplicate on eosin methylene blue (EMB) agar (Merck KGaA, Darmstadt, Germany) and incubated at 37°C overnight. Following incubation, the colonies that showed a green metallic sheen were enumerated to determine the number of Escherichia coli per gram of sample (CFU/g) that colonized the intestine. The results were calculated and compared among groups to determine intestinal colonization by Escherichia coli. For Escherichia coli shedding in feces, freshly voided feces were aseptically collected from trays and weighed, followed by processing as described as for intestinal colonization (Carrillo et al., 2005; Janež and Loc-Carrillo, 2013).
Viable bacterial count in internal organs and blood of COBB 500 broiler chicks
Viable bacterial count in organs (spleen and lungs) and blood of birds that died during the course of the study and those sacrificed at the end of the experiment were determined as described with little modifications (Huang and Matsumoto, 1999). Organs were aseptically excised and weighed. One gram of each organ was homogenized in 9 mL of PBS using stomacher (Lab Lemco 400, Seward, Worthington, United Kingdom). A 10-fold serial dilution was made in PBS from which 100 ul of homogenate were plated on Congo Red agar in triplicates and incubated at 37°C for 24 hours. The colonies were counted to determine the colony-forming unit per gram or milliliter (CFU/g/mL) in each organ or blood.
RESULTS
Clinical signs of COBB 500 broiler chicks infected with APEC
The clinical signs observed in the experimental birds included inappetance, ruffled feathers, rales and gasping for air, sitting on the hocks, and diarrhea. Similarly, some birds were depressed or dishevelled in appearance with swollen heads. These signs were minor in the C-ΦKAZ14 NP-treated group compared with the naked bacteriophage ΦKAZ14-treated and -untreated control groups.
Weekly body weights of COBB 500 broiler chicks infected with APEC
No differences were observed among the groups in the body weights of birds on d 7 post challenge. The mean body weight of birds treated with naked phage and C-ΦKAZ14 NP therapy, birds treated with ΦKAZ14 phage, and the untreated control group was not significantly different (P ≤ 0.05). Similar results were observed on d 14 post challenge. However, even with infection, the body weight continued to increase gradually from d 7 to d 14 to d 21 post challenge, when the experiment was terminated (Table 1a). This increase despite infection was attributed to the positive impact of C-ΦKAZ14 NP therapy on the mean weekly body weight of the birds. A slight difference in body weight was observed between birds treated with naked bacteriophage and those treated with C-ΦKAZ14 NPs on d 21 post challenge, and the difference among the treated groups and the untreated control group was significant (P ≤ 0.05). These results were not different from the replicate group (Table 1b).
Table 1a.
Body weight change (g) mean ± SEM triplicates of COBB 500 broiler chicks infected with pathogenic Escherichia coli and treated with C-ΦKAZ14 NPs.
| Days post infection | |||
|---|---|---|---|
| Group | 7 | 14 | 21 |
| Untreated control | 140 ± 20a | 240 ± 30b | 240 ± 30b |
| ΦKAZ14 phage | 170 ± 10b,c | 290 ± 20b,c | 550 ± 80c |
| C-ΦKAZ14 NPs | 160 ± 20b | 270 ± 30b | 600 ± 80a |
ΦKAZ14 phage = naked bacteriophage, C-ΦKAZ14 NPs = chitosan bacteriophage-encapsulated nanoparticles.
a–cvalues represent the mean ± SEM triplicate of survivors per cage. Values within a column with different superscripts differ significantly (P ≤ 0.05). Values represent the body weights of survivors.
Table 1b.
Body weight change (g) Mean ± SEM triplicates of COBB 500 broiler chicks infected with pathogenic Escherichia coli and treated with C-ΦKAZ14 NPs (replicate).
| Days post infection | |||
|---|---|---|---|
| Group | 7 | 14 | 21 |
| Untreated control | 140 ± 20a | 240 ± 30b | 240 ± 30b,c |
| ΦKAZ14 phage | 170 ± 10b,c | 290 ± 20b,c | 550 ± 80b |
| C-ΦKAZ14 NPs | 160 ± 20b | 270 ± 30b | 600 ± 80a,c |
ΦKAZ14 phage = naked bacteriophage, C-ΦKAZ14 NPs = chitosan bacteriophage-encapsulated nanoparticles.
a–cvalues represent the mean ± SEM triplicate of 12 birds per cage. Values within a column with different superscripts differ significantly (P ≤ 0.05). Values represent the body weights of survivors.
Mortality in COBB 500 broiler chicks
The birds treated with C-ΦKAZ14 NPs had a mortality of 16.7% (2/12) with a survival rate of 83.33%, indicating that the treatment with C-ΦKAZ14 NPs lowered the mortality in Escherichia coli-challenged birds treated with C-ΦKAZ14 NPs (Table 2a ). In the group treated with only naked ΦKAZ14, the mortality was 50% and the survival rate was 50% (6/12). However, in the untreated control group, the mortality rate was 58% (7/12) with a survival rate of 42%. The mortality in Escherichia coli-challenged untreated birds was higher 58.33% (7/12) than those challenged with Escherichia coli and treated with C-ΦKAZ14 NPs or naked ΦKAZ14 bacteriophage. The C-ΦKAZ14 NP-treated group had the lowest negligible mortality throughout the duration of the study.
Table 2a.
Mortality rate in COBB 500 broiler chicks infected with pathogenic Escherichia coli and treated with C-ΦKAZ14 NPs.
| Mortality rate | Protection | |||
|---|---|---|---|---|
| Group | Mortality | Survivors | (%) | rate |
| Untreated control | 7 | 5 | 58.33 | 42.00 |
| ΦKAZ14 phage | 6 | 6 | 50.00 | 50.00 |
| C-ΦKAZ14 NPs | 2 | 10 | 16.70 | 83.33 |
ΦKAZ14 phage = naked bacteriophage, C-ΦKAZ14 NPs = chitosan bacteriophage encapsulated nanoparticles. Total number of chicks per group (n) = 12.
Table 2b.
Mortality rate in COBB 500 broiler chicks infected with pathogenic Escherichia coli and treated with C-ΦKAZ14 NPs (replicate).
| Mortality rate | Protection | |||
|---|---|---|---|---|
| Group | Mortality | Survivors | (%) | rate |
| Untreated control | 7 | 5 | 58.33 | 42.00 |
| ΦKAZ14 phage | 6 | 6 | 50.00 | 50.00 |
| C-ΦKAZ14 NPs | 2 | 10 | 16.70 | 83.33 |
ΦKAZ14 phage = naked bacteriophage, C-ΦKAZ14 NPs = chitosan bacteriophage encapsulated nanoparticles. Total number of chicks per group (n) = 12.
Bacterial colonization and shedding in the feces of infected Cobb 500 broiler chicks
On d 7, the fecal shedding of Escherichia coli decreased among the groups (P ≤ 0.05). Fecal shedding of Escherichia coli in the feces of C-ΦKAZ14 NP-treated chicks decreased when compared with the ΦKAZ14 bacteriophage-treated group and the untreated control group (Table 3a). A comparable decrease in the bacterial shedding among the groups was observed from d 7 until d 21 post challenge (P ≤ 0.05). On d 21 post challenge, the bacterial shedding rate continued to differ between the group with lowest shedding rate observed in the C-ΦKAZ14 NP-treated group compared with the naked ΦKAZ14 bacteriophage-treated and untreated control groups. Furthermore, from d 7 to d 21 post challenge, the bacterial shedding was different between the ΦKAZ14 bacteriophage-treated and untreated control groups (P ≤ 0.05). Interestingly, fecal shedding of Escherichia coli continued to be lower (P ≤ 0.05) in the C-ΦKAZ14 NP-treated group from d 7 to d 21 post challenge compared with the ΦKAZ14 phage-treated group and the untreated control.
Table 3a.
Weekly bacterial shedding rate (CFU) mean ± SEM triplicates in feces of COBB 500 broiler chicks infected with pathogenic Escherichia coli and treated with C-ΦKAZ14 NPs.
| Days post challenge | ||||
|---|---|---|---|---|
| Group | 7 | 14 | 21 | Colonization |
| Untreated control | 2.35 × 109 ± 0.05a | 2.29 × 108 ± 0.01a | 2.31 × 108 ± 0.01a | 2.30 × 109 ± 0.02a |
| ΦKAZ14 phage | 1.95 × 105 ± 0.04b | 1.88 × 105 ± 0.04b | 1.95 × 104 ± 0.03b | 1.76 × 105 ± 0.03b |
| C-ΦKAZ14 NPs | 1.58 × 103 ± 0.06c | 1.22 × 103 ± 0.04b | 1.09 × 102 ± 0.10b,c | 0.79 × 103 ± 0.10c |
ΦKAZ14 phage = naked bacteriophage, C-ΦKAZ14 NPs = chitosan bacteriophage-encapsulated nanoparticles.
a–cvalues represent the mean ± SEM triplicate of 12 birds per cage. Values within a column with different superscripts differ significantly (P ≤ 0.05). Values represent the body weights of survivors.
Similarly, a decrease in bacterial colonization was observed. The efficacy of treatment was more pronounced in the groups treated with C-ΦKAZ14 NPs and ΦKAZ14 compared with the untreated control group. A decrease in bacterial colonization (P ≤ 0.05) was observed between the C-ΦKAZ14 NP-treated group and the naked ΦKAZ14-treated and untreated control groups. In the same vein, these results were not different with the replicate group (Table 3b).
Table 3b.
Weekly bacterial shedding rate (CFU) mean ± SEM triplicates in faeces of COBB 500 broiler chicks infected with pathogenic Escherichia coli and treated with C-ΦKAZ14 NPs.
| Days post challenge | ||||
|---|---|---|---|---|
| Group | 7 | 14 | 21 | Colonization |
| Untreated control | 2.35 × 109 ± 0.05a | 2.29 × 108 ± 0.01a | 2.31 × 108 ± 0.01a | 2.30 × 109 ± 0.02a |
| ΦKAZ14 phage | 1.95 × 105 ± 0.04b | 1.88 × 105 ± 0.04b | 1.95 × 104 ± 0.03b | 1.76 × 105 ± 0.03b |
| C-ΦKAZ14 NPs | 1.58 × 103 ± 0.06c | 1.22 × 103 ± 0.04b | 1.09 × 102 ± 0.10b,c | 0.79 × 103 ± 0.10c |
ΦKAZ14 phage = naked bacteriophage, C-ΦKAZ14 NPs = chitosan bacteriophage-encapsulated nanoparticles.
a–cvalues represent the mean ± SEM triplicate of 12 birds per cage. Values within a column with different superscripts differ significantly (P ≤ 0.05). Values represent the body weights of survivors.
Viable bacterial count in lung, spleen and blood of infected COBB 500 broiler chicks
In the control group, viable counts of APEC O1:K1:H7 increased from d 7 to 28 in the lung, spleen, and blood. In the ϕKAZ14 bacteriophage-treated group, a decrease in viable counts of APEC O1:K1:H7 was observed from d 7 to 28 dpi. The level of decrease in APEC O1:K1:H7 in organs was apparent in the lung followed by the spleen and blood. On 28 dpi a drastic decrease in viable counts of APEC O1:K1:H7 was observed compared with d 7 to 21, respectively. However, in the C- ϕKAZ14NP-treated group viable counts of APEC O1:K1:H7 were low in the blood, spleen, and lung on d 7 to 21, respectively. The least viable counts of APEC O1:K1:H7 were observed in the blood on 28 dpi followed by the spleen and lung, respectively. There was no difference observed between the results in the main and replicate group (Table 4a,b).
Table 4a.
Mean ± SD viable count of Escherichiia coli (O1:K1:H7) isolated from organs and blood of infected 500 COBB broiler chicks.
| Spleen | Lung | Bloodc | ||
|---|---|---|---|---|
| Group | DPI | Mean CFU/g(mL) | Mean CFU/g(mL) | Mean CFU/g(mL) |
| Control | 7 | 94.67 ± 8.32a | 103.33 ± 10.40a | 104.33 ± 9.29a |
| 14 | 154.33 ± 42.47a | 161.66 ± 43.68a | 162 ± 43.55 | |
| 21 | 399 ± 13.52a | 417 ± 52.6a | 432 ± 72.64 | |
| 28 | 464 ± 53.73b | 470.33 ± 50.9b | 496.33 ± 76.13 | |
| ΦKAZ14 | 7 | 90.66 ± 2.30a | 100.67 ± 0.57a | 93 ± 3.46 |
| 14 | 80.67 ± 2.08a | 94 ± 3.46a | 90 | |
| 21 | 60a | 86.67 ± 10.01a | 79.33 ± 1.15 | |
| 28 | 56.33 ± 5.68b | 77 ± 4.35b | 80 | |
| C- ΦKAZ14NP | 7 | 86 ± 5.19a | 87.33 ± 4.16a | 79 ± 3 |
| 14 | 56.67 ± 4.16a | 66.33 ± 4.50a | 47.33 ± 8.32 | |
| 21 | 34.33 ± 20.64a | 44.67 ± 15.53a | 34 ± 16.52 | |
| 28 | 13 ± 3.46 | 34 ± 16.52b | 9.33 ± 1.15 |
a= including birds that died before sacrifice, b= all survivor birds before sacrifice, c= all live birds before mortality and sacrifice, DPI = Day post infection, ϕKAZ14 = unprotected bacteriophage, C-φKAZ14NP = chitosan nanoparticle protected bacteriophage.
Table 4b.
Mean ± SD viable count of Escherichiia coli (O1:K11:H7) isolated from organs and blood of infected 500 COBB broiler chicks (replicate group).
| Spleen | Lung | Bloodc | ||
|---|---|---|---|---|
| Group | DPI | Mean CFU/g(mL) | Mean CFU/g(mL) | Mean CFU/g(mL) |
| Control | 7 | 94.67 ± 8.32a | 103.33 ± 10.40a | 104.33 ± 9.29a |
| 14 | 154.33 ± 42.47a | 161.66 ± 43.68a | 162 ± 43.55 | |
| 21 | 399 ± 13.52a | 417 ± 52.6a | 432 ± 72.64 | |
| 28 | 464 ± 53.73b | 470.33 ± 50.9b | 496.33 ± 76.13 | |
| ΦKAZ14 | 7 | 90.66 ± 2.30a | 100.67 ± 0.57a | 93 ± 3.46 |
| 14 | 80.67 ± 2.08a | 94 ± 3.46a | 90 | |
| 21 | 60a | 86.67 ± 10.01a | 79.33 ± 1.15 | |
| 28 | 56.33 ± 5.68b | 77 ± 4.35b | 80 | |
| C-ΦKAZ14NP | 7 | 86 ± 5.19a | 87.33 ± 4.16a | 79 ± 3 |
| 14 | 56.67 ± 4.16a | 66.33 ± 4.50a | 47.33 ± 8.32 | |
| 21 | 34.33 ± 20.64a | 44.67 ± 15.53a | 34 ± 16.52 | |
| 28 | 13 ± 3.46 | 34 ± 16.52b | 9.33 ± 1.15 |
a= including birds that died before sacrifice, b= all survivor birds before sacrifice, c= all live birds before mortality and sacrifice, DPI = Day post infection, ϕKAZ14 = unprotected bacteriophage, C-φKAZ14NP = chitosan nanoparticle protected bacteriophage.
DISCUSSION
The current research demonstrates the efficacy of C-ΦKAZ14 NP therapy for the biological control and reduction of bacterial colonization and shedding in feces of COBB 500 broiler chickens infected with APEC. The findings are discussed.
Colibacillosis was experimentally reproduced with APEC in one-week-old COBB broiler chicks using an Avian pathogenic Escherichia coli type strain (APEC O1:K1:H7). The strain was previously isolated from the lung of a chicken clinically diagnosed with coli septicaemia (Johnson et al., 2007). The clinical symptoms associated with this experimentally induced APEC infection were inappetence, ruffled feathers, rales and gasping for air, sitting on the hocks, and diarrhea. Similarly, some birds were depressed and dishevelled in appearance. These clinical signs diminished in the groups treated with C-ΦKAZ14 NPs compared with the untreated control group. In a similar study, Lau et al. (2010) showed that bacteriophage therapy alleviates severe clinical Colibacillosis in chicks.
The body weights of birds on d 7 post challenge decreased in all groups. During the initial stage of infection, inappetance in the affected birds could lead to decrease in growth performance as a result of reduced feed intake (Gomis et al., 1997). However, increase in body weight was observed in the C-ΦKAZ14 NP-treated group compared with the untreated control on d 14 post challenge. This might be due to the effect of treatment with C-ΦKAZ14 NPs, which reduced the severity of infection and improved appetence and feed intake leading to recovery and weight gain. These findings were consistent with the reports of Oliveira et al., (2010) who observed an increase in the body weight of the bacteriophage-treated group compared with the untreated control group. Similarly, Atterbury et al. (2007) showed that bacteriophage administered at a high dosage was beneficial to growth performance by reducing Salmonella enteritidis and Salmonella typhimurium in the cecal contents. This result indicates that bacteriophage administered at a high concentration could improve feed efficiency and growth, leading to increase in body weight.
In the current study, the C-ΦKAZ14 NP-treated group exhibited a lower mortality and a higher survival rate compared with the group that received naked ΦKAZ14 bacteriophage (mortality of 50.0 and 16.7%, and survival of 50.0 and 83.33%, respectively). These results were lower when compared with the untreated control (with mortality and survival of 58.33 and 42.00%, respectively) (Table 3a,b). This success could be attributed to the therapy with C-ΦKAZ14 NPs. This might be due to due to the protection of naked phages from inactivation by gastric acid and enzymes (Ma et al., 2008). It is likely that in the naked ΦKAZ14 bacteriophage-treated group, some of the administered phages were inactivated by acids or enzymes; as such, the challenged birds were overwhelmed by infection, leading to a mortality of 50%. Carvalho et al. (2010) showed protecting phages against the effects of acids or gastric juice increase their effects.
On colonization of challenged birds by Escherichia coli and the effects of bacteriophage treatment, the treatment with C-ΦKAZ14 NPs was observed to reduce bacterial colonization. Lower concentrations of the Escherichia coli strain (O1:K11:H7) were observed in the C-ΦKAZ14 NP-treated group compared with untreated control birds (P < 0.05, N = 12). This finding is in concordance with the reports of Borie et al. (2009) who showed the effects of a phage cocktail in reducing Salmonella enteritidis colonization in chicks, and that bacteriophage supplementation reduces salmonella colonization in broiler chickens; similarly, inclusion of bacteriophage in feed reduces cecal Escherichia coli colonization in broilers (Huff et al., 2004; Atterbury et al., 2007). It was also observed that fecal shedding of Escherichia coli (O1:K11:H7) in chicks treated with C-ΦKAZ14 NPs continued to decrease (P ≤ 0.05) from d 7 to 21 post challenge compared with chicks treated with naked ΦKAZ14 phage and the untreated control group. To lend credence to this finding, Wang et al. (2011) observed that supplementation of the broiler diet with bacteriophage increases lactobacillus and decreases Escherichia coli and Salmonella species in excreta.
A decrease in viable counts of APEC O1:K1:H7 was observed in the C- ΦKAZ14NP-treated group compared with the ΦKAZ14 and untreated control groups. Low viable counts of APEC O1:K1:H7 were observed in the blood, spleen, and lung on 28-d pi. However, at the beginning of the study on d 7 pi the viable count was high in the untreated control group. These differences were attributed to the effect of C- ΦKAZ14NP treatment. Huang and Mutsumoto (1999) observed higher viable counts of APEC O1:K1:H7 in the lung followed by liver, spleen, and blood in a vaccination evaluation study. However, a lower viable count was observed in the liver and spleen of the vaccinated birds than in the control. In the current study, a decrease in the viable APEC O1:K1:H7 count was observed in the internal organs and the blood of the group treated with C- ΦKAZ14 NP compared with ΦKAZ14 and untreated control groups. However, a higher viable count of APEC O1:K1:H7 was observed in the blood followed by the lung and spleen of the infected, untreated control group. This result showed that the therapeutic effect of C- ΦKAZ14 NP could be measured in an in vivo viable count method that requires small samples and produces quantitative results in a reproducible manner compared to the method of assessing efficacy by mortality or lesion (Huang and Matsumoto, 1999). The fact that the lowest viable count was observed in the blood of C- ΦKAZ14NP- and ΦKAZ14-treated groups showed that blood is a good medium in which ΦKAZ14 bacteriophage could act to cause lysis of APEC O1:K1:H7 used in the challenge experiment. According to Brüssow (2005), and Górski and Weber-Dabrowska (2005), bacteriophages can migrate through the mucosal surface and even across the blood-brain barrier, thereby providing good protection to the host. The adsorption of a phage to its host bacteria is more efficient in a highly fluid environment such as blood, as compared with viscous and solid environments (Joerger, 2003). Thus, this could be the reason that viable counts were low in the blood of C- ΦKAZ14 NP- and ΦKAZ14-treated groups. But, viable counts were observed to be high in the blood of the untreated control group. A viable count was observed to be high in the lungs suggesting that the lung is the prime target of APEC O1:K1:H7 before it gains entry and spreads to major organs causing Colibacillosis, as such a high rate of viable count from this predilection site was possible. However, the effect of therapy with C- ΦKAZ14NP and ΦKAZ14 was observed to decrease the viable count in the treated groups compared with the untreated control. Similarly, a viable count of APEC O1:K1:H7 in the spleen decreased from 7-d pi to 28-d pi. A decrease in viable counts was observed in C- ΦKAZ14NP followed by ΦKAZ14-treated groups and was highest in the untreated control group. Even though a higher viable count was observed in the spleen in this group, the viable count is the lowest when it is compared with the lungs. The spleen serves both as a phagocytic filter as well as an antibody-producing organ that helps to remove bacteria from the bloodstream or sequester bacteria that are not as well opsonized (Bohnsack and Brown, 1986); thus, a lower rate of isolation in the spleen is possible even without treatment with C- ΦKAZ14NP and ΦKAZ14.
CONCLUSION
In conclusion, the effects of C-ΦKAZ14 NPs, a potential oral therapy for the treatment or control of Colibacillosis caused by Avian pathogenic Escherichia coli, have been demonstrated. Various concerns associated with orally administered bacteriophages have been expressed by researchers. Orally administered bacteriophage could be inactivated by enzymes and acids along the gastrointestinal tract. In this study, the ΦKAZ14 bacteriophage was encapsulated in chitosan nanoparticles referred to as C-ΦKAZ14 NPs. The application of C-ΦKAZ14 NPs showed promise in the biological control of Colibacillosis. There was a reduction in the bacterial colonization of the chicken intestines and in shedding in the feces of the affected birds. In all, the significance of this study in the production of healthy chickens, enhancing the safety of foods of poultry origin and improving public health and food security, can never be overemphasized. These findings have shed light on these areas.
Acknowledgments
This work was partly funded by the Research University Grant Scheme (RUGS) grant number 9329400, University Putra Malaysia, Selangor, Malaysia.
REFERENCES
- Antao E. M., Glodde S., Li G., Sharifi R., Homeier T., Laturnus C., Wieler L. H. The chicken as a natural model for extra intestinal infections caused by avian pathogenic Escherichia coli (APEC) Microb. Pathogen. 2008;45:361–369. doi: 10.1016/j.micpath.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Atterbury R. J., Van Bergen M. A. P., Ortiz F., Lovell M. A., Harris J. A., De Boer A., Barrow P. A. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl. Environ. Microbial. 2007;73:4543–4549. doi: 10.1128/AEM.00049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes H. J., Gross W. B. Colibacillosis. In: Calnek B. W., Barnes H. J., Beard C. W., McDougald L. R., Saif Y. M., editors. Diseases of Poultry. Iowa State University Press; Ames, IA: 1997. pp. 131–141. [Google Scholar]
- Barnes J., Nolan L. K., Vaillancourt J.-P. Colibacillosis. In: Saif Y. M., Fadly A. M., Glisson J. R., McDougald L. R., Nolan L. K., Swayne D. E., editors. Diseases of Poultry. 12th ed. Blackwell Publishing Ltd; Garsington Road, Oxford OX4 2DQ, UK: 2008. p. 691. [Google Scholar]
- Bohnsack J. F., Brown E. J. The role of the spleen in resistance to infection. Annu. Rev. Med. 1986;37:49–59. doi: 10.1146/annurev.me.37.020186.000405. [DOI] [PubMed] [Google Scholar]
- Borie C., Sánchez M. L., Navarro C., Ramírez S., Morales M. A., Retamales J., Robeson J. Aerosol spray treatment with bacteriophages and competitive exclusion reduces Salmonella enteritidis infection in chickens. Avian Dis. 2009;53:250–254. doi: 10.1637/8406-071008-Reg.1. [DOI] [PubMed] [Google Scholar]
- Brüssow H. Phage therapy: The Escherichia coli experience. Microbial. 2005;151:2133–2140. doi: 10.1099/mic.0.27849-0. [DOI] [PubMed] [Google Scholar]
- Carrillo C. L., Atterbury R. J., El-Shibiny A., Connerton P. L., Dillon E., Scott A., Connerton I. F. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 2005;71:6554–6563. doi: 10.1128/AEM.71.11.6554-6563.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C. M., Gannon B. W., Halfhide D. E., Santos S. B., Hayes C. M., Roe J. M., Azeredo J. The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens. BMC microbial. 2010;10:232. doi: 10.1186/1471-2180-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy T., Sharma C. P. Chitosan-as a biomaterial. Biomater. Artif. Cells Artif. Organs. 1990;18:1–24. doi: 10.3109/10731199009117286. [DOI] [PubMed] [Google Scholar]
- Chibani-Chennoufi S., Sidoti J., Bruttin A., Kutter E., Sarker S., Brüssow H. In vitro and in vivo bacteriolytic activities of Escherichia coli phages: Implications for phage therapy. Antimicrob. Agents Chemother. 2004;48:2558–2569. doi: 10.1128/AAC.48.7.2558-2569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delicato E. R., de Brito B. G., Gaziri L. C. J., Vidotto M. C. Virulence-associated genes in Escherichia coli isolates from poultry with Colibacillosis. Vet. Microbial. 2003;94:97–103. doi: 10.1016/s0378-1135(03)00076-2. [DOI] [PubMed] [Google Scholar]
- Ewers C., Janßen T., Kießling S., Philip H. C., Wieler L. H. Molecular epidemiology of avian pathogenic Escherichia coli (APEC) isolated from coli septicemia in poultry. Vet. Microbial. 2004;104:91–101. doi: 10.1016/j.vetmic.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Gomis S. M., Watts T., Riddell C., Potter A. A., Allan B. J. Experimental reproduction of Escherichia coli cellulitis and septicemia in broiler chickens. Avian Dis. 1997:234–240. [PubMed] [Google Scholar]
- Górski A., Weber-Dabrowska B. The potential role of endogenous bacteriophages in controlling invading pathogens. Cell. Mol. life Sci. 2005;62:511–519. doi: 10.1007/s00018-004-4403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S., Seino H., Akiyama Y., Nonaka I. Progress in biomedical polymers. Springer; US: 1990. Chitosan: A biocompatible material for oral and intravenous administrations; p. 283.p. 290. [Google Scholar]
- Huang H. J., Matsumoto M. Immunity against Escherichia coli infection in chickens assessed by viable bacterial counts in internal organs. Avian Dis. 1999:469–475. [PubMed] [Google Scholar]
- Huff G. R., Huff W. E., Rath N. C., Tellez G. Limited treatment with β-1, 3/1, 6-glucan improves production values of broiler chickens challenged with Escherichia coli. Poult. Sci. 2006;85:613–618. doi: 10.1093/ps/85.4.613. [DOI] [PubMed] [Google Scholar]
- Huff W. E., Huff G. R., Rath N. C., Balog J. M., Donoghue A. M. Therapeutic efficacy of bacteriophage and Baytril (enrofloxacin) individually and in combination to treat Colibacillosis in broilers. Poult. Sci. 2004;83:1944–1947. doi: 10.1093/ps/83.12.1944. [DOI] [PubMed] [Google Scholar]
- Huff W. E., Huff G. R., Rath N. C., Donoghue A. M. Method of administration affects the ability of bacteriophage to prevent Colibacillosis in 1-day-old broiler chickens. Poult. Sci. 2013;92:930–934. doi: 10.3382/ps.2012-02916. [DOI] [PubMed] [Google Scholar]
- Janež N., Loc-Carrillo C. Use of phages to control Campylobacter spp. J. Microbial. Methods. 2013;95:68–75. doi: 10.1016/j.mimet.2013.06.024. [DOI] [PubMed] [Google Scholar]
- Joerger R. D. Alternatives to antibiotics: Bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 2003;82:640–647. doi: 10.1093/ps/82.4.640. [DOI] [PubMed] [Google Scholar]
- Johnson T. J., Kariyawasam S., Wannemuehler Y., Mangiamele P., Johnson S. J., Doetkott C., Nolan L. K. The genome sequence of avian pathogenic Escherichia coli strain O1: K1: H7 shares strong similarities with human extra intestinal pathogenic E. coli genomes. J. Bacteriol. 2007;189:3228–3236. doi: 10.1128/JB.01726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikabo A. A., AbdulKarim S. M., Abas F. Chitosan nanoparticles as carriers for the delivery of ΦKAZ14 bacteriophage for oral biological control of colibacillosis in chickens. Molecules. 2016;21:256. doi: 10.3390/molecules21030256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapczyk J., Krowczynski L., Pawlik B., Liber Z. Chitin and Chitosan: Sources, Chemistry, Biochemistry, Physical Properties and Applications. Elsevier Applied Science; London: 1989. Pharmaceutical dosage forms with chitosan; p. 665.p. 670. [Google Scholar]
- Lau G. L., Sieo C. C., Tan W. S., Hair-Bejo M., Jalila A., Ho Y. W. Efficacy of a bacteriophage isolated from chickens as a therapeutic agent for Colibacillosis in broiler chickens. Poult. Sci. 2010;89:2589–2596. doi: 10.3382/ps.2010-00904. [DOI] [PubMed] [Google Scholar]
- Levy S. B. Antibiotic resistance: consequences of inaction. Clin. Infect. Dis. 2001;33:24–S129. doi: 10.1086/321837. [DOI] [PubMed] [Google Scholar]
- Levy S. B. Factors impacting on the problem of antibiotic resistance. J. Antimicrob. Chemother. 2002;49:25–30. doi: 10.1093/jac/49.1.25. [DOI] [PubMed] [Google Scholar]
- Ma Y., Pacan J. C., Wang Q., Xu Y., Huang X., Korenevsky A., Sabour P. M. Microencapsulation of bacteriophage Felix O1 into chitosan-alginate microspheres for oral delivery. Appl. Environ. Microbial. 2008;74:799–4805. doi: 10.1128/AEM.00246-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira A., Sereno R., Azeredo J. In vivo efficiency evaluation of a phage cocktail in controlling severe Colibacillosis in confined conditions and experimental poultry houses. Vet. Microbial. 2010;146:303–308. doi: 10.1016/j.vetmic.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Piercy D. W. T., West B. Experimental Escherichia coli infection in broiler chickens: course of the disease induced by inoculation via the air sac route. J. Comp. Pathol. 1976;86:203–210. doi: 10.1016/0021-9975(76)90044-x. [DOI] [PubMed] [Google Scholar]
- Simon M. S. Reducing pathogenic E. coli infection by vaccination. World. Poult. 25:online version. 2009 [Google Scholar]
- Struszczyk H., Wawro D., Niekraszewicz A. Adv. chitin. chitosan. Elsevier Applied Science; London: 1991. Biodegradability of chitosan fibres; p. 580. [Google Scholar]
- Wang G., Pan L., Zhang Y., Wang Y., Zhang Z., Lü J., Zhou P., Fang Y., Jiang S. Intranasal delivery of cationic PLGA Nano/micro particles-loaded FMDV DNA vaccine encoding IL-6 elicited protective immunity against FMDV challenge. PloS one. 2011;6:e27605. doi: 10.1371/journal.pone.0027605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Maurer J. J., Hubert S., De Villena J. F., McDermott P. F., Meng J., White D. G. Antimicrobial susceptibility and molecular characterization of avian pathogenic Escherichia coli isolates. Vet. Microbial. 2005;107:215–224. doi: 10.1016/j.vetmic.2005.01.021. [DOI] [PubMed] [Google Scholar]


