Abstract
Because the ureter arises from the mesonephric or Wolffian duct (WD), the WD opening should migrate inferiorly along the urogenital sinus or future urethra. However, this process of descent has not been evaluated morphometrically in previous studies and we know little about intermediate morphologies for the descent. In the present work, serial sagittal sections of 15 specimens at gestational age 6–12 weeks and serial horizontal sections of 20 specimens at 6–10 weeks were analyzed. Monitoring of horizontal sections showed that, until 9 weeks, a heart-, lozenge- or oval-shape of the initial urogenital sinus remained in the bladder and urethra. Thus, the future bladder and urethra could not be distinguished by the transverse section or plane. The maximum width of the urogenital sinus or bladder at 6–10 weeks was 0.8 mm, although its supero-inferior length reached 5 mm at 10 weeks. During earlier stages, however, the medial shift of the WD was rather evident. Depending on the extent of upward growth of the bladder smooth muscle, the descent of the vas deferens became evident at 10–12 weeks. Development of the urethral rhabdosphincter likely resulted in the differentiation of urogenital sinus into the urethra and bladder before formation of the bladder neck with 3-layered smooth muscles. Development of the prostate followed these morphological changes, later accelerating the further descent of the WD opening. Because of their close topographical relationships, slight anomalies or accidents of the umbilical cord at 10–12 weeks may have a significant effect on normal anatomy.
Keywords: Mesonephric duct, Wolffian duct, Urogenital sinus, Bladder, Vas deferens, Human development
Introduction
The ureter arises from the mesonephric or Wolffian duct (WD) and, depending on the progressive absorption of the WD into the urogenital sinus, the ureter and WD become separated [1,2,3]. The absorption process was recently revealed in photographs of serial embryonic sections [4]. The initial absorption results in a pair of wing-like posterior protrusions of the future bladder. In contrast to the ureteral orifice, which remains at the tip of the protrusion, the WD appears to move medially and inferiorly. In adult males, however, the mesonephric duct-derived vas deferens opens to the urethra at the colliculus seminalis 3 or more centimeters below the ureteral openings. Similar to vaginal descent [5], the descent of the WD or vas deferens in embryos and/or fetuses should occur along the developing bladder and urethra.
At present, the process of descent of the WD or vas deferens, as determined by distance below the bladder relative to weeks of gestation, remains unclear. Rather than differentiating of urogenital sinus into the bladder and urethra, the later development of the prostate may separate the WD opening from the bladder neck. The urogenital sinus- or WD-derived structure in the bladder trigone has been immunohistochemically examined in animal models [6,7]. Briefly, WD-derived epithelium undergoes apoptosis and is replaced in the trigone by sinus-derived epithelium. However, the timing and intermediate morphologies during descent remain to be determined in human embryos and fetuses. Therefore, descent was morphometrically evaluated by assessing sagittal sections of 15 specimens at approximately 6–8 and 10–12 weeks of gestation, and topographical changes along the transverse axis were determined by examining horizontal sections of 20 specimens at 6–8 and 9–10 weeks of gestation.
Materials and Methods
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). The embryonic sections were from the large collection kept at the Embryology Institute, Universidad Complutense, Madrid. The specimens in the collection were products of miscarriages and ectopic pregnancies managed at the Department of Obstetrics at the university. In our previous study about vaginal descent [5], in the collection, female specimens were listed up according to a morphology containing a single thick, central dauct (Műllerian duct and its derivative) with a pair of thin dicts (WD remnants). Therefore, we easily chose male specimens for the present study.
The descent of the WD or vas deferens was strictly estimated by examining serial sagittal sections of nine embryos of crown-rump length (CRL) 21–35 mm (gestational age, 6–8 weeks) and of six fetuses of CRL 50–70 mm (gestational age 10–12 weeks). Topographical changes along the transverse axis were assessed in detail by examining horizontal sections of 12 embryos of CRL 15–30 mm (gestational age, 6–8 weeks) and of eight fetuses of CRL 35–45 mm (gestational age, 9–10 weeks). All sections were stained with hematoxylin and eosin, orange-G or silver stain. The study protocol was approved by the university ethics committee (No. B08/374).
Results
Assessments of sagittal sections
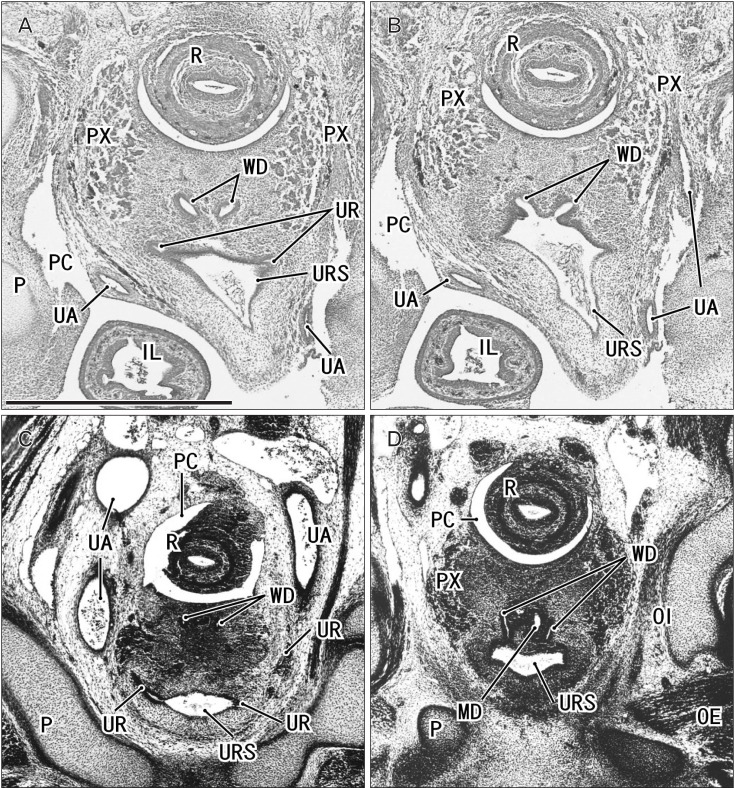
Examination of sagittal sections clearly demonstrated a supero-inferior distance between the WD or vas deferens and the ureteral opening to the urogenital sinus or bladder (Figs. 1, 2). This distance increased at the start of development of smooth muscles in the upper part of the urogenital sinus at 8–9 weeks of gestation (Fig. 1C). Subsequently, folded epithelia suggested differentiation of the bladder from the urogenital sinus (Figs. 2, 3). The supero-inferior length of the bladder appeared to rapidly increase, extending toward the umbilicus. Smooth muscle development started earlier in the bladder than in the rectum (Fig. 1C). Despite the delay, well-differentiated smooth muscle layers developed much earlier in the rectum than in the small intestine (Fig. 2A, C). Smooth muscles were also present in the urachus at 10–12 weeks. The ureteral opening was consistently located slightly above the pubis, but the distance from the ureteral opening to the upper margin of the pubis was less than 0.1 mm at 6–8 weeks. This distance increased depending on the upward growth of the bladder but remained less than 0.2 mm at 10–12 weeks. In contrast, the WD orifice descended from a level slightly above the pubis to a level in the middle of the pubis until 8 weeks (Fig. 2C, D). Since the pubis was almost 2.8–3.3 mm in height at 12 weeks, the distance from the upper margin of the pubis to the WD opening reached 1.5 mm. The umbilical artery attached to the bladder at the rapidly growing upper part (Fig. 2D, E). A three-layered smooth muscle configuration specific to the bladder neck had not yet formed at 12 weeks.
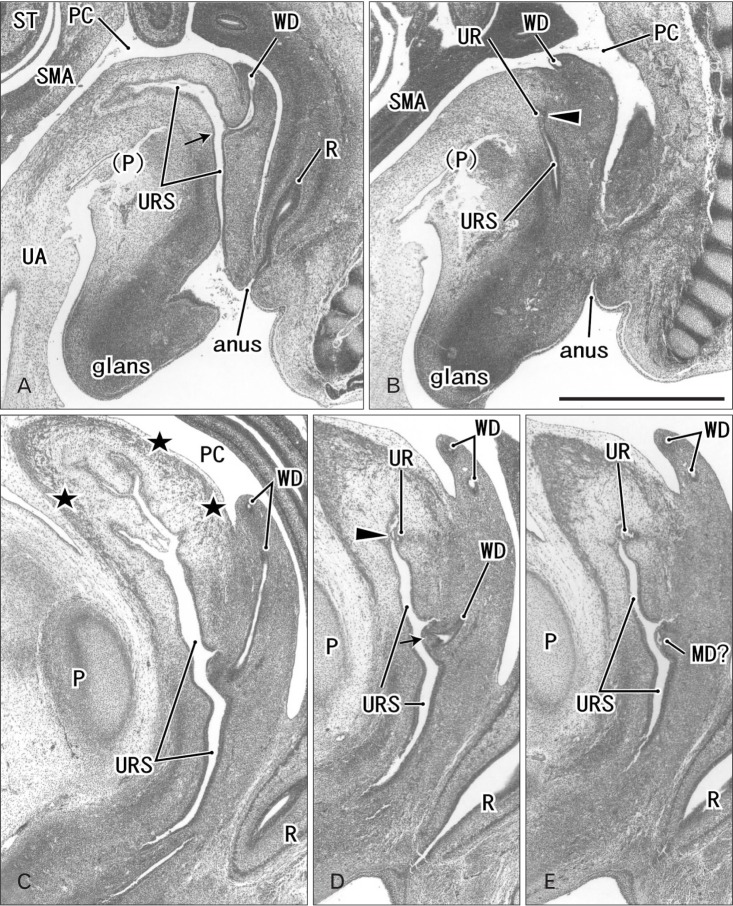
Fig. 1. Sagittal sections of two embryos before differentiation of urogenital sinus into the bladder and urethra. (A, B) A specimen of crown-rump length (CRL) 24 mm (7 weeks). (C–E) A specimen of CRL 35 mm (8 weeks). Hematoxylin and eosin staining. The left-hand side of each panel corresponds to the anterior side of the pelvis. Panel (A) (panel C) is the most medial side of panels (A) and (B) (panels C–E). Intervals between panels are 0.3 mm (A–B), 0.4 mm (C–D), and 0.2 mm (D–E), respectively. All panels were prepared at the same magnification. Scale bar in panel B=1 mm. (A, B) The Wolffian duct (WD) opening (arrow) is located less than 0.1 mm inferior and 0.3 mm medial side of the ureteral opening (arrowhead; UR). (D) The WD opening (arrow) is located 0.5 mm inferior and less than 0.1 mm medial side of the ureteral opening (arrowhead). (C) Smooth muscles start differentiation in the future bladder wall (stars), but the epithelium appears to be a common type of the urogenital sinus (URS). MD, Műllerian duct or paramesonephric duct; P, pubis; PC, peritoneal cavity; R, rectum; SMA, mesentery containing the superior mesenteric artery; ST, stomach; UA, umbilical artery; WD, Wolffian duct or mesonephric duct.
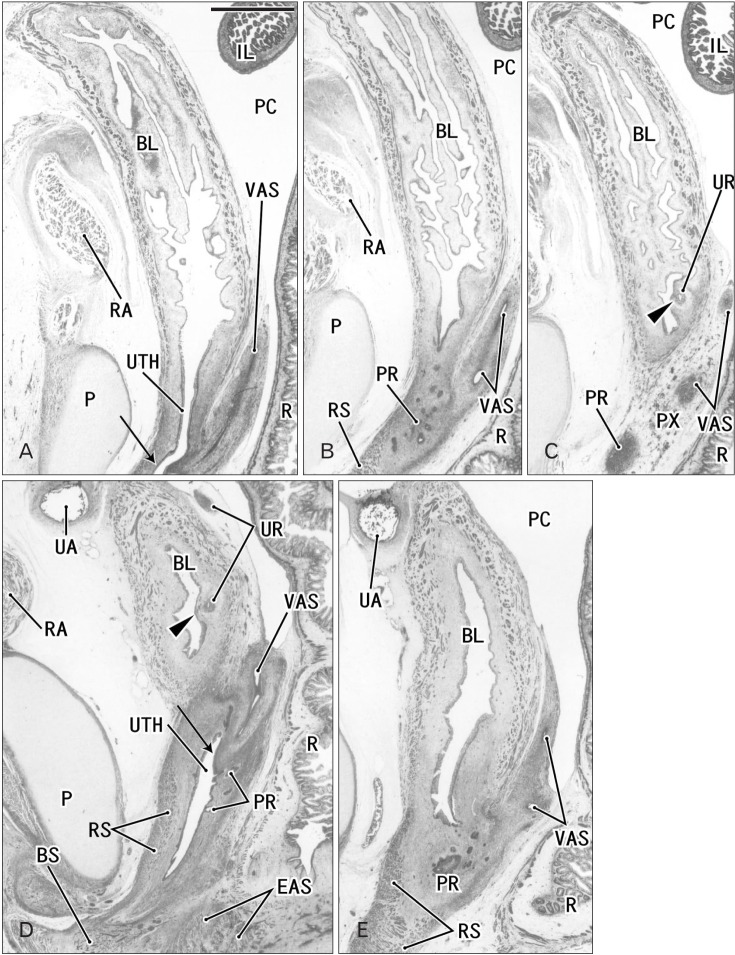
Fig. 2. Sagittal sections of two fetuses after differentiation of urogenital sinus into the bladder and urethra. (A–C) A specimen of crown-rump length (CRL) 56 mm (10 weeks). (D, E) A specimen of CRL 65 mm (12 weeks). Hematoxylin and eosin staining. The left-hand side of each panel corresponds to the anterior side of the pelvis. Panel (A) (panel D) is the most medial side of panels (A–C) (panels D and E). Intervals between panels are 1.1 mm (A–B), 1.5 mm (B–C), and 0.5 mm (D–E), respectively. All panels were prepared at the same magnification. Scale bar in panel A=1 mm. (A–C) The Wolffian duct (WD) opening (arrow) is located 2.2 mm inferior and 2.6 mm medial side of the ureteral opening (arrowhead; UR). (D) The WD opening (arrow) is located 1.9 mm inferior and less than 0.1 mm medial side of the ureteral opening (arrowhead). Smooth muscles are seen in the bladder wall (BL in all panels; dark red color), while the urethra (UTH) accompanies the rhabdophincter (RS in panels B, D, and E). The prostate (PR) starts developing as sprouts from the urethra in both specimens. EAS, external anal sphincter; IL, ileum of the intestine; P, pubis; PC, peritoneal cavity; PX, pelvic autonomic nerve plexus; R, rectum; RA, rectus abdominis muscle; UA, umbilical artery; VAS, vas deferens.
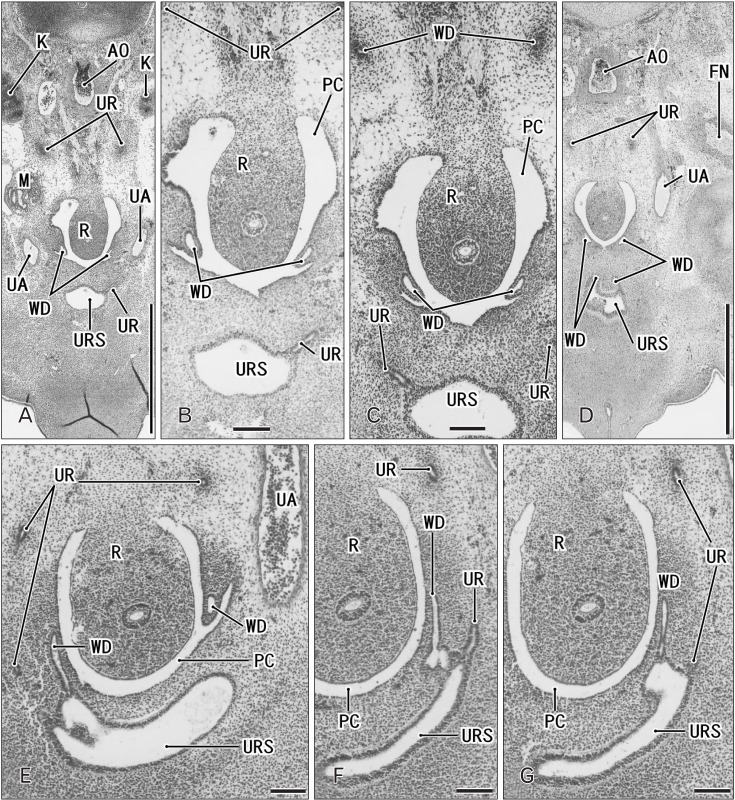
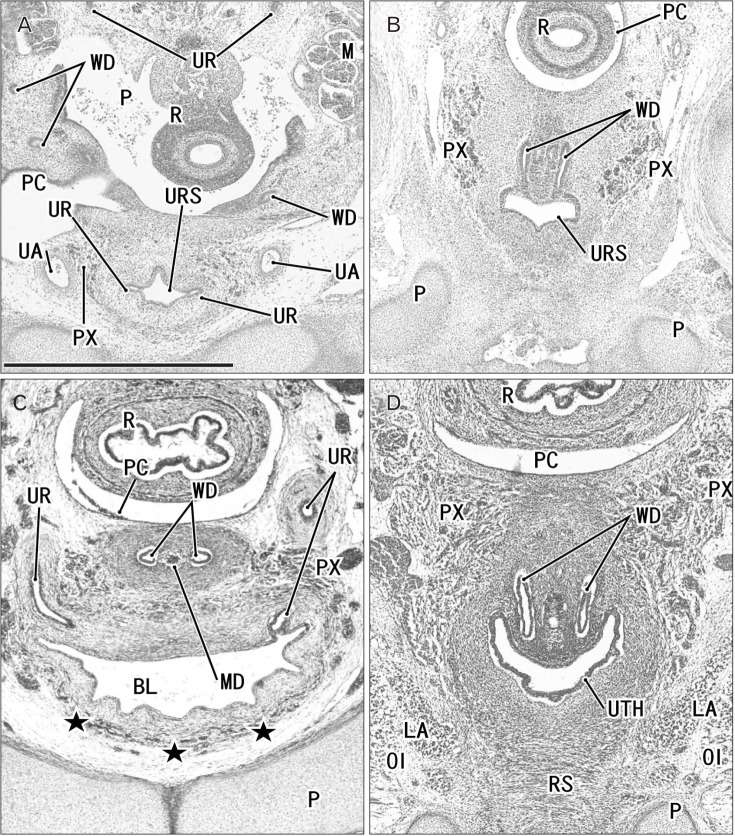
Fig. 3. Horizontal sections of two embryos at 6 weeks before differentiation of urogenital sinus into the bladder and urethra. (A–D) A specimen of crownrump length (CRL) 15 mm. (E–G) A specimen of CRL 17 mm. Hematoxylin and eosin staining. The upper side of each panel corresponds to the posterior side of the pelvis. Panel A (panel E) is the most superior side of panels (A–D) (panels E–G). Panel (B) is a higher magnification view of the central part of panel (A), panel (C) displays a plane 0.1 mm inferior side of panel (A) and 0.2 mm superior side of panel (D). Intervals between panels are 0.2 mm (E–F) and 0.1 mm (F–G), respectively. Scale bars=1 mm in panels A and D, 0.1 mm in panels B, C, and E–G. (A–D) The Wolffian duct (WD) opening is located 0.2–0.3 mm inferior and 0.1–0.2 mm medial side of the ureteral opening (UR). (F) The WD opening is located in the same supero-inferior level as and in 0.1 mm medial side of the ureteral opening. Because of the inferiorly hanging course, the unilateral ureter is cut at multiple sites in all panels. AO, aorta; FN, femoral nerve; K, definite kidney (metanephros); M, mesonephros; PC, peritoneal cavity; R, rectum; UA, umbilical artery; URS, urogenital sinus.
Assessments of horizontal sections
Horizontal sections clearly demonstrated changes in the transverse section or plane of the urogenital sinus, bladder and urethra (Figs. 3, 4, 5, 6, 7). In place of site-dependent morphology, however, individual variations were observed in the transverse shapes of the urogenital sinuses especially of the upper part of it, the future bladder.
Fig. 4. Horizontal sections of three embryos at 7 weeks before differentiation of urogenital sinus into the bladder and urethra. (A, B) A specimen of crown-rump length (CRL) 21 mm. (C, D) A specimen of CRL 22 mm. (E, F) A specimen of CRL 26.5 mm. Hematoxylin and eosin staining. The upper side of each panel corresponds to the posterior side of the pelvis. Panels (A), (C), and (E) display the plane 0.2 mm, 0.3 mm, or 0.4 mm superior to panels (B), (D), or (F), respectively. All panels were prepared at the same magnification. Scale bar in panel B=1 mm. (A, B) The Wolffian duct (WD) opening is located 0.2 mm inferior and less than 0.1 mm medial side of the UR. (C, D) The WD opening is located 0.3 mm inferior and 0.1 mm medial side of the ureteral opening (UR). (E, F) The WD opening is located 0.3–0.4 mm inferior and less than 0.1 mm medial side of the UR. Because of the inferiorly hanging course, the unilateral ureter is cut at multiple sites in panels (A), (C), and (E). LA, levator ani muscle; M, mesonephros; MD, Műllerian duct or paramesonephric duct; P, pubis; PC, peritoneal cavity; PX, pelvic autonomic nerve plexus; R, rectum; UA, umbilical artery; URS, urogenital sinus.
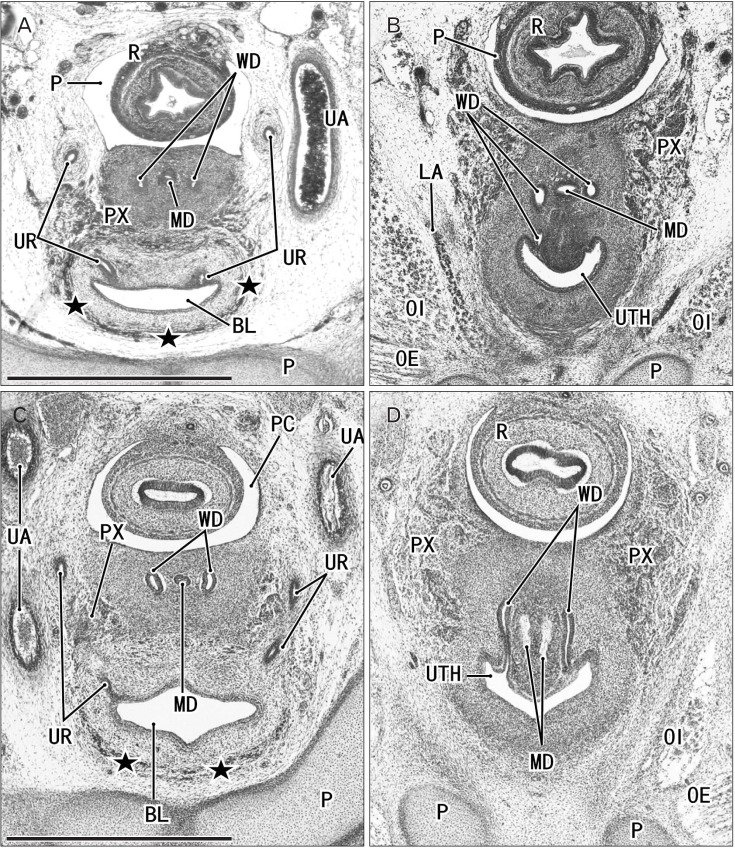
Fig. 5. Horizontal sections of two embryos at 7 weeks before differentiation of urogenital sinus into the bladder and urethra. (A, B) A specimen of crownrump length (CRL) 26 mm. (C, D) Another specimen of CRL 26 mm. Orange-G staining (A, B) and silver staining (C, D). The upper side of each panel corresponds to the posterior side of the pelvis. (A, B) The urogenital sinus (URS) is dilated and protrudes anteriorly due to unknown reason. Panels (A) and (C) display the plane 0.1 mm superior to panels (B) and (D), respectively. In both specimens, the Wolffian duct (WD) opening is located 0.1 mm inferior and 0.1 mm medial side of the ureteral opening (UR). All panels were prepared at the same magnification. Scale bar in panel A=1 mm. IL, ileum of the intestine; K, definite kidney (metanephros); MD, Műllerian duct or paramesonephric duct; OE, obturator externus muscle; OI, obturator internus muscle; P, pubis; PC, peritoneal cavity; PR, prostate; PX, pelvic autonomic nerve plexus; R, rectum; UA, umbilical artery.
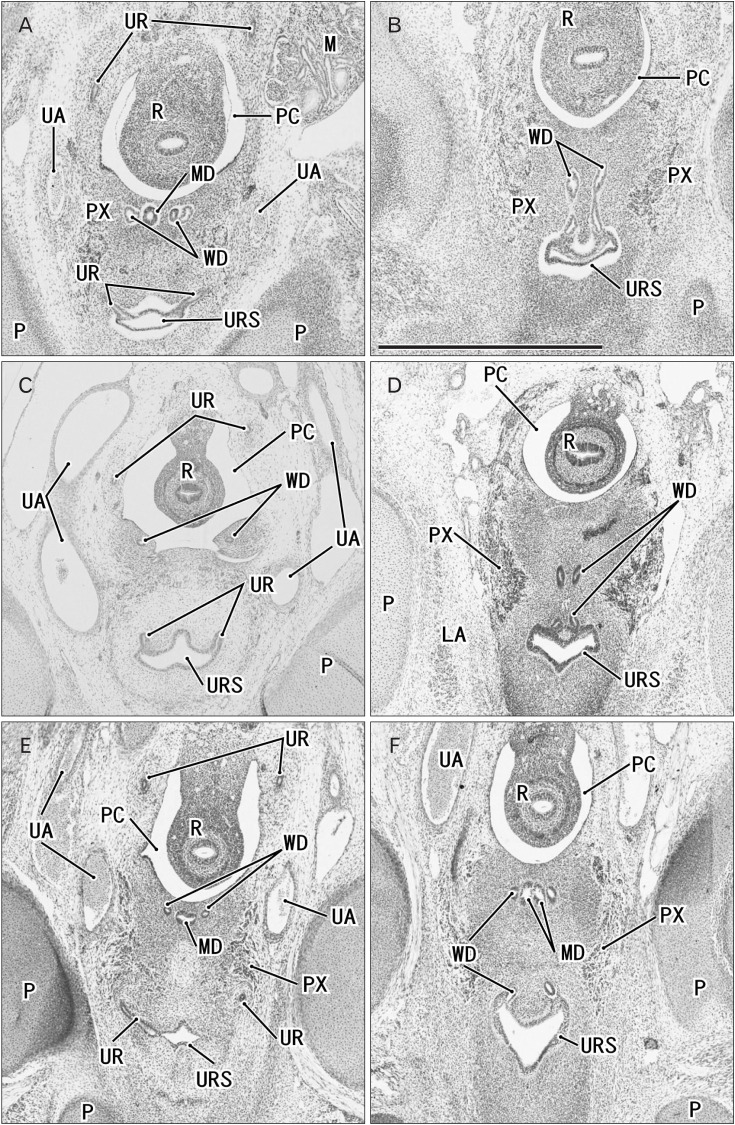
Fig. 6. Horizontal sections of two embryos at 8 weeks after differentiation of urogenital sinus into the bladder and urethra. (A, B) A specimen of crown-rump length (CRL) 29 mm. (C, D) A specimen of CRL 28 mm. Hematoxylin and eosin staining (A–C) and Orange-G staining (D). The upper side of each panel corresponds to the posterior side of the pelvis. Panels (A) and (C) display the plane 0.3 mm superior to panels (B) and (D), respectively. (C, D) In spite of the similar epithelium between sites, smooth muscles (stars) are evident in the bladder anterior wall (BL), while rhabdosphincter muscles (RS) develop along the urethra (UTH). In both specimens, the Wolffian duct (WD) opening is located 0.3 mm inferior and less than 0.1 mm medial side of the ureteral opening (UR). All panels were prepared at the same magnification. Scale bar in panel A=1 mm. BL, bladder; LA, levator ani muscle; M, mesonephros; MD, Műllerian duct or paramesonephric duct; OI, obturator internus mus c l e ; P, pubi s ; PC, peritoneal cavity; PX, pelvic autonomic nerve plexus; R, rectum; UA, umbilical artery; URS, urogenital sinus.
Fig. 7. Horizontal sections of two fetuses at 9 weeks after differentiation of urogenital sinus into the bladder and urethra. (A, B) A specimen of crownrump length (CRL) 39 mm. (C, D) A specimen of CRL 35 mm. Hematoxylin and eosin staining. The upper side of each panel corresponds to the posterior side of the pelvis. Panels A (or panel C) displays the plane 0.6 mm (or 0.8 mm) superior to panel B (or D), respectively. In both specimens, smooth muscles (stars) are evident in the bladder anterior wall (BL). In panels A and B (or C and D), the Wolffian duct (WD) opening is located 0.6 mm (or 0.8 mm) inferior and 0.1 mm medial side of the ureteral opening (UR). All panels were prepared at the same magnification. Scale bar in panel A=1 mm. LA, levator ani muscle; MD, Műllerian duct or paramesonephric duct; OE, obturator externus muscle; OI, obturator internus muscle; P, pubis; PC, peritoneal cavity; PX, pelvic autonomic nerve plexus; R, rectum; UA, umbilical artery; UTH, urethra.
At 6 weeks (Fig. 3), the ureter and WD together opened to the urogenital sinus at its wing-like superolateral ends, with the ureteral opening consistently located on the side immediately lateral to the WD (Fig. 3F). At 7 weeks (Figs. 4, 5), because of the medial shift, the WD orifice was located in the dorsal, rather than the lateral, aspect of the urogenital sinus. Since both the ureter and WD extended inferiorly in a hanging manner, these structures were cut at both the anterior and posterior sites in a single section (Figs. 3A, E, 4A, B, E). Throughout development, the section containing the WD opening to the urogenital sinus frequently also contained an almost straight course of the duct at the bottom of the hanging course (Figs. 4B, D, E, 5D, 6, 7). At 7 weeks, the development of the pelvic autonomic nerve plexus started on the inferior side of the ureteral opening. Although it was sometimes dilated (Fig. 5A, B), the urogenital sinus exhibited a heart-, lozenge- or oval-shape, and, because of individual variations, the future bladder and urethra could not be distinguished by the transverse shape of the lumen.
At 8 and 9 weeks (Figs. 6, 7), the epithelium of the future bladder (near the ureteral opening) was identical to the epithelium of the future urethra (near the WD orifice). Moreover, the initial lozenge shape of the urogenital sinus was maintained until 9 weeks (Fig. 7C). The earliest evidence of smooth muscle development in the future bladder was found in a specimen at 8 weeks, along with the development of the rhabdosphincter muscles along the future urethra (Fig. 6C, D). Although smooth muscles became evident first in the upper part of the bladder (Fig. 2), they were restricted to the anterior wall at lower levels, including the ureteral openings (Figs. 6C, 7A, C). Folded epithelia of the bladder were rarely seen in horizontal sections, and the evidence was limited at 8–9 weeks (Fig. 6C). Thus, at 8–9 weeks, it was still difficult to discriminate between the bladder and urethra in the transverse plane of the lumen. In addition, at the same age, double layered smooth muscles were evident in the rectum. The umbilical artery was attached to the bladder at the lateral aspect (Figs. 4A, C, 5C, 6A). Because of unexpected skipping of sections (a failure of serial sections), the supero-inferior distance from the ureter to the vas deferens tended to be lower when estimated using horizontal than sagittal sections.
Descent of the WD opening
The maximum width of the urogenital sinus or bladder was limited to 0.8 mm (e.g., Fig. 7A) in horizontal sections (6–10 weeks). However, its supero-inferior length was much greater, reaching 5 mm at 10 weeks (Fig. 2A).
Depending on the drastic upward growth of the bladder, constructed of smooth muscle, the descent of the WD opening became evident at 10–12 weeks (Fig. 8). During earlier stages, however, the medial shift of the WD opening was evident, although the absolute distance remained less than 0.2 mm because of the small size of the urogenital sinus (maximum width, 0.4 mm at 7–8 weeks). The greater supero-inferior distance observed (>1.8 mm) was based on measurements of sagittal sections (Fig. 8). At 10–12 weeks, the bladder expanded along both the supero-inferior and transverse axes, whereas the thickness of the urethra remained constant. Thus, due to the growth of the bladder, the distance between the ureteral opening and WD opening was also increased. These observations are summarized in Fig. 9, which overestimates the age-dependent difference in width and underestimates the age-dependent difference in supero-inferior length. Smooth muscle development in the bladder as well as the development of the rhabdosphincter likely resulted in a differentiation of urogenital sinus into the bladder and urethra, followed by the descent of the vas deferens. Development of the prostate followed these morphological changes.
Fig. 8. Correlation between a gestational age and a topographical relation between the ureter and the Wolffian duct or vas deferens. One dot (a simple dot or a dot with circle) corresponds to one specimen. Thirty-two of the 35 specimens observed were evaluated because, in 3 specimens, a complete set of serial sections were not available. The dot with circle indicates a fact that, in the specimen, bladder smooth muscles as well as urethral rhabdosphincter muscles were evident. Depending on drastic development and growth of these muscles, the descent of the vas deferens rapidly occurs at 10–11 weeks. This change are not correlate with individual variations (Figs. 3,4,5,6,7) in the transverse shape and size of the urogenital sinus, bladder and urethra.
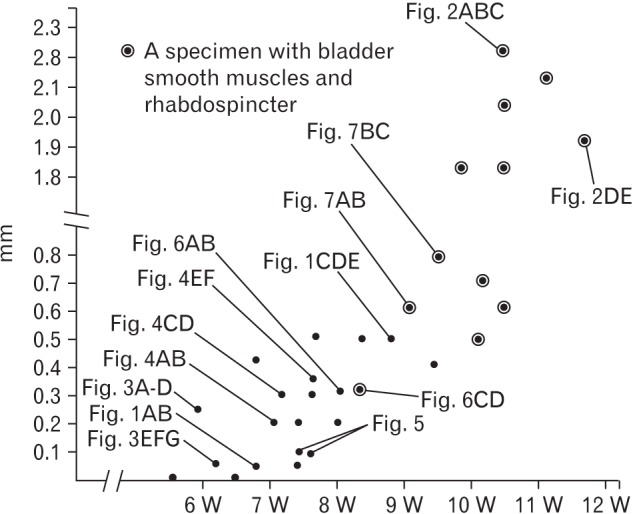
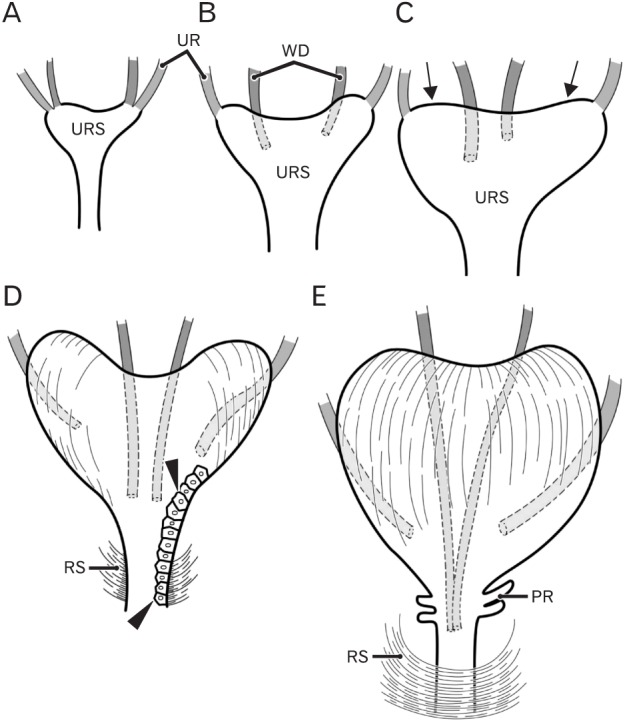
Fig. 9. Schematic representations of the descent of the Wolffian duct (WD) or vas deferens. Anterior view. At 6 weeks (A), the WD (red) opens to the urogenital sinus at a site adjacent to the ureteral orifice (UR; green). At 7–8 weeks (B, C), rather than descent, there are individual variations in the WD position along the mediolateral axis as well as in left/right difference in morphology of the urogenital sinus (URS). The future bladder and urethra are not discriminated in the sinus. At 8–9 weeks, the bilateral upper angles (arrows) of the URS start upward growth toward the umbilicus. At 9 weeks (D), depending on development of smooth muscles in the bladder as well as rhapdosphincter muscles of the urethra (RS), the descent of the vas deferens becomes evident. However, the epithelium is still same (arrowheads) between the future bladder and urethra. At 10–11 weeks (E), a drastic upward growth of bladder smooth muscles as well as a developing prostate (PR) accelerates the descent of the vas.
Discussion
This study demonstrated intermediate morphologies during the descent of WD opening at the colliculus seminalis. Due to the drastic upward growth of the smooth muscle-containing bladder, the descent of the WD opening became evident at 10–12 weeks. Growth of the urogenital sinus was largely directed along an oblique supero-inferior axis toward the umbilicus: the width of the bladder was limited to 0.8 mm in contrast to its 5 mm length at 10 weeks. Therefore, fewer developmental changes in the urogenital sinus than expected were observed in the horizontal sections. Similarly, the epithelium of the future bladder was identical to the epithelium of the future urethra. Conversely, differentiation of urogenital sinus into the bladder and urethra did not start from their transverse shapes but from the muscles associated with them. Thus, the smooth muscles developing in the bladder wall as well as the striated muscles along the urethra seemed to be essentially important for the site-dependent differentiation of the sinus. Attachment of the WD to the future urethra seemed to induce this difference in muscles. Smooth muscle differentiation in the upper half of the urogenital sinus near the urachus was likely to start earliest in the body. Thus, more strictly, the WD seemed to suppress any positive role of the urogenital sinus or urachus, favoring smooth muscle differentiation. These morphological changes were followed by development of the prostate and, later, by a further, much greater descent of the WD opening. These suggested processes differ markedly from vaginal descent, in which the distal end of the Műllerian duct appears to attach to and slide actively downward along the urethral wall [5].
Our recent study showed individual morphologic variations at the joining of the WD and ureter among similar-sized specimens at 5–6 weeks [4]. In this study, we observed that the urogenital sinus had a reversed Y-shape, the arms of which appeared to be derived from the WDs. Actually, all of the specimens in this study had bilateral wing-like protrusions to receive the WD and ureter. Our previous study hypothesized that, when absorption of the WD reached the distal terminal of the ureter, the wing-like parts would enlarge posteriorly for further involvement of the duct with little or no incorporation of the distal ureter [4]. Absorption of the ureter into the sinus or bladder was unlikely along such a long distance (>0.5 mm). In fact, the distance from the ureteral opening to the upper margin of the pubis was almost stable (0.1–0.2 mm) at 6–12 weeks despite the drastic increase in body size. The increased distance between the ureteral opening and WD opening seemed to be largely dependent on the de novo production of the bladder wall, especially by smooth muscles before birth, as well as by an increased size of the prostate after birth.
The bladder appeared to grow toward the umbilicus possibly due to a mechanical stress from the umbilical cord [8]. Growth or elongation of the urachus in the umbilical cord seemed to accelerate smooth muscle development in the bladder wall. The urachus also contained many smooth muscles. Combinations of abnormalities in the urachus and abnormalities of the bladder and urethra, such as undescended bladder, sagittal septum of the bladder and urethral stenosis, have been reported [9]. Indeed, defects in the lower abdominal wall, as well as abnormal traction of the umbilical cord, seen in omphalocele, were most likely to have a deleterious effect on normal development from the urogenital sinus to the bladder [10,11]. Recent advances in ultrasound techniques revealed urachus defects in fetuses in utero [12,13]. However, abnormalities in the vas deferens and/or colliculus seminalis, even if present, would be found after puberty. A slight abnormality or accident in the umbilical cord or umbilicus may be associated with small changes in the topographical anatomy of the bladder, urethra and vas deferens. These slight changes may result in marked disturbances in male sexual function.
Overall, in contrast to the ureteral opening, which remains almost in its initial position in the urogenital sinus or bladder, the WD orifice at the colliculus seminalis was found to descend due to muscle differentiation and development in the bladder and along the urethra.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scirntific Research (JSPS KAKENHI No. 16K08435) from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
References
- 1.Hamilton WJ, Mossman HW. Human embryology. 4th ed. London: Williams & Wilkins; 1978. pp. 321–322. [Google Scholar]
- 2.O'Rhahilly R, Műller F. Human embryology and teratology. 2nd ed. New York: Wiley-Liss; 1996. pp. 194–201. [Google Scholar]
- 3.Moore KL, Persaud TV. The developing human: clinically oriented embryology. 6th ed. Philadelphia: Saunders; 1998. pp. 244–260. [Google Scholar]
- 4.Naito M, Hinata N, Rodriguez-Vazquez JF, Murakami G, Aizawa S, Fujisawa M. Absorption of the Wolffian duct and duplicated ureter by the urogenital sinus: morphological study using human fetuses and embryos. BJU Int. 2015;116:135–141. doi: 10.1111/bju.13006. [DOI] [PubMed] [Google Scholar]
- 5.Masumoto H, Rodríguez-Vázquez JF, Verdugo-López S, Murakami G, Matsubara A. Fetal topographical anatomy of the female urethra and descending vagina: a histological study of the early human fetal urethra. Ann Anat. 2011;193:500–508. doi: 10.1016/j.aanat.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Mendelsohn C. Using mouse models to understand normal and abnormal urogenital tract development. Organogenesis. 2009;5:306–314. doi: 10.4161/org.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka ST, Ishii K, Demarco RT, Pope JC, 4th, Brock JW, 3rd, Hayward SW. Endodermal origin of bladder trigone inferred from mesenchymal-epithelial interaction. J Urol. 2010;183:386–391. doi: 10.1016/j.juro.2009.08.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evatt EJ. A contribution to the development of the prostate gland in the human female, and a study of the homologies of the urethra and vagina of the sexes. J Anat Physiol. 1911;45(Pt 2):122–130. [PMC free article] [PubMed] [Google Scholar]
- 9.Parrott TS, Gray SW, Skandalakis JE. Bladder and urethra. In: Skandalakis JE, Gray SW, editors. Embryology for Surgeons. 2nd ed. Baltimore: Williams & Wilkins; 1994. pp. 671–717. [Google Scholar]
- 10.Suson KD, Novak TE, Gupta AD, Benson J, Sponseller P, Gearhart JP. Neuro-orthopedic manifestations of the omphalocele exstrophy imperforate anus spinal defects complex. J Urol. 2010;184(4 Suppl):1651–1655. doi: 10.1016/j.juro.2010.03.085. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Hwang SE, Rodríguez-Vázquez JF, Murakami G, Cho BH. Liver agenesis with omphalocele: a report of two human embryos using serial histological sections. Pediatr Dev Pathol. 2014;17:431–440. doi: 10.2350/14-05-1484-OA.1. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs F, Picone O, Levaillant JM, Mabille M, Mas AE, Frydman R, Senat MV. Prenatal diagnosis of a patent urachus cyst with the use of 2D, 3D, 4D ultrasound and fetal magnetic resonance imaging. Fetal Diagn Ther. 2008;24:444–447. doi: 10.1159/000174572. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff A, Calvo-Garcia MA, Baregamian N, Levitt MA, Lim FY, Hall J, Peña A. Prenatal counseling for cloaca and cloacal exstrophy-challenges faced by pediatric surgeons. Pediatr Surg Int. 2012;28:781–788. doi: 10.1007/s00383-012-3133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]