Abstract
Fixed drug eruption (FDE) is a common hypersensitivity reaction characterized by recurrent, well-circumscribed, erythematous patches that arise at the same site as a result of systemic drug exposure. However, fixed food eruption (FFE), a lesion triggered by food ingestion, is a rare allergy that was first defined in 1996. Based on their anti-inflammatory and anti-oxidant properties, the fruit and leaves of Actinidia arguta, the hardy kiwi, are widely consumed across Korea, Japan, and China. This report describes the first case of FFE caused by hardy kiwi leaves, known as Daraesun in Korean, confirmed by oral provocation tests and skin biopsy.
Keywords: Fixed food eruption, actinidia arguta, food allergy
INTRODUCTION
Fixed drug eruption (FDE) is a cutaneous hypersensitivity reaction that tends to recur at the same site upon re-exposure to the offending drug. FDE usually presents as single or multiple erythematous plaques that resolve, leaving post-inflammatory hyperpigmentation. More rarely, certain foods can induce similar symptoms, and the term fixed food eruption (FFE) was first used in 1996 to describe a case in which strawberries caused a fixed lesion.1 Since then, cases of FFE have been reported for 10 other foods; cheese crisps,2 lentils,3 lactose,4 quinine,5 peanuts,6 cashew nuts,7 asparagus,8,9 Japanese sand lance,10 tonic water,11 fish, and seafood.12 However, clinical presentations vary according to the primary site and extent of lesions, and results from in vitro and in vivo studies are inconsistent. This report describes a patient with FFE induced by Actinidia arguta, followed by a literature review on the underlying mechanisms of FFE.
CASE REPORT
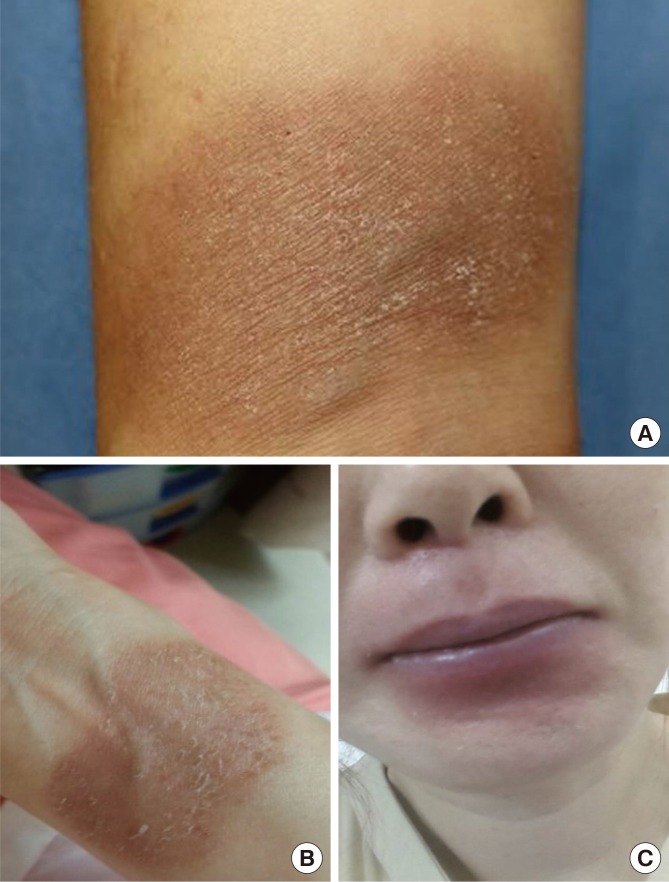
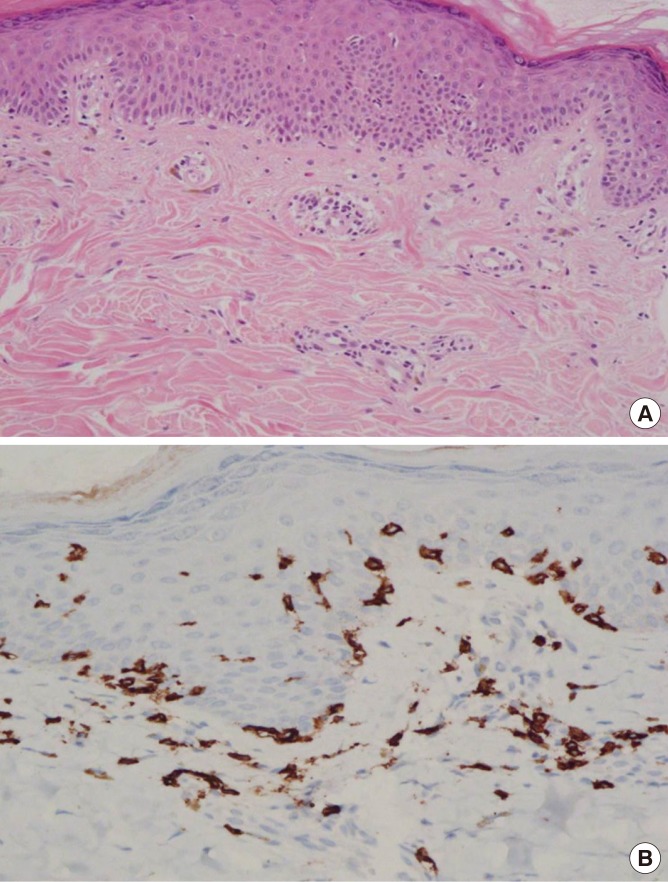
A 43-year-old woman presented with well-demarcated eruptions on the wrist and perioral lesions that had recurred for 1.5 years. She experienced 5 episodes a day after consuming boiled hardy kiwi leaves (Fig. 1A and C). She had a history of green kiwi oral allergy syndrome (Fig. 1B) with low sensitization to birch-alder mix in MAST (Class 1), but no history of latex allergy. No medications had been taken before the lesion developed. After 4 weeks without specific treatment, the skin rash diminished, leaving hyperpigented, scaly lesions (Fig. 2A). Further 4 weeks later when the skin lesions had resolved, open oral food provocation tests and skin tests were performed. Skin pricks with commercial extracts of food allergens were all negative, whereas prick-to-prick tests with cooked hardy kiwi leaves and fresh green kiwi fruits were positive. An autologous serum skin test for detection of histamine release from basophils or mast cells was negative. A patch test at the previous lesion site was suggested, but the patient declined for cosmetic reasons. An open oral food provocation test with 200 g of boiled hardy kiwi leaves was performed (Fig. 1C). Immediately after ingestion, the patient had mild oral itching and throat tightness. After 12 hours, pruritic erythematous eruptions developed in the previously affected areas (Fig. 2B and C). Three days after the oral provocation test, a skin biopsy was taken from the active skin lesion. The results showed perivascular lymphocytic and eosinophilic infiltration with elevated melanophage levels (Fig. 3A). Immunohistological analysis of the effector T cell response induced by FFE was positive for CD8+ T-cells (Fig. 3B). The patient was diagnosed with Actinidia arguta-induced FFE, which was confirmed by an oral provocation test and skin biopsy. We recommended that the patient avoid exposure to this fruit and its leaves.
Fig. 1. (A) Actinidia arguta (hardy kiwi) vegetable. (B) The larger Actinidia deliciosa (green kiwi fruit) in back compared to the Actinidia arguta in front (cited image from: https://en.wikipedia.org/wiki/Actinidia_arguta). (C) Boiled Actinidia arguta vegetable for oral provocation tests.
Fig. 2. (A) Recurrent localized erythemas with hyperpigmentation on the patient’s right wrist. (B, C) Twenty-four hours after Actinidia arguta oral provocation test (B, typical fixed eruption on the wrist; C, perioral eruption with contact dermatitis).
Fig. 3. (A) Superficial perivascular lymphocytic and slightly eosinophilic infiltration with increased dermal melanophages by hematoxylin-and-eosin staining (×200). (B) The immunohistochemical staining shows the intraepidermal CD8+ T cells resident in the skin lesion (×400).
DISCUSSION
This is the first case report of FFE caused by hardy kiwi leaves (Actinidia arguta), and cross-reactivity with green kiwi (Actinidia deliciosa) was shown. According to a previous in vitro study, in which sera from cases with an allergy to green or gold (Actinidia chinensis) kiwi fruits were analyzed for Immunoglobulin E (IgE) binding to hardy kiwi by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), immunoblotting and enzyme-linked immunosorbent assay (ELISA), IgE cross-reactivity was detected for both raw and heat-processed hardy kiwi.13
The classification and pathophysiological mechanisms of FFE are yet to be fully clarified. Although it is uncertain whether the immune responses underlying FFE and FDE are identical, FDE is a delayed-type reaction mediated by an increase in intraepidermal CD8+ T cells compared with normal levels.14 Among the 14 case reports of FFE, 5 had a skin biopsy taken from the active skin lesion. Three of these cases displayed a T cell-dependent pathway with induction of IFNγ-producing CD8+ T cells in the dermis and epidermis.3,8,9 One case exhibited co-expression of neutrophils and eosinophils,6 and another case had neutrophilic polymorphonuclear perivascular infiltration.12
Taken together with the clinical features and test results, the patient showed oral allergy syndrome and fixed food eruption at the same time, but it is not currently known whether they are independent of each other or share the same mechanism. Our case appears to manifest as a combination of both type I and IV hypersensitivity reactions. In the present case, an IgE mechanism was indicated by the following findings: (1) positive for prick-to-prick tests, (2) reproducible urticarial lesion with oral allergy syndrome, and (3) eosinophilic infiltration in the epidermis or dermis was observed on skin biopsy. Furthermore, according to a recent report on FFE induced by peanut and cashew, the patient showed serum-specific IgE to peanut on immunoblot analysis.6 In contrast, the clinical features of FFE in the present case were representative of a type IV cell-mediated hypersensitivity reaction, as suggested by the following: (1) immunohistochemical staining revealed the presence of intraepidermal CD8+ T cells; (2) the mean length of time from food intake to the onset of symptoms was approximately 12 to 24 hours; and (3) among the 14 previously reported FFE cases, 8 underwent a patch test and 6 were positive (75%).
These findings support the notion that FFE may be attributed to a combination of type I and IV hypersensitivity reactions. After exposure to kiwi fruit or birch pollen, the patient was sensitized to antigen-presenting cells. Type 2 T helper cells generate specific IgE antibodies, resulting in oral allergy syndrome, allergic rhinitis, and asthma. However, CD8+ T cells that reside in FFE lesions have a crucial role in the development of localized tissue immune responses and hyperpigmentation. In the resting state, intraepidermal CD8+ T cells remain quiescent for protective immunity. After food consumption, the induction of food-specific CD8+ T cells leads to apoptosis of keratinocytes and increased dermal melanophages, causing the typical skin lesion of FFE. Interestingly, 3 years earlier, our patient had undergone a car accident and the region of traumatized skin was consistent with that of the FFE wrist lesion. This phenomenon may be directly related to the strong local immune memory elicited by the effector-memory function of intraepidermal CD8+ T cells.15 It is essential to collect more cases for future research to confirm the role of mast cells and CD8+ T cells in FFE.
To the best of our knowledge, this is the first case report published on FFE caused by Actinidia arguta leaves. Although FFE is a rare food allergy, clinicians should be aware that food may be responsible in cases with fixed eruptions in the absence of drug intake. Considering that Actinidia arguta has cross-reactivity with other species of kiwi fruit, it is recommended that the patient avoid consumption of all species of kiwi fruit.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Kelso JM. Fixed food eruption. J Am Acad Dermatol. 1996;35:638–639. doi: 10.1016/s0190-9622(96)90698-2. [DOI] [PubMed] [Google Scholar]
- 2.Hatzis J, Noutsis K, Hatzidakis E, Bassioukas K, Perissios A. Fixed drug eruption in a mother and her son. Cutis. 1992;50:50–52. [PubMed] [Google Scholar]
- 3.Yanguas I, Oleaga JM, González-Güemes M, Goday JJ, Soloeta R. Fixed food eruption caused by lentils. J Am Acad Dermatol. 1998;38:640–641. doi: 10.1016/s0190-9622(98)70136-7. [DOI] [PubMed] [Google Scholar]
- 4.Tsuruta D, Sowa J, Kobayashi H, Ishii M. Fixed food eruption caused by lactose identified after oral administration of four unrelated drugs. J Am Acad Dermatol. 2005;52:370–371. doi: 10.1016/j.jaad.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Muso Y, Kentarou O, Itami S, Yoshikawa K. Fixed eruption due to quinine: report of two cases. J Dermatol. 2007;34:385–386. doi: 10.1111/j.1346-8138.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 6.Parker AL, Pinson ML, Wohltmann WE, Gomez R. Fixed food eruption caused by peanut and cashew: A case report and review of the literature. J Allergy Clin Immunol Pract. 2015;3:119–122. doi: 10.1016/j.jaip.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima S, Kidou M, Ihn H. Fixed food eruption caused by cashew nut. Allergol Int. 2008;57:285–287. doi: 10.2332/allergolint.C-07-58. [DOI] [PubMed] [Google Scholar]
- 8.Volz T, Berner D, Weigert C, Röcken M, Biedermann T. Fixed food eruption caused by asparagus. J Allergy Clin Immunol. 2005;116:1390–1392. doi: 10.1016/j.jaci.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Gaus BM, Scheiba N, Schäkel K. Asparagus-induced fixed food eruptions mimicking cutaneous lupus. Acta Derm Venereol. 2014;94:731–732. doi: 10.2340/00015555-1773. [DOI] [PubMed] [Google Scholar]
- 10.Tsuruta D, Sowa J, Kobayashi H, Ishii M. Fixed food eruption caused by Japanese sand lance. Clin Exp Dermatol. 2009;34:e309–e310. doi: 10.1111/j.1365-2230.2009.03263.x. [DOI] [PubMed] [Google Scholar]
- 11.Leleu C, Boulitrop C, Bel B, Jeudy G, Vabres P, Collet E. Quinoline Yellow dye-induced fixed food-and-drug eruption. Contact Dermat. 2013;68:187–188. doi: 10.1111/cod.12019. [DOI] [PubMed] [Google Scholar]
- 12.Waton J, Splingard B, Barbaud A. A new entity: the neutrophilic fixed food eruption. Contact Dermatitis. 2011;65:44–47. doi: 10.1111/j.1600-0536.2010.01845.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Lucas JS, Hourihane JO, Lindemann J, Taylor SL, Goodman RE. Evaluation of IgE binding to proteins of hardy (Actinidia arguta), gold (Actinidia chinensis) and green (Actinidia deliciosa) kiwifruits and processed hardy kiwifruit concentrate, using sera of individuals with food allergies to green kiwifruit. Food Chem Toxicol. 2006;44:1100–1107. doi: 10.1016/j.fct.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316–321. doi: 10.1097/ACI.0b013e32832cda4c. [DOI] [PubMed] [Google Scholar]
- 15.Shiohara T, Mizukawa Y, Teraki Y. Pathophysiology of fixed drug eruption: the role of skin-resident T cells. Curr Opin Allergy Clin Immunol. 2002;2:317–323. doi: 10.1097/00130832-200208000-00005. [DOI] [PubMed] [Google Scholar]