Abstract
The calyx of Held is a giant nerve terminal that forms a glutamatergic synapse in the auditory pathway. Due to its large size, it offers a number of advantages for biophysical studies, including voltage-clamp of both pre- and postsynaptic compartments and the loading with indicator dyes and caged compounds. Three aspects of recent findings on the calyx are reviewed here, each of which seems to have only subtle consequences for nerve-evoked excitatory postsynaptic currents: vesicle heterogeneity, refractoriness of release sites, and superpriming. Together, they determine short-term plasticity features that are superficially similar to those expected for a simple vesicle pool model. However, detailed consideration of these aspects may be required for the correct mechanistic interpretation of data from synapses with normal and perturbed function, as well as for modeling the dynamics of short-term plasticity.
Main Text
Since the early work of Katz and colleagues (1, 2, 3), an understanding of the mechanisms that underlie neurotransmitter release has been a prime goal in cellular and molecular neuroscience. Research over the last three decades of the twentieth century was aimed at refining the original view of Katz, who posited that there are a certain “number of units available” at the nerve terminal with “a number of units responding to one impulse” (3). Units, in this context, are the quanta of neurotransmitter, released by exocytosis of individual synaptic vesicles. Katz and colleagues based their view on the finding that the coefficient of variation of the measured neuromuscular end-plate potential upon repeated stimulation was smaller than expected for a Poisson distribution, and thus proposed a binominal process to describe the release of neurotransmitter quanta (3). Vere-Jones (4) refined this view, which originally did not distinguish between sites for accepting release-ready vesicles and the actual “number of units available”. Such a distinction is necessary to describe a dynamic situation, where the random process of consumption of units is balanced by a random renewal process. Therefore, Vere-Jones (4) postulated a fixed number of sites representing the binominal N. Sites can either be occupied with vesicles or empty at the time of arrival of an action potential (AP) and the expectation value for the number of release events during an AP is then given by a product of three quantities: N × pocc × psucc, where pocc is the probability of occupancy and psucc is the probability that a vesicle attached to a site will successfully release. In this view, what is generally called the readily releasable pool (RRP) is the product N × pocc, and what is often called pr (or pves), is psucc. However, a formal treatment of vesicular release statistics, considering consumption and renewal of vesicles (5), actually proved that release probability, as provided by standard methods of mean/variance analysis, reports the product pocc × psucc.
Given the Katz’s original view and Vere-Jones' refinement, the synaptic output y upon arrival of an action potential is:
| (1) |
with q representing the quantal size, which is the response of the postsynapse to the release of a single neurotransmitter quantum.
In this review, I will argue that the framework of Eq. 1 is a representation of the vesicular release process, based on which various aspects of synaptic physiology can be decomposed, namely:
q: This represents primarily postsynaptic aspects (not further discussed here).
psucc: This is the mechanism of triggering vesicular release, including Ca2+-dependence of release, which is an important aspect of facilitation (a temporary increase in psucc), with superpriming as a mechanism of increasing psucc in a fraction of vesicles.
pocc: This represents the dynamics of vesicle consumption and resupply, including short-term depression as a depletion of vesicles, or else, a reduction of pocc. This aspect is often represented in models of short-term plasticity as the fractional occupancy or depletion of a pool of vesicles. Rapid pool refilling may be required for a synapse to display net paired-pulse facilitation.
N: This is the number of sites, which in the framework of pool models represents the maximal size of the pool.
Many biophysical models that explicitly or implicitly build on such a concept have been published in the last 60 years (6, 7, 8, 9, 10, 11). Before discussing the parameters involved individually, I will try to demonstrate the simplicity and power of this approach considering the superposition of facilitation and short-term depression of a synapse during a high-frequency train of APs.
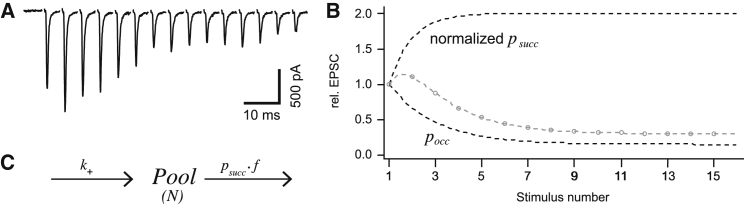
We consider the simple case of just one homogenous pool of 200 release sites (N = 200), all of which are occupied at rest and partially depleted during a stimulus train. Fig. 1 A shows a recording from the calyx of Held synapse (see below), which illustrates postsynaptic responses when such a glutamatergic synapse is stimulated at high frequency: excitatory postsynaptic currents (EPSCs) first increase in amplitude (facilitation) and subsequently decrease, approaching a certain steady-state level.
Figure 1.
Short-term plasticity. (A) A sequence of excitatory postsynaptic currents in response to afferent fiber stimulation at 200 Hz (data provided by H. Taschenberger; EPSCs were recorded in the presence of 1 mM kynurenic acid in the bath solution to minimize AMPAR desensitization and saturation and stimulation artifacts were blanked for clarity). (B) Schematic diagram of the expectations for normalized EPSC amplitudes of such an experiment based on Eq. 1. (Gray circles) Amplitude values, which were calculated as the product of psucc and pocc. The value psucc was assumed to double its initial value during facilitation, while pocc decreases due to vesicle consumption. The product shows the same pattern of facilitation, followed by depression, as the train of EPSCs to the left. (C) A simple diagram of a single-pool model. A pool of vesicles occupying N release sites is assumed to be depleted by release, which is triggered by a train of stimuli at frequency f. Each stimulus depletes a fraction, psucc, of the remaining vesicles and the vacated sites are reoccupied with a rate constant k+. If N is the number of sites and n is the time-dependent number of release-ready vesicles, then .
Fig. 1 B shows the predictions of Eq. 1 for the amplitudes measured in such an experiment as the product of an exponentially rising psucc (for facilitation) and a decaying pocc (due to consumption of vesicles). The rise in psucc might be caused by facilitation of calcium current (12), while the decrease in pocc may simply be a consequence of consumption of vesicles. pocc would eventually decay to zero if there were no refilling of the pool. Therefore, and to accommodate the finding that EPSCs decay to a certain steady-state level, it was assumed in the calculation of Fig. 1 that the pool is refilled with a rate constant k+ according to the equation shown in Fig. 1 C. In that equation, the quantity pool is N × pocc in terms of Eq. 1. At later times, when psucc has reached a constant value, the kinetic equations for Pool (see legend to Fig. 1) can be readily solved, such that the pool approaches a steady state with time constant:
| (2) |
and
| (3) |
Here, the subscript ss denotes steady state. The process of consumption and refilling, which in reality is a discrete process, was replaced for simplicity by a continuous process with rate constants that agree with the time-average of the discrete ones.
The simplified concept of a pool model provides some valuable insights. Unfortunately, however, it rarely describes the details of a real synapse. In practice, one finds significant deviations and specifications. With respect to the parameters of Eq. 1, the most common ones are:
N: There is hardly any synapse with a single homogeneous pool of release-ready vesicles. Instead, in many cases two or three pools must be assumed for a satisfactory description of results, and such discrete pools may well represent the artificial subdivision of a pool that is actually more or less continuous in its properties (11). Also, the formalism outlined here deals exclusively with the RRP in the nomenclature of Alabi and Tsien (13). The recycling pool and the resting pool, as defined by experiments with styryl dyes and Phluorins, are assumed not to be significantly depleted during the relatively weak forms of stimulation considered here.
psucc: Each pool mentioned above may have its own psucc, and this may be influenced by changes in AP-waveforms, modulation of calcium current (ICa), buildup of intracellular Ca2+ concentration ([Ca2+]) during repetitive activity, and modulation of intrinsic release willingness on various timescales (facilitation, augmentation, posttetanic potentiation; see below).
pocc: Even in the simplest case (Eq. 2), this probability mirrors all the complications listed above for psucc. Representing the balance between vesicle consumption and supply, it depends on the process of pool-refilling, which can rarely be accurately described by a constant k+, but involves processes that depend on [Ca2+] and possibly on the filling state of other vesicle pools that feed the RRP (see Alabi and Tsien (13) and Miki et al. (14)).
Q: Quantal size is mainly determined by properties of the postsynaptic neuron (see review by Borst and Soria van Hoeve (15)), but also depends on the neurotransmitter content of vesicles (16).
The calyx of Held
The calyx of Held is an axiomatic nerve terminal in the auditory pathway that makes a glutamatergic connection with a single principal neuron in the medium nucleus of the trapezoid body (17). Due to its large size, it offers many unique possibilities for biophysical analyses of neurotransmitter release, including simultaneous voltage clamp of pre- and postsynaptic compartments (18), and loading of the presynaptic terminal via a recording patch pipette with [Ca2+]-indicator dyes and caged compounds. Following first patch recordings by Forsythe (17), numerous studies exploiting these possibilities have appeared (see Neher (11) and Borst and Soria van Hoeve (15) for review). Here, I will summarize some recently discovered phenomena that register prominently in invasive biophysical experiments but have relatively subtle consequences for physiological AP-induced responses of unperturbed nerve terminals—subtle in the sense that their effects on AP-evoked release are not readily discernable from what one would expect based on the simple model of Fig. 1. Nevertheless, I will argue that consideration of insights developed in calyx research will strongly influence the mechanistic interpretation of data from glutamatergic synapses in general.
The secret life of slow vesicles
This topic refers to N in Eq. 1 and the question of whether a single homogenous pool can explain all aspects of calyx data. While many models of glutamatergic short-term plasticity have described dynamic changes at the synapse with relatively simple equations (6, 19, 20), it became clear early on that the time-course of neurotransmitter release from a voltage-clamped calyx is quite complex. A steplike depolarization applied to the calyx terminal elicits an almost steplike Ca2+ influx, which—according to the simplest form of a single-pool model—should result in a surge of release that decays exponentially due to depletion of the vesicle pool. Instead, a double exponential decay is observed, with a fast component with ∼3–5 ms time constant and another one ∼10-fold slower (21). Although the amplitude of the slow component is quite small, its total contribution to release is approximately the same as that of the first component. This led to the proposal that there are two vesicle pools of approximately equal size, each of which can release its vesicles, albeit with ∼10-fold different release rates (21). Each pool contains ∼3 vesicles per active zone (AZ) and there are ∼500 AZs on a calyx terminal (22).
Other interpretations are, of course, possible. The slow component of release may not be the result of autonomous release from a separate pool, but the consequence of depletion of a replacement pool that initially supplies new vesicles to the releasing pool at high rates (large k+) and thereby maintains a certain plateau of release. However, as the replacement pool is depleted, the vesicle supply decreases, leading to a further slow decay of release. Such a process is conveniently described by a sequential pool model with two pools in series. Alternatively, initially fast releasing vesicles may influence remaining vesicles to decrease their release probability (lowering, psucc), or the release apparatus may adapt to the ongoing stimulus. Such adaptation has actually been postulated for several types of synapses (see Zucker and Regehr (7) for review). However, specific evidence for such a mechanism at the calyx has not been put forward. Rather, several studies confirmed that a total number of vesicles, equivalent or higher than the sum of the slowly (SRP) and fast-releasing pool (FRP) of Neher and Sakaba (8) can be released within a few milliseconds, if strong enough stimulation is applied. For such strong stimulation, either prolonged step depolarization to the potential of maximum ICa, possibly at increased external [Ca2+] (23, 24), or release of Ca2+ from caged-Ca2+ by flash photolysis was used (25, 26). Such rapid release of six or more vesicles per AZ indicates that both SRP and FRP harbor release-competent vesicles. These results do not prove that SRP vesicles can release, because a final step of rendering a vesicle release competent may well be in the millisecond range if accelerated by high [Ca2+] (14, 27), but some arguments discussed below speak against such an interpretation. Nevertheless, more recent data indicate that the parallel nature of SRP and FRP is not sufficient to describe all aspects of calyx data.
Upon depletion by strong stimulation, the SRP refills rapidly (in the 50–100 ms time range) and the FRP refills slowly (several 100 ms to s, depending on [Ca2+]) (21). More recently, Lee et al. (28) demonstrated that SRP vesicles can convert rapidly into FRP vesicles. By depleting the FRP with a medium-strength stimulus and recording the content of both pools during recovery, it was shown that the SRP size transiently decreases while the FRP refills. This was interpreted to indicate that the FRP refills at the expense of the SRP and that the transition rate constant between SRP and FRP must be quite fast. The transition requires an intact actin network, whose disruption dramatically reduces the FRP-refilling rate constant. Accordingly, the transient dip in the SRP size is strongly reduced by actin disassembly. Beyond demonstrating a rapid transition of vesicles from SRP to FRP, these findings (28) also speak against the idea that the slow component represents a release-incompetent precursor stage of the FRP, because actin disassembly does not affect the SRP size (28). A decrease and slow-down of the slow component would be expected if it were just due to upstream depletion.
Together, these findings indicate that there are two release-competent pools (SRP and FRP) and that SRP vesicles can convert to become part of the FRP. The finding that this conversion depends on an intact cytoskeleton supports the previous proposal (29) that the SRP harbors vesicles that are perfectly release-competent but located unfavorably with respect to Ca2+ channels. Liberation of privileged FRP-sites will allow SRP vesicles to move rapidly into such empty slots by the help of an active process. This mechanism may be described as “positional priming”. Alternatively, it was argued, based on Ca2+-uncaging studies, that the SRP is mediated by vesicles that are less sensitive to Ca2+ and need an additional step of molecular priming to respond rapidly (26).
The presence of slowly responding vesicles has only subtle effects on AP-evoked release because these vesicles are hardly touched by the short [Ca2+]-transients produced by an AP. In fact, Sakaba (30) showed that the synchronous release during a 100 Hz stimulus train is predominantly carried by FRP vesicles. In the framework of the positional priming concept, the short-lived local nanodomain of elevated [Ca2+] during an AP will not reach the unfavorably located SRP vesicles. Nevertheless, during high-frequency trains of APs, [Ca2+] nanodomains will build up and spread spatially, such that SRP vesicles eventually contribute to release, albeit in a less synchronized manner (30). Besides their contribution to asynchronous release, there is another important consequence of the existence of SRP vesicles: due to the rapid conversion between SRP and FRP, they rapidly occupy vacated FRP sites and provide for sustained release during repetitive activity.
Site clearing and refractoriness of active zones
SRP vesicles can mediate very rapid refilling of privileged release sites after these have been vacated by preceding exocytosis. Experiments on voltage-clamped terminals, however, have again set a limit to the speed of such recovery. Accumulating evidence over the last years has revealed a role of the endocytotic machinery in the rapid regeneration of release-ready vesicles. This started with experiments on the Drosophila mutant shibire (31), which shows temperature-dependent paralysis due to a mutation in dynamin, a key component of the endocytotic vesicle budding machinery. The consequent loss of recycled vesicles, may well delay the refilling of AZ sites. However, Kawasaki et al. (31) noticed that flies become paralyzed very rapidly after shifting them to nonpermissive temperatures—much faster than expected if a loss of recycling vesicles were the reason for the paralysis. Also, electron microscopy showed numerous reserve vesicles in nerve terminals of paralyzed flies. This led to the conclusion that perturbation of endocytosis may have a backlash effect on the reuse of release sites by preventing the removal of vesicular components from such sites. Such refractoriness of release sites after their use had already been discussed by Betz (32) and Katz (33).
More recent analyses of voltage-clamped calyx data demonstrated that recovery of the FRP after prolonged depolarization is retarded not only by interfering with dynamin but also by perturbation of multiple other proteins involved in endocytosis, including Intersectin and AP2 (33, 34, 35). Early on it had already been shown that FRP recovery under such conditions is Ca2+-dependent and involves Ca2+-calmodulin (21). A study on KI-mice expressing a calmodulin-binding-deficient Munc13-1 variant then demonstrated a slow-down of FRP recovery after massive stimulation, very similar to the effects of perturbing molecules involved in endocytosis (36). This was proposed to indicate that Munc13 is involved in site-clearing and that the Ca2+-dependence of this process is mediated by calmodulin-Munc13 interaction. For a full characterization of this site-clearing process, it is important to note that it only affects FRP recovery but not SRP recovery, and that site-clearing is much faster than full endocytosis. The latter happens on a 1–10 s timescale (35), while refilling of release sites takes only a few hundred milliseconds. Thus, site-clearing must be a rapid step between exo- and endocytosis, its perturbation leading to strong retardation of site-refilling, in agreement with the conclusions of a recent study using tissue-specific dynamin-1 deletion (37). The finding (21), that only FRP sites are affected by such manipulations, points to a more important role of an intact AZ structure for the recovery of FRP vesicles, while SRP vesicles appear to be more loosely coupled to the AZ, not requiring intact special sites for docking and priming.
Why, then, can the effects of perturbed site-clearing be considered subtle for normal physiology? One explanation is that it constitutes a type of ceiling to the refilling rate and that this ceiling is rarely reached during afferent fiber stimulation. In line with this argument is the fact that in the KI-mice mentioned above (36), a phenotypic change can only be observed at the highest stimulation frequencies, while it is readily detectable in step-depolarization experiments under voltage-clamp. Likewise, for the first demonstration of a Ca2+-dependent refilling of vesicle pools at the calyx (38), synapses had to be stimulated with 300 Hz and/or APs had to be broadened by the application of TEA. Thus, site-clearing sets some limitation to neurotransmitter release, which may become relevant only during extremely strong stimulation. Nevertheless, characterizing its properties is important for an understanding of the molecular processes that take place before and after exocytosis.
Superpriming
So far, I have argued that glutamate release at the calyx of Held occurs via two pools of vesicles, SRP and FRP, which differ in their release and refilling rates. An important conclusion is, that nerve-evoked release is predominantly mediated by FRP vesicles, while SRP vesicles play a predominantly indirect role as a source of rapidly-to-recruit vesicles for the refilling of empty FRP sites. Recent experiments, however (39,40), have brought up the need to subdivide the FRP into a normally primed component, called SVn (SV for synaptic vesicles and subscript n for normally) and a superprimed component, called SVs. Superprimed vesicles are characterized by enhanced release probability and by slow recovery. It is not clear, presently, whether the superprimed state is reached by maturation of SVn, as described by a model with two pools in sequence, or represents a second vesicle type or else special docking sites for vesicles that can mediate release in parallel to that of SVns. Given the fact (to be discussed below) that normal priming is fast (100 ms timescale) and superpriming is slow (s timescale), it is hard to distinguish experimentally between these alternatives. In any case, the characteristic feature of SVs is their high release probability that relates to the quantity psucc of Eq. 1.
Superpriming was first invoked by Schlüter et al. (41) to describe the effects of quadruple knockout of all Rab3 isoforms. Experiments in hippocampal autapses showed that loss of Rab3 causes a deficit in neurotransmitter release during the very early phase of high-frequency stimulation. A careful analysis of time-courses of release with a variety of stimulation protocols led to the conclusion that mutants lack a component of release that is carried by a subset of primed, fusion-competent vesicles of high release probability and a significant delay in the supply of these modified vesicles. The term “superpriming” was chosen to describe these properties.
Heterogeneity in vesicle pools of hippocampal synapses had been studied before extensively by various laboratories (41, 42, 43, 44, 45, 46), with many indications pointing toward a subset of vesicles that can be released by afferent fiber stimulation with high release probability. In particular, the work of Hanse and Gustafsson (46) characterized a subpool of vesicles, the so-called preprimed pool, that displayed many of the properties of superprimed vesicles, as described here. At the calyx of Held, superpriming was first invoked by Lee et al. (39), who voltage-clamped presynaptic terminals and applied a paired-pulse protocol to first deplete the FRP and subsequently study its reappearance at various interpulse intervals (ISIs). They found that at an ISI of 750 ms, half of the FRP had recovered. However, when comparing the time course of the recovered response with that measured at a rested synapse, they noticed that it was slower by a factor of 1.7. With longer ISIs, both amplitude and speed of response increased, but the latter with a very slow time constant of ∼4 s. Further, Lee et al. (39) found that the recovery of the amplitude (number of FRP vesicles) displayed pharmacological features that were very different from those of the recovery of speed (Ca2+ sensitivity of FRP vesicles). The former was slowed down by disrupting the cytoskeleton (see above) and by calmodulin inhibitors, while the latter was sensitive to modulators of the PLC/DAG signaling pathway. Specifically, it was strongly accelerated by a DAG analog. Lee et al. (39) concluded that vesicles undergo a slow process of superpriming after their recruitment to the readily releasable pool.
Very recently a study at calyx terminals revealed some interesting features that are readily explained by superpriming (40). Afferent fiber stimulation at a range of frequencies was used and EPSCs were recorded at the voltage-clamped postsynaptic cell. First, the authors noticed that there is a remarkable heterogeneity between Calyces with respect to their short-term plasticity. Some synapses showed strong short-term depression (STD), others a sequence of facilitation and depression. As shown previously at other glutamatergic synapses (42, 44, 47), those with large initial EPSCs tended to display STD, while those with low initial EPSCs were facilitating. Secondly, the study confirmed earlier results (16) indicating that application phorbol esters (PdBu, a mimic of the second messenger diacylglycerol) increases synaptic strength and converts those synapses that initially display facilitation to depressing ones. Strikingly, all differences between initial amplitude—either due to intrinsic variation or induced by PdBu—vanished rapidly during stimulation. EPSCs, which initially were different by up to a factor of 6, became very similar in amplitude after just three high-frequency stimuli. Also, when a high-frequency stimulus train (100 Hz) was preceded by a few conditioning stimuli at low frequencies (10 Hz), differences between synapses during the high-frequency response were very much reduced.
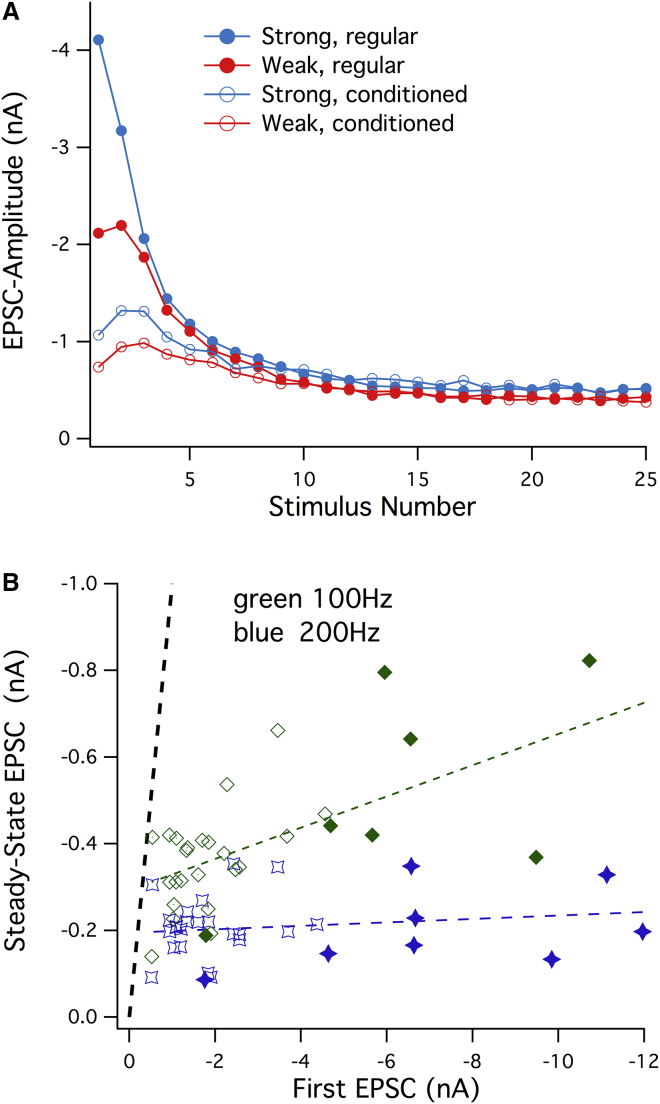
Fig. 2 A shows amplitudes of EPSCs during 100 Hz trains both for synapses with different initial amplitudes and with/without conditioning. Amplitudes converge toward a common steady-state value of −0.5 nA, although initial values vary between −0.8 and −4 nA. The results find a unifying explanation by the hypothesis that natural variability and potentiation by PdBu are caused by variations in superpriming. Given the finding that superpriming is restricted to a subpool of vesicles (41) the natural variations may just signify that, at rest, a variable fraction of vesicles reach the superprimed state, and that this fraction is increased by PdBu. Because superpriming vesicles have high release probability (see below), and are regenerated only slowly (39), the superprimed vesicles (SVss) will rapidly be depleted during high-frequency trains, and leave normally primed vesicles (SVns) behind. The convergence of traces in Fig. 2 A indicates that SVns are quite similar in number, for the two groups of synapses shown.
Figure 2.
EPSC amplitudes converge during high-frequency stimulation. (A) EPSC amplitudes during 100 Hz stimulus trains are plotted against stimulus number for regular trains (solid symbols) and trains preceded by 10 conditioning stimuli at 10 Hz (circles). Averages from two groups of synapses (three each) are shown, one with relatively large initial amplitudes (blue symbols), one with smaller initial amplitudes (red symbols). Differences between the two groups disappear after two EPSCs have been elicited. Likewise, conditioning prepulses reduce the differences between synapses and turn STD into facilitation (data replotted from Taschenberger et al. (40)). Both phenomena can be explained by the rapid depletion of a small pool of superprimed vesicles with high release probability and slow recovery kinetics. (B) Steady-state EPSC amplitudes during 200 Hz (blue symbols) and 100 Hz (green symbols) stimulus trains are plotted against first EPSCs. Each symbol represents one synapse. (Solid symbols) From recordings in 1 μM PdBu; (open symbols) from the control. (Dashed lines) Linear fits to 100 and 200 Hz. At 200 Hz, there is little correlation between steady state and initial amplitudes, although the latter span a 20-fold amplitude range. (Black dashed trace) Identity line (data replotted from Taschenberger et al. (40)).
In Fig. 2 B, steady-state EPSC amplitudes from 22 synapses are plotted against first EPSCs for frequencies of 100 and 200 Hz. Values fluctuate around the linear fits. However, for 200 Hz the fluctuations are not at all correlated with the initial values, plotted on the x axis—an indication that the process generating sustained release is distinct from the one controlling superpriming (Fig. 2 B). For the case of 100 Hz stimulation some correlation between steady-state and first EPSC-amplitude is observed, indicating that during longer interstimulus intervals some superpriming can take place at various degrees in different cells. A detailed analysis of EPSCs at several frequencies (40) showed that SVns have high psucc between 0.4 and 0.5 and are regenerated slowly in the s time range, while SVss have ∼5 times lower psucc and are regenerated in the 100 ms range. Furthermore, the superpriming rate was strongly enhanced by PdBu and during high-frequency stimulation, which was interpreted as a [Ca2+]-mediated effect (40). Intrinsic variability between synapses may reflect the action of slow transmitter systems (48), modulating the abundance of SVss.
The concept of superpriming sheds light onto the fastest forms of short-term plasticity: STD and short-term facilitation (see below). Slower forms of synaptic plasticity have been studied extensively in the last 50 years. Apart from the very long-term forms, two forms of intermediate plasticity have received attention: augmentation and posttetanic potentiation (PTP). The former modulates synaptic strength in the 100 ms to s range, while PTP, when elicited by a seconds-long stimulation period, subsequently decays in the s to min range (49, 50, 51). Taschenberger et al. (40) tested whether superpriming may be involved in these two forms of plasticity. First, they noticed that the superpriming timescale in the sub-s to s range, together with its [Ca2+] dependence, very well qualifies to mediate at least some of the augmentation observed during and after burstlike activity, which elevates [Ca2+] transiently. Second, they elicited PTP and studied short-term plasticity in the PT-potentiated state. They found that under PTP, synapses behave exactly as if they had an increased superprimed component, while the SVn pool stayed constant. Taken together, the data indicate that the same mechanism (superpriming) underlies PTP, potentiation by PdBu, and the natural variation between individual synapses.
Facilitation: a matter of psucc or of pocc?
The decrease or absence of PPF has traditionally been associated with increased release probability (42, 44, 47). A likely reason for such a correlation is the simple consequence of high psucc, that the remaining pool after a first EPSC is strongly depleted. A second EPSC may therefore be smaller than the first one even if psucc is higher during the second response. To see net facilitation of the second EPSC in a pair, the balance between these two effects must be positive. A hypothetical synapse with only SVss could hardly display PPF because its psucc is near 0.5. A second EPSC could only reach a paired-pulse ratio of 1 or larger if psucc during the second response were psucc ≈ 1, unless rapid replacement of released vesicles occurred. However, for SVns with a psucc in the range 0.1–0.2, a positive balance in the paired-pulse ratio can very well be obtained as long as the relative increase in psucc exceeds a value of 20%. In consequence, strong PPF was observed during conditioned trains (Fig. 2 A) during which the SVs pool is largely depleted, with only SVns remaining.
However, recent findings complicate this explanation, which has so far neglected the possibility that some refilling of release sites may happen in the interval between the two responses considered. Lee et al. (28) found that PPF at 200 Hz stimulation is converted to depression when actin networks are disassembled. Likewise, Miki et al. (14) found this to be the case in a study of glutamatergic synapses between parallel fibers and molecular layer interneurons in rat cerebellum. They further concluded, based on fluctuation analysis, that a release site vacated by exocytosis has a 60% chance of being reoccupied even if the stimulus interval is only 5 ms. Disrupting the cytoskeleton strongly retarded this replacement process, just as it blocked rapid conversion of vesicles from SRP into FRP at the calyx. The newly recruited vesicles most likely show up as SVns, mediating the sustained response during trains. Such reloading of vesicles is well in line with previous findings at cerebellar mossy fiber terminals (52) and other glutamatergic synapses (46, 53). Given rapid reloading, facilitation should not be viewed as purely reflecting psucc but rather as the result of a balance among consumption of vesicles, replacement, and enhancement of psucc of those vesicles present at the time of arrival of an AP. In this sense, slow forms of facilitation, such as observed in tonic synapses at the crayfish neuromuscular junction, were modeled in terms of an increasing pocc (54), which was assumed to be very low in these synapses at rest and to increase during stimulation due to elevated [Ca2+]. Such slowly facilitating synapses, including hippocampal mossy fiber synapses, may be viewed as synapses without a superprimed pool and very low resting pocc. The value psucc, on the other hand, may be modulated by residual Ca2+ (55), saturation of endogenous Ca2+ buffer (56, 57), and by the well-known modulation of ICa by second messenger pathways (58).
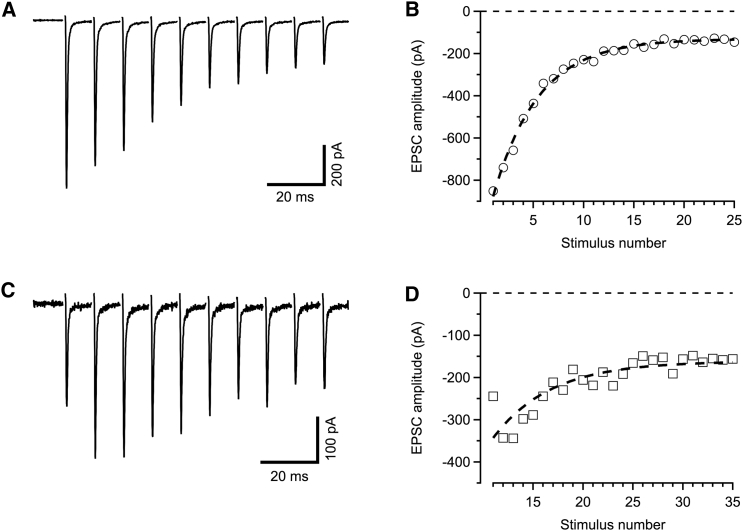
What justifies the findings described here (superpriming, rapid reloading, and facilitation as a change in both pocc and psucc) to be deemed as subtle? Why is superpriming largely disregarded in the present literature—although described more than 10 years ago? If the interpretation offered here correctly describes the situation at the calyx of Held, then these effects obviously are not subtle individually. However, together they lead to responses during regular train stimulation that are remarkably similar to those expected for simpler models (19, 59). Although superprimed vesicles of rested synapses have a psucc that is 4–5 times higher than those of SVns (0.5 vs. 0.1–0.2), the latter ones facilitate, enhancing their contribution during the first few responses in a stimulus train, while SVss are depleted (40). Both an increase in psucc and the rapid reloading of SVns contribute to this effect. Together, contributions of SVns and SVss add up to a time course that is barely distinguishable from a single exponential decay, maybe preceded by one or two EPSCs showing net facilitation. Only more complex stimulus patterns, such as a sequence of low-frequency conditioning stimuli, followed by a high-frequency train, reveal some of the complexities. Fig. 3 presents an example, in which a simple pool model according to Fig. 1 C readily describes the response of a calyx synapse to a 100 Hz stimulus train, while it completely fails to describe the conditioned response.
Figure 3.
Simulations of regular and conditioned EPSC-trains with a simple pool model. (A and C) Original EPSC-traces during 100 Hz stimulation, applying either a regular train (A) or the same train after 10 conditioning stimuli at 10 Hz (C); same recording conditions as in Fig. 1 A. (B and D) Peak amplitudes of EPSCs are plotted against stimulus numbers (100 Hz episodes only) for the two trains shown in (A) and (C) together with the predictions of a simple model as shown in Fig. 1C, assuming constant psucc and k+. The best fit of the model to the regular train (B, dashed line) predicts an exponential decay with a time constant of 4.48 interstimulus intervals. Deviations between data and model are minor. If the same model is applied to the conditioned train, however, the prediction (D, dashed line) completely fails to simulate the initial facilitating phase (note the first data point at −240 pA). This might be corrected by assuming an increase in psucc between stimuli 1 and 2 (as assumed in Fig. 1B). However, under that assumption, the simulation for the regular train (B) would fail to predict the initial decay.
Conclusions
Studies at the calyx of Held revealed a number of features that control various aspects of neurotransmitter release. These include very rapid reloading of vacated release sites, slow generation of a special population of superprimed vesicles, and a limitation of the maximum throughput during sustained activity due to refractoriness of release sites. Coincidentally, these different influences add up to an overall behavior that is hardly distinguishable from the predictions of a very simple single pool model of release, as shown in the scheme of Fig. 1 C—as long as one restricts the analysis of synaptic function to the commonly used tests, such as the application of high-frequency trains at two or three different frequencies. This situation may be considered fortunate, because it allows the description of synaptic functions in neuronal networks with simple models (19, 20). For investigators interested in synaptic mechanisms, however, a refined view is required, which also considers specialized stimulation patterns (stimulation in a wide frequency range and conditioned high frequency trains) and a multitude of literature findings on heterogeneity between vesicles (41, 42, 43, 44, 45, 46). Furthermore, when considering the influence of diffusely projecting modulatory transmitter systems on network properties, the distinction between several pools, one of which can be modulated by such influences, may be helpful.
Nevertheless, the original Katz view, as represented by Eq. 1, can still be considered as a framework for decomposing overall synaptic function into several separate mechanistic aspects. For the calyx of Held, however, the following specifications are required:
The quantity N (number of release sites) describes not a single vesicle pool, but three distinct ones including a slowly releasing pool (SRP), which contributes only little to nerve-evoked EPSCs and serves as a reservoir of release-competent vesicles for rapid reloading of the fast-releasing pool (FRP). The FRP, on the other hand, needs to be subdivided into a pool that holds normally primed vesicles (SVns) and another one that holds superprimed vesicles (SVss). Various forms of short- and medium-term plasticity can be understood as modulations of the abundance of SVss.
The priming rate constant k+ can no longer be understood as representing a simple sequence of docking and priming of vesicles, but needs to be viewed as a parameter that describes a complex process, involving rapid conversion of SRP vesicles into SVns. The high speed of this process depends on an intact cytoskeleton and is limited by site clearing, a process during which a release site is refractory for reuse, during massive use of a synapse. Site clearing depends on a variety of proteins that are commonly associated with endocytosis or with steps between exo- and endocytosis.
The release probability, psucc, is no longer a single number but has a high value at rest for SVss (≈0.5) and a low value (0.1–0.2) for SVns. Facilitation during high-frequency stimulation increases psucc of both types of vesicles and is enhanced by rapid reloading of vesicles.
Although many aspects of the findings at the calyx of Held, summarized here, have been observed at other glutamatergic synapses, researchers in the field seem to be reluctant to adopt all this complexity. It should be evident, however, that attention to the subtle effects that showed up in calyx research may allow a much more detailed view of molecular mechanisms and may help to better understand the phenotypes that result from perturbations of synaptic protein function.
Acknowledgments
I thank Bert Sakmann, Manfred Lindau, Nils Brose, and Richard W. Tsien for valuable comments on the article, Holger Taschenberger for providing original data, and Stefan Hallermann for feedback and improving figures.
Editor: Brian Salzberg.
References
- 1.Fatt P., Katz B. Spontaneous subthreshold activity at motor nerve endings. J. Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 2.Del Castillo J., Katz B. Statistical factors involved in neuromuscular facilitation and depression. J. Physiol. 1954;124:574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Castillo J., Katz B. Quantal components of the end-plate potential. J. Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vere-Jones D. Simple stochastic models for the release of quanta of transmitter from a nerve terminal. Aust. J. Stat. 1966;8:53–63. [Google Scholar]
- 5.Scheuss V., Neher E. Estimating synaptic parameters from mean, variance, and covariance in trains of synaptic responses. Biophys. J. 2001;81:1970–1989. doi: 10.1016/S0006-3495(01)75848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liley A.W., North K.A. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J. Neurophysiol. 1953;16:509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- 7.Zucker R.S., Regehr W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 8.Neher E., Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Regehr W.G. Short-term presynaptic plasticity. Cold Spring Harb. Perspect. Biol. 2012;4:a005702. doi: 10.1101/cshperspect.a005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta Roy R., Stefan M.I., Rosenmund C. Biophysical properties of presynaptic short-term plasticity in hippocampal neurons: insights from electrophysiology, imaging and mechanistic models. Front. Cell. Neurosci. 2014;8:141. doi: 10.3389/fncel.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neher E. Merits and limitations of vesicle pool models in view of heterogeneous populations of synaptic vesicles. Neuron. 2015;87:1131–1142. doi: 10.1016/j.neuron.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Müller M., Felmy F., Schneggenburger R. A limited contribution of Ca2+ current facilitation to paired-pulse facilitation of transmitter release at the rat calyx of Held. J. Physiol. 2008;586:5503–5520. doi: 10.1113/jphysiol.2008.155838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alabi A.A., Tsien R.W. Synaptic vesicle pools and dynamics. Cold Spring Harb. Perspect. Biol. 2012;4:a013680. doi: 10.1101/cshperspect.a013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miki T., Malagon G., Marty A. Actin- and myosin-dependent vesicle loading of presynaptic docking sites prior to exocytosis. Neuron. 2016;91:808–823. doi: 10.1016/j.neuron.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 15.Borst J.G., Soria van Hoeve J. The calyx of Held synapse: from model synapse to auditory relay. Annu. Rev. Physiol. 2012;74:199–224. doi: 10.1146/annurev-physiol-020911-153236. [DOI] [PubMed] [Google Scholar]
- 16.Hori T., Takai Y., Takahashi T. Presynaptic mechanism for phorbol ester-induced synaptic potentiation. J. Neurosci. 1999;19:7262–7267. doi: 10.1523/JNEUROSCI.19-17-07262.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsythe I.D. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J. Physiol. 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borst J.G.G., Helmchen F., Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J. Physiol. 1995;489:825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsodyks M.V., Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc. Natl. Acad. Sci. USA. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varela J.A., Sen K., Nelson S.B. A quantitative description of short-term plasticity at excitatory synapses in layer 2/3 of rat primary visual cortex. J. Neurosci. 1997;17:7926–7940. doi: 10.1523/JNEUROSCI.17-20-07926.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakaba T., Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32:1119–1131. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- 22.Sätzler K., Söhl L.F., Lübke J.H. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J. Neurosci. 2002;22:10567–10579. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J.Y., Wu L.G. Fast kinetics of exocytosis revealed by simultaneous measurements of presynaptic capacitance and postsynaptic currents at a central synapse. Neuron. 2001;30:171–182. doi: 10.1016/s0896-6273(01)00271-9. [DOI] [PubMed] [Google Scholar]
- 24.Leão R.M., von Gersdorff H. Synaptic vesicle pool size, release probability and synaptic depression are sensitive to Ca2+ buffering capacity in the developing rat calyx of Held. Braz. J. Med. Biol. Res. 2009;42:94–104. doi: 10.1590/s0100-879x2009000100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wölfel M., Schneggenburger R. Presynaptic capacitance measurements and Ca2+ uncaging reveal submillisecond exocytosis kinetics and characterize the Ca2+ sensitivity of vesicle pool depletion at a fast CNS synapse. J. Neurosci. 2003;23:7059–7068. doi: 10.1523/JNEUROSCI.23-18-07059.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wölfel M., Lou X., Schneggenburger R. A mechanism intrinsic to the vesicle fusion machinery determines fast and slow transmitter release at a large CNS synapse. J. Neurosci. 2007;27:3198–3210. doi: 10.1523/JNEUROSCI.4471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter A.M., Pinheiro P.S., Sørensen J.B. A sequential vesicle pool model with a single release sensor and a Ca2+-dependent priming catalyst effectively explains Ca2+-dependent properties of neurosecretion. PLOS Comput. Biol. 2013;9:e1003362. doi: 10.1371/journal.pcbi.1003362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J.S., Ho W.K., Lee S.H. Actin-dependent rapid recruitment of reluctant synaptic vesicles into a fast-releasing vesicle pool. Proc. Natl. Acad. Sci. USA. 2012;109:E765–E774. doi: 10.1073/pnas.1114072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadel K., Neher E., Sakaba T. The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron. 2007;53:563–575. doi: 10.1016/j.neuron.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Sakaba T. Roles of the fast-releasing and the slowly releasing vesicles in synaptic transmission at the calyx of Held. J. Neurosci. 2006;26:5863–5871. doi: 10.1523/JNEUROSCI.0182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawasaki F., Hazen M., Ordway R.W. Fast synaptic fatigue in shibire mutants reveals a rapid requirement for dynamin in synaptic vesicle membrane trafficking. Nat. Neurosci. 2000;3:859–860. doi: 10.1038/78753. [DOI] [PubMed] [Google Scholar]
- 32.Betz W.J. Depression of transmitter release at the neuromuscular junction of the frog. J. Physiol. 1970;206:629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz B. On neurotransmitter secretion. Interdiscip. Sci. Rev. 1993;18:359–364. [Google Scholar]
- 34.Sakaba T., Kononenko N.L., Haucke V. Fast neurotransmitter release regulated by the endocytic scaffold intersectin. Proc. Natl. Acad. Sci. USA. 2013;110:8266–8271. doi: 10.1073/pnas.1219234110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosoi N., Holt M., Sakaba T. Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron. 2009;63:216–229. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Lipstein N., Sakaba T., Brose N. Dynamic control of synaptic vesicle replenishment and short-term plasticity by Ca2+-calmodulin-Munc13-1 signaling. Neuron. 2013;79:82–96. doi: 10.1016/j.neuron.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Mahapatra S., Fan F., Lou X. Tissue-specific dynamin-1 deletion at the calyx of Held decreases short-term depression through a mechanism distinct from vesicle resupply. Proc. Natl. Acad. Sci. USA. 2016;113:E3150–E3158. doi: 10.1073/pnas.1520937113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L.Y., Kaczmarek L.K. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature. 1998;394:384–388. doi: 10.1038/28645. [DOI] [PubMed] [Google Scholar]
- 39.Lee J.S., Ho W.K., Lee S.H. Superpriming of synaptic vesicles after their recruitment to the readily releasable pool. Proc. Natl. Acad. Sci. USA. 2013;110:15079–15084. doi: 10.1073/pnas.1314427110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taschenberger H., Woehler A., Neher E. Superpriming of synaptic vesicles as a common basis for intersynapse variability and modulation of synaptic strength. Proc. Natl. Acad. Sci. USA. 2016;113:E4548–E4557. doi: 10.1073/pnas.1606383113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlüter O.M., Basu J., Rosenmund C. Rab3 superprimes synaptic vesicles for release: implications for short-term synaptic plasticity. J. Neurosci. 2006;26:1239–1246. doi: 10.1523/JNEUROSCI.3553-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobrunz L.E., Stevens C.F. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 43.Hessler N.A., Shirke A.M., Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- 44.Murthy V.N., Sejnowski T.J., Stevens C.F. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 45.Rosenmund C., Clements J.D., Westbrook G.L. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 46.Hanse E., Gustafsson B. Vesicle release probability and pre-primed pool at glutamatergic synapses in area CA1 of the rat neonatal hippocampus. J. Physiol. 2001;531:481–493. doi: 10.1111/j.1469-7793.2001.0481i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Debanne D., Guérineau N.C., Thompson S.M. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J. Physiol. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Jong A.P., Verhage M. Presynaptic signal transduction pathways that modulate synaptic transmission. Curr. Opin. Neurobiol. 2009;19:245–253. doi: 10.1016/j.conb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Magleby K.L. Facilitation, augmentation, and potentiation of transmitter release. Prog. Brain Res. 1979;49:175–182. doi: 10.1016/S0079-6123(08)64631-2. [DOI] [PubMed] [Google Scholar]
- 50.Genc O., Kochubey O., Schneggenburger R. Munc18-1 is a dynamically regulated PKC target during short-term enhancement of transmitter release. eLife. 2014;3:e01715. doi: 10.7554/eLife.01715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Perez E., Wesseling J.F. Augmentation controls the fast rebound from depression at excitatory hippocampal synapses. J. Neurophysiol. 2008;99:1770–1786. doi: 10.1152/jn.01348.2007. [DOI] [PubMed] [Google Scholar]
- 52.Saviane C., Silver R.A. Fast vesicle reloading and a large pool sustain high bandwidth transmission at a central synapse. Nature. 2006;439:983–987. doi: 10.1038/nature04509. [DOI] [PubMed] [Google Scholar]
- 53.Hallermann S., Silver R.A. Sustaining rapid vesicular release at active zones: potential roles for vesicle tethering. Trends Neurosci. 2013;36:185–194. doi: 10.1016/j.tins.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan B., Zucker R.S. A general model of synaptic transmission and short-term plasticity. Neuron. 2009;62:539–554. doi: 10.1016/j.neuron.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J. Physiol. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Felmy F., Neher E., Schneggenburger R. Probing the intracellular calcium sensitivity of transmitter release during synaptic facilitation. Neuron. 2003;37:801–811. doi: 10.1016/s0896-6273(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 57.Blatow M., Caputi A., Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. doi: 10.1016/s0896-6273(03)00196-x. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi T. Dynamic aspects of presynaptic calcium currents mediating synaptic transmission. Cell Calcium. 2005;37:507–511. doi: 10.1016/j.ceca.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 59.Mongillo G., Barak O., Tsodyks M. Synaptic theory of working memory. Science. 2008;319:1543–1546. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]