Abstract
Although endoscopic transurethral resection of the prostate (TURP) is a well-established procedure as a treatment for benign prostatic hyperplasia, its complications remain a concern. Among these, coagulopathy may be caused by the absorption of irrigating fluid. This study aimed to evaluate such phenomenon using a rotational thromboelastometry (ROTEM).
A total of 20 patients undergoing TURP participated in this study. A mixture of 2.7% sorbitol–0.54% mannitol solution and 1% ethanol was used as an irrigating fluid, and fluid absorption was measured via the ethanol concentration in expired breath. The effects on coagulation were assessed by pre- and postoperative laboratory blood tests, including hemoglobin, hematocrit, platelet count, international normalized ratio of prothrombin time (PT-INR), activated partial thromboplastin time, electrolyte, and ROTEM.
INTEM-clotting time (INTEM-CT) was significantly lengthened by 14% (P = 0.001). INTEM-α-angle was significantly decreased by 3% (P = 0.011). EXTEM-clot formation time was significantly prolonged by 18% (P = 0.008), and EXTEM-maximum clot firmness (EXTEM-MCF) was significantly decreased by 4% (P = 0.010). FIBTEM-MCF was also significantly decreased by 13% (P = 0.015). Moreover, hemoglobin (P < 0.001), hematocrit (P < 0.001), platelet counts (P < 0.001), potassium (P = 0.024), and ionized calcium (P = 0.004) were significantly decreased, while PT-INR (P = 0.001) was significantly increased after surgery. The amount of irrigating fluid absorbed was significantly associated with the weight of resected prostatic tissue (P = 0.001) and change of INTEM-CT (P < 0.001).
As shown by the ROTEM analysis, the irrigating fluid absorbed during TURP impaired the blood coagulation cascade by creating a disruption in the coagulation factor activity or by lowering the coagulation factor concentration via dilution.
Keywords: blood coagulation, rotational thromboelastometry, transurethral resection of prostate
1. Introduction
Transurethral resection of the prostate (TURP) is considered to be the most effective surgical procedure for symptomatic benign prostate hyperplasia (BPH).[1,2] However, concerns still remain regarding its complications caused by the absorption of irrigating fluid, such as hyponatremia, pulmonary edema, severe bleeding, and myocardial arrhythmia.[3–6] In particular, coagulopathy occurs when an excessive volume of irrigating fluid is absorbed. Serious postoperative hematuria, accompanied with hemorrhagic symptoms, has been reported, which may result from disseminated intravascular coagulopathy.[7] Although hemostatic problems after TURP are common and implicated in the morbidity of the procedure,[8,9] the mechanism regarding the effect of irrigating fluid on coagulopathy remains unclear.
Intravascular fluid administered during surgery—whether crystalloid or colloid—is known to influence blood coagulation.[10–12] Irrigating fluid used during a TURP procedure is absorbed at a rate of 10 to 30 mL/min,[4] which is not a small amount. Therefore, it is important to investigate whether irrigating fluid affects blood coagulation to contribute to perioperative bleeding. Presently, to the best of our knowledge, there are no studies on the correlation between the absorption of irrigating fluid and blood coagulation using a point-of-care hemostasis monitoring method. One such monitoring method—rotational thromboelastometry (ROTEM)—employs a viscoelastic device to assess blood clot formation.
The purpose of this study is to enhance our understanding of the relationship between irrigating fluid and blood coagulation in patients undergoing TURP. Based on the hypothesis that absorbed irrigating fluid causes, a change in the coagulation cascade, we performed this prospective observational study using ROTEM analysis.
2. Methods and materials
After obtaining an approval from the Institutional Review Board at Seoul National University Bundang Hospital (Seongnam-si, South Korea, approval on August 7, 2013, B-1307/211-001), this prospective and observational study was registered in the Clinical Research Information Service (http://cris.nih.go.kr, KCT0001216). Written informed consent was obtained from all subjects.
This study was conducted in patients with a physical status of 1 or 2, according to the American Society of Anesthesiologists’ (ASA) criteria, undergoing TURP for BPH under spinal anesthesia. Preoperative exclusion criteria were as follows: anticoagulation or antithrombotic therapy, hematologic disease, pulmonary diseases, such as pulmonary edema or effusion, infectious disease, renal disorders, hepatic disorders, alcohol abuse, or malignant disease.
Upon arrival in the operating room, standard monitoring, including pulse oximetry, electrocardiogram and noninvasive arterial pressure, was applied before the induction of anesthesia. All patients first received an intravenous administration of 0.03 mg/kg midazolam, and then spinal anesthesia using 2.0 to 3.0 mL of 0.5% hyperbaric bupivacaine (Marcaine, Astra-Zeneca, Stockholm, Sweden) mixed with 10 to 20 μg of fentanyl intrathecally. We treated hypotension (arterial blood pressure <20% of baseline or <90 mm Hg) caused by spinal anesthesia with 5 to 10 mg of ephedrine or 10 to 30 μg of phenylephrine. Ringer lactate solution was used to maintain fluid volume during the operation, at a rate of 5 mL/kg/h. A forced-air warming blanket (Bair Hugger 52200, Arizant Healthcare Inc., 3M Company, Eden Prairie, MN) was applied to all patients.
TURP was conducted by 1 operator using a 24 F continuous irrigating resectoscope. A mixture of 2.7% sorbitol–0.54% mannitol (Urosol, CJ Pharma., Seoul, South Korea) and 1% ethanol (w/v), which was included to measure the fluid absorption via ethanol concentration in expired breath (EB ethanol), was used as an irrigating fluid. Immediately after TURP, the saline solution was used as the irrigating fluid through a 22 F 2-way urinary catheter until the urine drained clearly.
Venous blood samples were taken from the patients twice: before (after confirming the sensory block by spinal anesthesia in the operating room) and after surgery (upon arrival to the postanesthetic care unit within 30 minutes of the operation). Venous blood was drawn using the standard 2-syringe technique to prevent contamination by tissue factors. After discarding the initial 5 mL drawn, the test blood sample was obtained immediately. Blood samples were collected in ethylenediaminetetraacetic acid–containing tubes (Becton Dickinson, Plymouth, UK) to determine the hemoglobin, hematocrit, and platelet counts, and in serum separator tubes to determine the electrolytes (sodium, potassium, chloride, and ionized calcium). Blood samples were put into citrate-containing bottles to determine the international normalized ratio of prothrombin time (PT-INR), activated partial thromboplastin time (aPTT), and for ROTEM analysis (Pentapharm, Munich, Germany). PT-INR and aPTT values were obtained using a STA-R Evolution analyzer (Diagnostica Stago Inc., Asnieres, France) with STA Neoplastine CI Plus and STA PTT AUTOMATE reagents (Diagnostica Stago Inc.), respectively.
ROTEM tests were conducted in accordance with the manufacturer's recommendations by 1 investigator. The analyses were performed automatically, and graphic results were drawn. Four parameters of ROTEM were obtained as follows: clotting time (CT), α-angle (α), clot formation time (CFT), and maximum clot firmness (MCF). The extrinsic and intrinsic coagulation cascades were assessed by EXTEM and INTEM, respectively, using the recommended reagents (star-TEM with in-TEM or ex-TEM). Changes in the fibrin polymerization and fibrinogen concentration were examined by a FIBTEM test using the fib-TEM and ex-TEM reagents. The appropriate reagents were added to 300 μL of citrated whole blood for each test by computer-assisted pipetting. These ROTEM analyses were conducted within 10 minutes of obtaining the test samples. All analyses were performed using ROTEM machine that was set at 37 °C. MCF measurements have a coefficient of variation of <3% for EXTEM, <5% for INTEM, and <6% for FIBTEM. For CFT, the coefficient of variation is <4% for EXTEM and <3% for INTEM. Coefficient of variation for angle alpha is <3% for EXTEM and <6% for INTEM. The coefficient of variation for CT is <15% for both EXTEM and INTEM.[13]
The amount of irrigating fluid absorbed can be estimated using the ethanol monitoring method, as described by Hahn and Ekengren.[14] EB ethanol was estimated using a breathalyzer, AlcoScan AL7000 (Sentech Korea Corp., Paju-si, Gyeonggi-do, South Korea), every 10 minutes during the operation, until the end of surgery. The breathalyzer was calibrated in accordance with the manufacturer's recommendations. To obtain sufficient breath samples, patients were asked to take a deep breath and exhale steadily and continuously into the mouthpiece of the breathalyzer. EB ethanol was used to calculate the volume of irrigating fluid absorbed, using the following formula: absorption (mL) = (2140 + 3430 × EB-ethanoli) × ΔEB-ethanol + (44 + 806 × EB-ethanoli),[14] where EB-ethanoli is the expired breath ethanol concentration at the beginning of any 10-minute period, and ΔEB-ethanol is the total change in the ethanol concentration during the same period. A decrease in ΔEB-ethanol is expressed as a negative number. The total volume of fluid absorbed during an operation was the sum of results taken every 10 minutes.
The estimated blood loss was calculated using the formulas described by Rosencher et al[15] with pre- and postoperative hematocrit values.
The primary outcomes were the results of INTEM, EXTEM, and FIBTEM. The secondary outcomes were hemoglobin, hematocrit, platelet count, PT-INR, aPTT, electrolytes (sodium, potassium, chloride, and ionized calcium), volume of absorbed irrigating fluid, operating time, resected prostatic tissue weight, and the total amount of irrigating fluid. The primary variable used for the power calculation was FIBTEM-MCF based on our pilot data. In general, FIBTEM-MCF is recommended to be used on the management of severe bleeding in patients undergoing surgery or trauma as a point-of care tool. Thus, for the purpose of this study, we focused on the change of FIBTEM-MCF to determine the sample size.[16,17] Assuming a 30% difference in the mean (standard deviation) of 14.1 (4.9) for the baseline values, while aiming for a power of 90% and a risk of 0.05 for type-1 errors, 17 patients were required. We included 20 patients to allow for a 15% dropout rate.
Statistical analyses were performed using a Wilcoxon signed rank test to make a comparison between the pre- and postoperative values. A correlation among the irrigating fluid absorbed and the operating time, anesthesia time, change of ROTEM parameters, and volume of irrigating fluid used was examined by Spearman rank correlation analysis. Possible predictor variables were entered in a multivariate linear regression analysis (including only univariate significant variables), with irrigating fluid absorbed as the dependent variable. The variables included were operating time, anesthesia time, change of INTEM-CT, resected prostatic tissue weight, and volume of irrigating fluid used. Multicollinearity was avoided by excluding the variables with a variance inflation factor of ≥10. Data were analyzed using SPSS ver. 22 (SPSS Inc., Chicago, IL) software, and a P value of <0.05 was considered statistically significant.
3. Results
A total of 20 patients, between September 2013 and July 2014, were included in this study (Fig. 1). There were no complications, that is, no hyponatremia, massive bleeding, or TURP syndrome. Patient characteristics and information on surgery and anesthesia are provided in Table 1.
Figure 1.
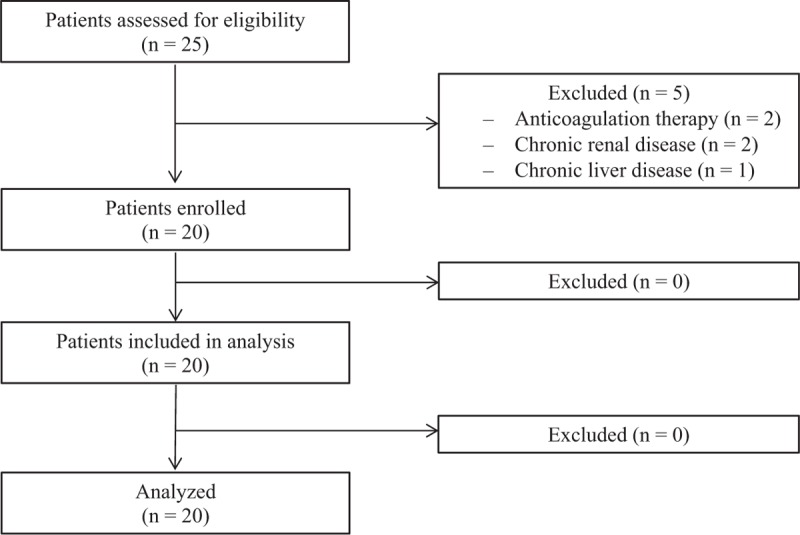
Flowchart.
Table 1.
Characteristic of patients, surgery and anesthesia (n = 20).
Data regarding ROTEM parameters are described in Table 2. INTEM-CT was significantly lengthened by 14% (P = 0.001). INTEM-α-angle was significantly decreased by 3% (P = 0.011). EXTEM-CFT was significantly prolonged by 18% (P = 0.008), and EXTEM-MCF was significantly decreased by 4% (P = 0.010). FIBTEM-MCF was also significantly decreased by 13% (P = 0.015). Although there were no significant changes in the other parameters of ROTEM, the overall variables showed a hypocoagulable tendency: INTEM-CFT and EXTEM-CT were prolonged by 13% (P = 0.070) and 7% (P = 0.239), respectively. Moreover, INTEM-MCF and EXTEM-α-angle were decreased by 4% (P = 0.091 and P = 0.110, respectively).
Table 2.
ROTEM parameters (n = 20).
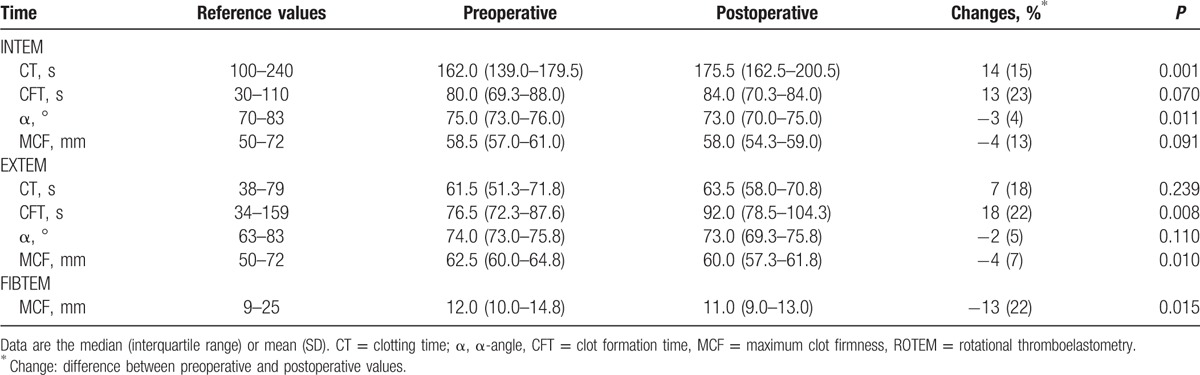
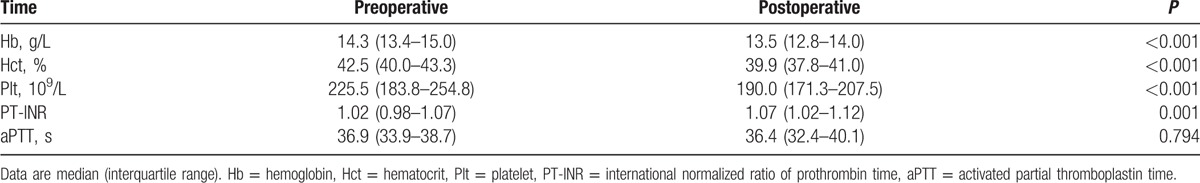
Table 3 shows the levels of blood counts and coagulation values in the pre- and postoperative periods. Hemoglobin (P < 0.001), hematocrit (P < 0.001), and platelet counts (P < 0.001) in the postoperative period were significantly lower compared with those in the preoperative period. The postoperative values of PT-INR was significantly greater than the preoperative values (P < 0.001), whereas preoperative and postoperative aPTT were comparable (P = 0.794).
Table 3.
Blood count and coagulation values (n = 20).
Sodium (P = 0.150) and chloride (P = 0.185) did not change significantly from before to after surgery (Table 4). The postoperative values of potassium (P = 0.024) and ionized calcium (P = 0.049) were significantly lower than the preoperative values (Table 4).
Table 4.
Electrolytes (n = 20).
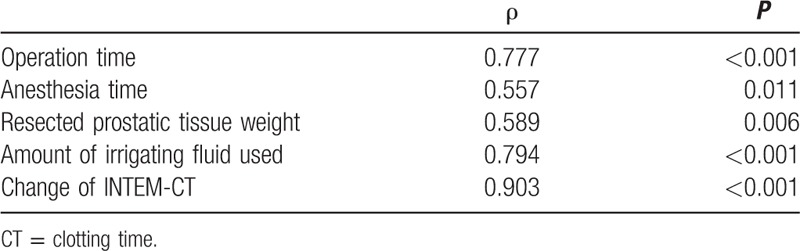
There were statistically significant correlations between the amount of absorbed irrigating fluid and operation (ρ = 0.777, P < 0.001) and anesthesia (ρ = 0.557, P = 0.011) time, resected prostatic tissue weight (ρ = 0.589, P = 0.006), volume of irrigating fluid used (ρ = 0.794, P < 0.001), and change of INTEM-CT (ρ = 0.903, P < 0.001) (Table 5).
Table 5.
Correlation (ρ) between the volume of absorbed irrigating fluid and other factors at the end of the surgery.
After adjustment of multicollinearity, the factors in the multivariate linear regression analysis that was associated with the amount of absorbed irrigating fluid were resected prostatic tissue weight (P = 0.001) and change of INTEM-CT (P < 0.001) (Table 6).
Table 6.
Multivariate linear regression analysis of association between absorption of irrigating fluid and other variables.

4. Discussion
In the present study, we investigated the blood coagulation status of before and after TURP using ROTEM. The prolonged postoperative values were shown in INTEM-CT and EXTEM-CFT. Moreover, INTEM-α-angle, EMTEM-MCF, and FIBTEM-MCF were decreased postoperatively. Moreover, we showed a positive association between the volumes of irrigating fluid absorbed and resected prostatic tissue weight and change of INTEM-CT.
CT is defined as the duration of time from the onset of measurement to the initiation of clot formation,[13] which includes thrombin formation and clot polymerization. Therefore, CT reflects the coagulation factor activity or concentration; and prolonged CT suggests a low coagulation factor activity or coagulation factor deficiency.[18] In the present study, CT of INTEM was lengthened significantly. The degree of change in CT of INTEM showed a strong positive association with the volume of irrigating fluid absorbed, which indicates an impairment of the intrinsic coagulation pathway. Therefore, the irrigating fluid absorbed seems to have an influence on both intrinsic pathways by directly decreasing the coagulation factor activity or lowering the coagulation factor concentration through the hemodilution effect. Such effect of irrigating fluid inducing coagulation cascade impairment may be one of the contributing factors for postoperative bleeding after TURP.
Interestingly, the ROTEM parameters demonstrated a hypocoagulable pattern (prolonged CT and CFT values, and decreased α-angle and MCF values), although not all variables were statistically significant. Bell et al[19] reported that the thromboelastogrphy (TEG) parameters reflect a hypercoagulable state during and after TURP and that this change is caused by the inhibition of the fibrinolytic system. Conversely, Nielsen et al[20] concluded that the fibrinolytic system is activated during TURP due to surgical trauma. Although these 2 studies demonstrated conflicting results regarding the change of the fibrinolytic system, both of these reports failed to provide information about the amount of irrigating fluid used and absorbed. Thus, it is difficult to fully comprehend how much of an impact the irrigating fluid had on the results between the 2 different aforementioned reports. The average amount of irrigating fluid absorbed was about 400 mL in this study, which corresponds to about 10% of the total blood volume. In general, adverse symptoms (i.e., nausea, vomiting, confusion, and arterial hypotension) are more common if glycine solutions of 1000 to 2000 mL are absorbed.[21,22] Based on the result from a correlation analysis—between the irrigating fluid absorbed and the changes in ROTEM—there may be greater coagulation pathway impairment (hypocoagulability) with increased volume of irrigating fluid absorption.
In the present study, the postoperative values of PT-INR were increased compared with the preoperative values. However, aPTT did not change significantly. Ahsan et al[23] described that PT prolongation was observed in 35% of patients immediately after TURP, and it was correlated with increased perioperative blood loss. Ozmen et al[24] reported that PT was prolonged and aPTT did not change after TURP, which is in line with our findings. However, the accuracy of conventional coagulation tests to significantly reflect in vivo hypocoagulability is uncertain.[25] In addition, PT and aPTT reflect only some parts of the coagulation system and do not provide information regarding the full coagulation pathway and balance between coagulation and anticoagulation.[26] Although PT-INR and aPTT have been known to be correlated with CT of ROTEM,[27,28] the interchangeability between the conventional coagulation test and the ROTEM analysis is still controversial.[29,30] On the other hand, it was reported that CT of EXTEM is correlated with disseminated intravascular coagulopathy by Koami et al.[31] We identified the effect of irrigating fluid on the coagulation cascade more clearly by showing a strong correlation between the volume of irrigating fluid absorbed and the degree of change in the EXTEM-CT value.
Hemoglobin and hematocrit levels were decreased postoperatively in the present study. A couple of possible causes can be suggested about the changes of hematological parameters. First, there were intraoperative bleedings. The mean estimated blood loss was 281 mL in the present study. Second is the dilution effect of irrigating fluid containing ethanol. There are several reports that the irrigation with isotonic saline containing 1% ethanol could influence the hematocrit and hemoglobin levels.[32,33]
We identified other possible factors, such as ionized calcium, that could affect blood coagulation during TURP. Serum ionized calcium decreased significantly after surgery in the present study, which could result from the dilution caused by irrigating fluid absorption. Calcium is an important coagulation cofactor involved in the intrinsic, extrinsic, and final common pathways, as well as the conversion of fibrinogen to fibrin in the coagulation cascade.[34] One in vitro study, using TEG analysis reported that significant hypocalcemia can cause hypocoagulation[35]; therefore, ionized calcium should be monitored during TURP to maintain hemostasis in the blood coagulation system.
There are a few limitations to this study that should be taken into consideration. First, only patients with ASA classification of 1 or 2 participated in the present study. This restriction may result in selection bias and may not be generalized to all patients. In general, patients with ASA classification of >2 may have an underlying disease, such as coronary artery disease, that requires antiplatelet or antithrombotic agents. These drugs, despite discontinuation for several days before surgery, can have an influence on the ROTEM analysis. Thus, we limited the condition of patients to only those with ASA classification of 1 or 2. Second, this study was conducted in patients undergoing monopolar TURP, using a mixture of 2.7% sorbitol–0.54% mannitol as an irrigating fluid. Therefore, further studies are needed to better understand the effect of 0.9% saline used in bipolar TURP as an irrigating fluid. Finally, the number of patients enrolled in this study was relatively small (20 patients). Although there were significant findings in the present study, further studies with a larger patient population are necessary.
In conclusion, irrigating fluid absorbed during TURP has significant effects on the blood coagulation cascade via the inhibition of the clotting factor activity or by lowering the coagulation factor concentration through hemodilution, as suggested by the ROTEM results. Further studies are required, however, to reveal the effect of absorption of large volumes of irrigating fluid on blood coagulation.
Footnotes
Abbreviations: aPTT = activated partial thromboplastin time, CFT = clot formation time, CT = clotting time, MCF = maximum clot firmness, PT-INR = international normalized ratio of prothrombin time, ROTEM = rotational thromboelastometry, TURP = transurethral resection of the prostate.
Data availability: All data are fully available from the published papers.
Funding/support: This study was supported by a grant (no. 02-2013-081) from the Research Fund of Seoul National University Bundang Hospital, South Korea. No other external funding declared.
The authors have no conflicts of interest to disclose.
References
- [1].Crowley AR, Horowitz M, Chan E, et al. Transurethral resection of the prostate versus open prostatectomy: long-term mortality comparison. J Urol 1995;153:695–7. [DOI] [PubMed] [Google Scholar]
- [2].Neal DE. Transurethral prostatectomy. Br J Surg 1994;81:484–5. [DOI] [PubMed] [Google Scholar]
- [3].Ran L, He W, Zhu X, et al. Comparison of fluid absorption between transurethral enucleation and transurethral resection for benign prostate hyperplasia. Urol Int 2013;91:26–30. [DOI] [PubMed] [Google Scholar]
- [4].Gravenstein D. Transurethral resection of the prostate (TURP) syndrome: a review of the pathophysiology and management. Anesth Analg 1997;84:438–46. [DOI] [PubMed] [Google Scholar]
- [5].Lira-Dale A, Maldonado-Avila M, Gil-Garcia JF, et al. Effect of intraprostatic epinephrine on intraoperative blood loss reduction during transurethral resection of the prostate. Int Urol Nephrol 2012;44:365–9. [DOI] [PubMed] [Google Scholar]
- [6].Charlton AJ. Cardiac arrest during transurethral prostatectomy after absorption of 1.5% glycine. A case report and review of the literature. Anaesthesia 1980;35:804–6. [DOI] [PubMed] [Google Scholar]
- [7].Friedman NJ, Hoag MS, Robinson AJ, et al. Hemorrhagic syndrome following transurethral prostatic resection for benign adenoma. Arch Intern Med 1969;124:341–9. [PubMed] [Google Scholar]
- [8].Doll HA, Black NA, McPherson K, et al. Mortality, morbidity and complications following transurethral resection of the prostate for benign prostatic hypertrophy. J Urol 1992;147:1566–73. [DOI] [PubMed] [Google Scholar]
- [9].Mebust WK, Holtgrewe HL, Cockett AT, et al. Transurethral prostatectomy: immediate and postoperative complications. A cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol 1989;141:243–7. [DOI] [PubMed] [Google Scholar]
- [10].Ruttmann TG, James MF, Aronson I. In vivo investigation into the effects of haemodilution with hydroxyethyl starch (200/0.5) and normal saline on coagulation. Br J Anaesth 1998;80:612–6. [DOI] [PubMed] [Google Scholar]
- [11].Mittermayr M, Streif W, Haas T, et al. Hemostatic changes after crystalloid or colloid fluid administration during major orthopedic surgery: the role of fibrinogen administration. Anesth Analg 2007;105:905–17. table of contents. [DOI] [PubMed] [Google Scholar]
- [12].Shin HJ, Na HS, Do SH. The effects of acute normovolaemic haemodilution on peri-operative coagulation in total hip arthroplasty. Anaesthesia 2015;70:304–9. [DOI] [PubMed] [Google Scholar]
- [13].Theusinger OM, Nurnberg J, Asmis LM, et al. Rotation thromboelastometry (ROTEM) stability and reproducibility over time. Eur J Cardiothorac Surg 2010;37:677–83. [DOI] [PubMed] [Google Scholar]
- [14].Hahn RG, Ekengren J. Absorption of irrigating fluid and height of fluid bag during transurethral resection of the prostate. Br J Urol 1993;72:80–3. [DOI] [PubMed] [Google Scholar]
- [15].Rosencher N, Kerkkamp HE, Macheras G, et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion 2003;43:459–69. [DOI] [PubMed] [Google Scholar]
- [16].Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol 2013;30:270–382. [DOI] [PubMed] [Google Scholar]
- [17].Lier H, Vorweg M, Hanke A, et al. Thromboelastometry guided therapy of severe bleeding. Essener Runde algorithm. Hamostaseologie 2013;33:51–61. [DOI] [PubMed] [Google Scholar]
- [18].Schochl H, Frietsch T, Pavelka M, et al. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma 2009;67:125–31. [DOI] [PubMed] [Google Scholar]
- [19].Bell CR, Cox DJ, Murdock PJ, et al. Thrombelastographic evaluation of coagulation in transurethral prostatectomy. Br J Urol 1996;78:737–41. [DOI] [PubMed] [Google Scholar]
- [20].Nielsen JD, Gram J, Fabrin K, et al. Lack of correlation between blood fibrinolysis and the immediate or post-operative blood loss in transurethral resection of the prostate. Br J Urol 1997;80:105–10. [DOI] [PubMed] [Google Scholar]
- [21].Olsson J, Nilsson A, Hahn RG. Symptoms of the transurethral resection syndrome using glycine as the irrigant. J Urol 1995;154:123–8. [PubMed] [Google Scholar]
- [22].Hahn RG. Fluid absorption in endoscopic surgery. Br J Anaesth 2006;96:8–20. [DOI] [PubMed] [Google Scholar]
- [23].Ahsan Z, Cartner R, English PJ. Coagulation tests in predicting haemorrhage after prostatic resection. Br J Urol 1993;72:201–6. [DOI] [PubMed] [Google Scholar]
- [24].Ozmen S, Kosar A, Sayin A, et al. Effect of transurethral resection of the prostate on blood coagulation test results. Urol Int 2003;70:27–30. [DOI] [PubMed] [Google Scholar]
- [25].Levi M, Meijers JC. DIC: which laboratory tests are most useful. Blood Rev 2011;25:33–7. [DOI] [PubMed] [Google Scholar]
- [26].Muller MC, Meijers JC, Vroom MB, et al. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review. Crit Care 2014;18:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Engstrom M, Rundgren M, Schott U. An evaluation of monitoring possibilities of argatroban using rotational thromboelastometry and activated partial thromboplastin time. Acta Anaesthesiol Scand 2010;54:86–91. [DOI] [PubMed] [Google Scholar]
- [28].Casutt M, Konrad C, Schuepfer G. Effect of rivaroxaban on blood coagulation using the viscoelastic coagulation test ROTEM. Anaesthesist 2012;61:948–53. [DOI] [PubMed] [Google Scholar]
- [29].Haas T, Spielmann N, Mauch J, et al. Comparison of thromboelastometry (ROTEM®) with standard plasmatic coagulation testing in paediatric surgery. Br J Anaesth 2012;108:36–41. [DOI] [PubMed] [Google Scholar]
- [30].Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost 2007;5:289–95. [DOI] [PubMed] [Google Scholar]
- [31].Koami H, Sakamoto Y, Ohta M, et al. Can rotational thromboelastometry predict septic disseminated intravascular coagulation? Blood Coagul Fibrinolysis 2015;26:778–83. [DOI] [PubMed] [Google Scholar]
- [32].Wettstein MS, Poyet C, Grossmann NC, et al. Absorption of irrigation fluid during XPS GreenLight laser vaporization of the prostate: results from a prospective breath ethanol monitoring study. World J Urol 2016;34:1261–7. [DOI] [PubMed] [Google Scholar]
- [33].Hermanns T, Grossmann NC, Wettstein MS, et al. Absorption of irrigation fluid occurs frequently during high power 532 nm laser vaporization of the prostate. J Urol 2015;193:211–6. [DOI] [PubMed] [Google Scholar]
- [34].Mann KG. Biochemistry and physiology of blood coagulation. Thromb Haemost 1999;82:165–74. [PubMed] [Google Scholar]
- [35].James MF, Roche AM. Dose-response relationship between plasma ionized calcium concentration and thrombelastography. J Cardiothorac Vasc Anesth 2004;18:581–6. [DOI] [PubMed] [Google Scholar]


