Abstract
Rationale:
Ecthyma gangrenosum (Eg) is a necrotic lesion that is mostly seen in immunocompromised patients. It reflects a severe sepsis, possibly caused by Pseudomonas aeruginosa (Pa).
Patient concerns:
A healthy 3-year-old girl admitted to the Pediatric Emergency Department presented a sepsis-associated purpura with neurological and respiratory distress.
Interventions:
An empiric antibiotherapy (anti-meningococcal) was prescribed.
Diagnoses:
Forty-eight hours after admission, blood and wound cultures were positive for Pa. As a result, the decision was made to change the antibiotic therapy.
Unfortunately, on day 3, the patient died. Exhaustive immunologic tests are presently being carried out.
Outcomes:
Eg caused by Pa is uncommon in healthy children, and purpura sepsis is usually caused by Neisseria meningitides infection.
Lessons:
Eg should be recognized rapidly so that the appropriate treatment can be prescribed as quickly as possible.
Keywords: children, ecthyma gangrenosum, Pseudomonas aeruginosa, sepsis
1. Introduction
Ecthyma gangrenosum (Eg) is a rare but typical skin manifestation, most commonly caused by Pseudomonas aeruginosa (Pa), an aerobic Gram-negative opportunistic pathogen that has a high risk of associated mortality in cases where the infection is systemic.[1,2] Although rare, the presence of Eg is indicative of a severe systemic infection with a potentially fatal prognosis.[3] These skin lesions may be seen on admission or can develop later. The recognition of Eg lesions permits the earliest possible introduction of the most effective antimicrobial therapy, which is a key prognostic factor for survival. Eg with Pa is rare in healthy children, and empiric antibiotic regimens used in sepsis are generally not effective.
2. Patient presentation
A 3-year-old girl was admitted to the emergency department with a 4-day history of fever, lethargy, impaired consciousness, and multiple necrotic lesions that began the day before admission. She had no recent history of contact with contagious diseases or foreign travel, no familial medical problems. She was not on any medication and had received the appropriate immunizations.
On arrival, she was in a poor condition. Blood pressure and oxygen saturation were undetectable. She was lethargic, with cyanosis of the extremities, and her capillary refill time was more than 4 seconds. Many purple necrotic lesions were visible on her legs, thorax, face, and genital areas. The largest was a 5-centimeter necrotic lesion surrounded by an intense erythematous ring (Fig. 1).
Figure 1.
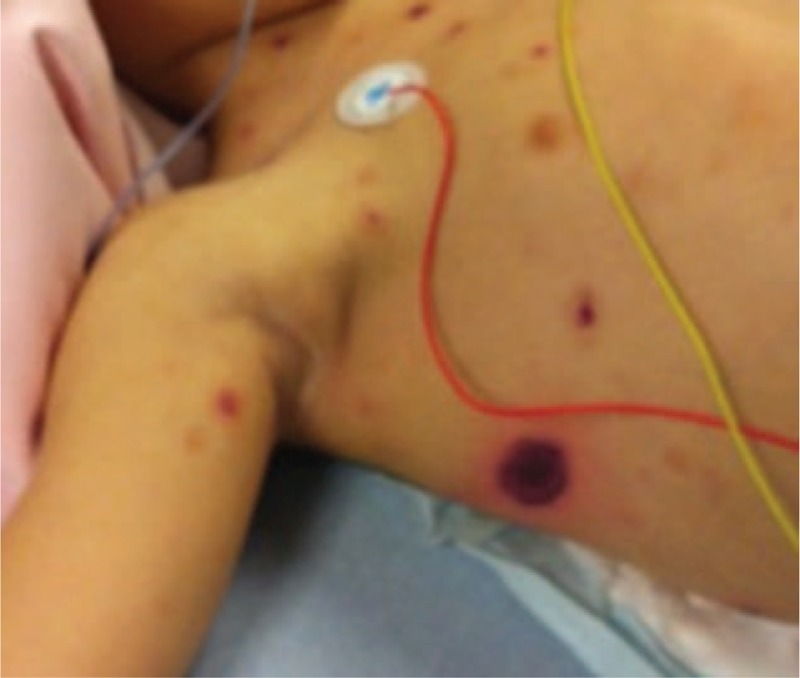
Necrotic skin lesion at day 4.
Initial resuscitation included 100 mg/kg intravenous (IV) ceftriaxone injection, 3 boluses of 20 mL/kg IV normal saline, and oxygen via a face mask.
Laboratory tests indicated leukopenia (1200 white blood cells/mm3) with an absolute neutrophil count of 200 cells/mm3, anemia (5 g/dL hemoglobin), and thrombocytopenia (9000 platelets/mm3) associated with a coagulation disorder with no sign of disseminated intravascular coagulation. An uncompensated metabolic acidosis, hyponatremia, renal failure, and increased liver enzyme levels were observed. The inflammatory markers, C-reactive protein and procalcitonin, were markedly raised, at 231 mg/L and 217 ng/mL, respectively. Chest x-ray revealed pulmonary edema. Blood and wound cultures were sent for laboratory analysis.
The patient was transferred to the Pediatric Intensive Care Unit with a diagnosis of meningococcal septic shock. As her respiratory distress worsened, she was intubated. Inotropic support was started to combat low blood pressure despite refilling and cardiac failure. One unit of packed blood cells and 3 units of packed platelets were transfused. During the first 2 days, she received 200 mg/kg/day cefotaxime IV, with the hypothesis of a Neisseria meningitidis infection. She was stable for the first 24 hours.
On day 3, Pa was identified in the blood and the skin lesion cultures, and the antibiotic regimen was immediately changed to 200 mg/kg/day ceftazidime IV. The skin lesions became more necrotic and spread further. The patient suffered from a brutal deterioration, with multiple organ failure, which necessitated hemodialysis, inotropic support, fresh frozen plasma transfusion, and intensive care management.
By day 4, severe skin lesions had spread even further and were necrotic and hemorrhagic, with blisters and ulcers underneath (Figs. 2 and 3). Despite an aggressive resuscitation, progression was rapidly fatal (Fig. 4).
Figure 2.
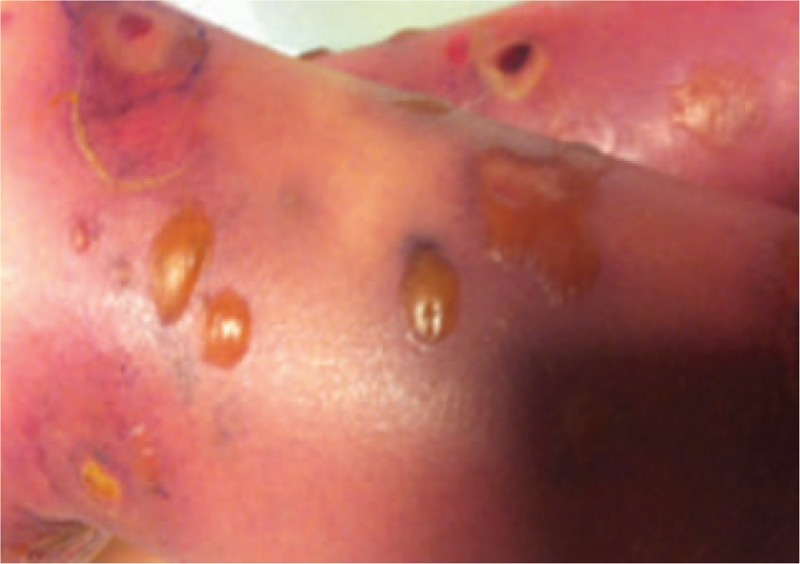
Bulla skin lesions at day 6.
Figure 3.
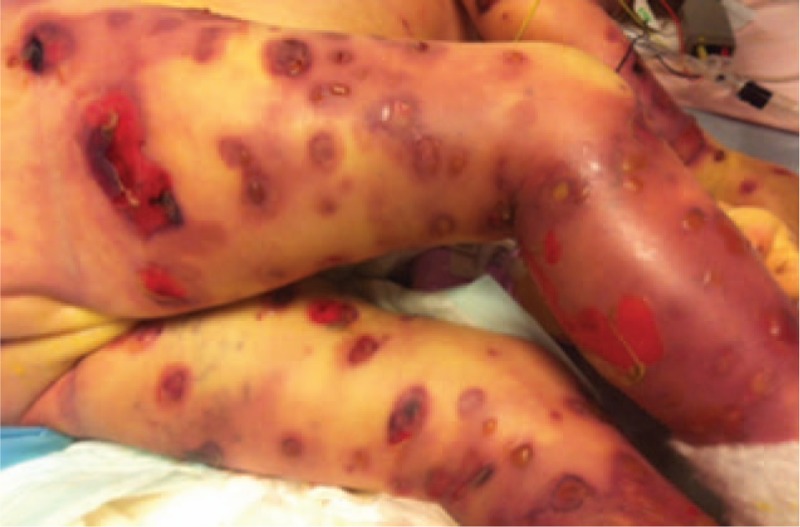
Bulla broken with ulcers underneath, hemorrhagic lesions day 8.
Figure 4.
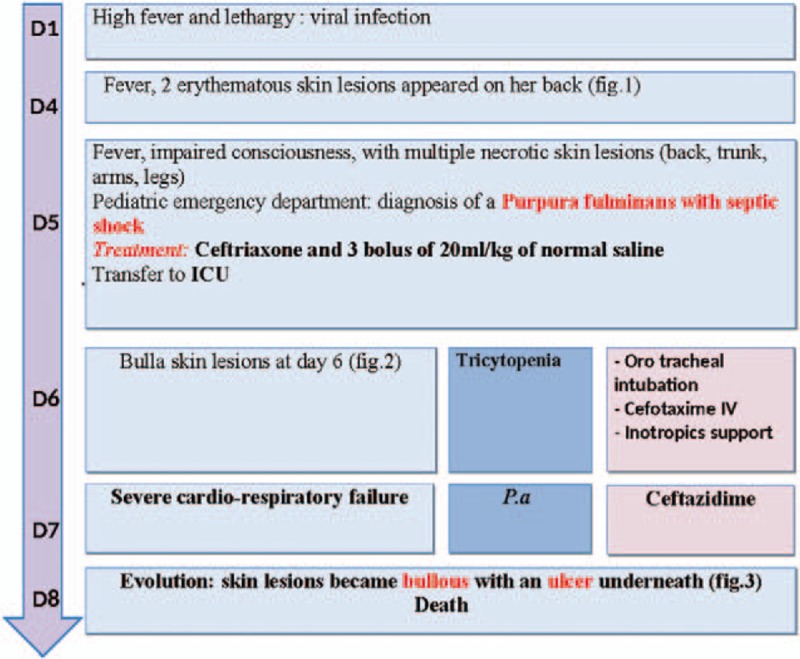
Timeline of interventions and outcomes.
Pa was isolated from 1 blood sample, 3 necrotic skin lesions, and a cerebrospinal fluid sample. The Pa identified in cerebrospinal fluid was resistant to ceftazidime; conversely, the blood and wound cultures were ceftazidime sensitive. Bone marrow aspiration was not performed. HIV serology was negative. Quantitative immunoglobulin levels revealed normal immunoglobulins (G, A, D, and M).
3. Discussion
Pa is an opportunistic bacterium, which can be found on the skin, in the nose and throat, and in the stools. It generally causes infection in immunocompromised patients with conditions such as neutropenia, immunodeficiency, and hypogammaglobulinemia.[4] The presence of Pa infection in healthy subjects is very uncommon.
In some reported cases of Pa sepsis in previously healthy children, most patients were male and less than 1 year old.[5,6] Fever, diarrhea, pneumonia, skin lesions (50%), and shock are the most relevant associated symptoms.[5,7] The reported overall mortality rate associated with Pa sepsis in children is variable, ranging from 20% to more than 50%.[6–8] Antibiotic treatments have been based mainly on anti-pseudomonas beta-lactam antibiotics and aminoglycosides, either alone or in association.
The presence of a viral infection or recent antibiotic therapy had been described as transient risk factors of Pa sepsis in immunocompetent children. Chusid and Hillmann postulated that a viral infection may directly alter the mucosal barrier of the gastrointestinal tract, and then reduce host defense. In addition, previous treatment with antibiotics may increase the number of Pa in the gastrointestinal tracts.[1,3,7,8,9]
Furthermore, it is possible that Pseudomonas organisms, by producing toxins, may trigger a transient neutropenic state. These toxins could inhibit granulocyte migration and cause like bone marrow suppression in healthy children.[3,8,10]
Whether this phenomenon represents a secondary immunosuppressed state or a predisposition to severe Pa infection in previously healthy children needs to be further evaluated. In our patient, neutropenia was probably induced by Pa infection rather than being a predisposing factor for the infection.[11]
Clinical presentations of community-acquired Pa sepsis show a geographical difference. Fever and diarrhea are the most common presentations in the Eastern world and are called Shanghai fever. However, fever and skin lesions are usually seen in the Western world (North America and Europe). Our European patient presented with Eg skin lesions, but not with diarrhea.[8,11]
Eg is a well-recognized cutaneous manifestation of Pa infection with or without septicemia.[9,12] It is described as an uncommon vasculitis, affecting the adventitia and media of blood vessels and caused from either hematogenous seeding of a pathogen, or direct inoculation through the skin.[1,13,14]
Eg appears as painless, erythematous, and purpuric macules in moist areas, which become nodular, bullous, or pustular with an indurated erythematous base and rim. Finally, they form gangrenous ulcers, with a gray-black eschar surrounded by an erythematous halo. The lesions mature within 12 hours and can co-exist at different stages of development. Fifty-seven percent of lesions occur in the gluteal and perineal regions, 30% involve the extremities, 12% the trunk and face.[1] Vaiman et al[15] analyzed Eg cases described in the literature from 1975 to 2014. Of the 167 published cases, Pa was detected in 123 (73.65%), and other bacterial etiologies were detected in 29 cases (17.35%), including Escherichia coli, Staphylococcus aureus, Aeromonas hydrophilia, and Mucor species. Of the 123 Eg cases with Pa etiology, sepsis was described in 72 cases (58.5%) and an absence of septicemia was reported for 51 cases (41.5%).
The prognosis for children with Pa infection is influenced by several risk factors.[16] The first of these is the presence of an unknown immune deficiency. Neutropenia below 500 cells/mm3 can predispose to a severe Pa infection,[17] and this seems to be associated with a higher mortality rate, even in previously healthy children. In a study reported by Huang et al, leukopenia was present in 24 (57%) of 43 children evaluated. Of the 10 cases that were fatal, 9 patients had leukopenia on admission.[7] Delay in introducing an appropriate treatment has also been associated with higher mortality.[18,19] In the study by Huang et al, 90% of the patients who died had not received an optimal antimicrobial treatment. Other risk factors include the presence of multiple Eg lesions and septic shock.
Prognosis in Pa infection is highly associated with early institution of optimal antibiotherapy.[7,8,11] Bodey et al[20] reported an overall cure rate of 67% for patients receiving appropriate antibiotics but only 14% for those receiving inappropriate antibiotics. Moreover, this retrospective analysis of Pseudomonas bacteremia cases indicated that in cases where optimal antimicrobial therapy was delayed (24–48 hours), the healing rate decreased from 74% to 46%.
Some authors propose a combination therapy as the preferred mode of treatment, based on synergisms between β-lactams and aminoglycosides, and the emergence of resistant strains with monotherapy.[21]
In our case, we suspect that Pa became rapidly resistant to ceftazidime, with the production of an inducible cephalosporinase. This is described in the literature as an increasingly well-recognized Pa phenomenon, which explains the importance of the correct application of antipseudomonal therapy.[22] Current recommendations for Pa infection are controversial. Bowers et al[23], from their study, suggested that there is no difference in mortality outcomes associated with the number of appropriate agents administered during initial empirical therapy for Pa bacteremia, as long as at least 1 agent is active.[23,24] The addition of an aminoglycoside to an antipseudomonal beta-lactam penicillin does not improve the clinical efficacy achieved with the beta-lactam penicillin alone.[22,25,26] While awaiting bacteriology results, empirical antimicrobial biotherapy should be started with anti-pseudomonal beta-lactam penicillin, and adjusted immediately once the bacterial culture results are known.[27]
A final point is the differentiation of Eg from Purpura fulminans caused by Neisseria meningitidis. These 2 skin lesions are different. Purpura fulminans lesions spread faster than Eg lesions and never become bullous. Moreover, antimeningococcal antibiotics (such as cefotaxime or ceftriaxone) are not efficient against Eg caused by Pa infection.
Physicians must be aware of the existence of Eg and should be able to recognize this skin lesion rapidly in order to prescribe the appropriate treatment, generally when the skin lesions become bullous.
4. Conclusion
This case report shows that Eg due to Pa infection can occur in a previously healthy child with no other medical issues. The main risk factor found to be connected to the severe Pa infection was neutropenia. A clinical picture of Eg in a previously healthy child should suggest a Pa infection. Eg is an informative skin lesion for diagnosing Pa sepsis and can present before the pathogen is identified. A careful and cautious physical examination is important and should be carried out in order to recognize this lesion. The early institution of the appropriate antimicrobial therapy, anti-pseudomonal beta-lactam penicillin, is important in terms of prognosis. This treatment must be adjusted immediately once bacterial culture results are known. A comprehensive immunologic evaluation should also be conducted.
Acknowledgments
The authors are grateful to Kate Vassaux, PhD, and Mrs Sara Jane Higgins for medical editing.
Footnotes
Abbreviations: Eg = ecthyma gangrenosum, HIV = human immunodeficiency virus, IV = intravenous, Pa = Pseudomonas aeruginosa.
SB and DD contributed equally to this study.
The authors have no funding and conflicts of interest to disclose.
Informed consent: The parents of the patient provided oral permission for publication of this case report.
References
- [1].Zomorrodi A, Wald ER. Ecthyma gangrenosum: considerations in a previously healthy child. Pediatr Infect Dis J 2002;21:1161–4. [DOI] [PubMed] [Google Scholar]
- [2].Goolamali SI, Fogo A, Killian L, et al. Ecthyma gangrenosum: an important feature of pseudomonal sepsis in a previously well child. Clin Exp Dermatol 2009;34:e180–2. [DOI] [PubMed] [Google Scholar]
- [3].Lian F. Clinicopathologic aspects of ecthyma gangrenosum in pediatric patients a case series and review of the literature. J Clin Anat Pathol 2013;20:1–5. [Google Scholar]
- [4].de Almeida JFL, Sztajnbok J, Troster EJ, et al. Pseudomonas aeruginosa septic shock associated with ecthyma gangrenosum in an infant with agammaglobulinemia. Rev Inst Med Trop São Paulo 2002;44:167–9. [DOI] [PubMed] [Google Scholar]
- [5].Viola L, Langer A, Pulitanò S, et al. Serious Pseudomonas aeruginosa infection in healthy children: case report and review of the literature. Pediatr Int 2006;48:330–3. [DOI] [PubMed] [Google Scholar]
- [6].Grisaru-Soen G, Lerner-Geva L, Keller N, et al. Pseudomonas aeruginosa bacteremia in children: analysis of trends in prevalence, antibiotic resistance and prognostic factors. Pediatr Infect Dis J 2000;19:959–63. [DOI] [PubMed] [Google Scholar]
- [7].Huang Y-C, Lin T-Y, Wang C-H. Community-acquired Pseudomonas aeruginosa sepsis in previously healthy infants and children: analysis of forty-three episodes. Pediatr Infect Dis J 2002;21:1049–52. [DOI] [PubMed] [Google Scholar]
- [8].Yin J, Li C-W, Luo N, et al. Pseudomonas aeruginosa sepsis associated with ecthyma gangrenosum in a previously healthy infant: a case report and literature review. Glob Pediatr Health 2015;2:2333794X15591566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chusid MJ, Hillmann SM. Community-acquired Pseudomonas sepsis in previously healthy infants. Pediatr Infect Dis J 1987;6:681–4. [DOI] [PubMed] [Google Scholar]
- [10].Ortí A, Escrig R, Pérez-Tamarit D, et al. Pseudomonas aeruginosa infection in a previously healthy infant. Clin Pediatr (Philadelphia) 2002;41:525–8. [DOI] [PubMed] [Google Scholar]
- [11].Chuang C-H, Wang Y-H, Chang H-J, et al. Shanghai fever: a distinct Pseudomonas aeruginosa enteric disease. Gut 2014;63:736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gargouri L, Maaloul I, Kamoun T, et al. Ecthyma gangrenosum: a manifestation of community-acquired Pseudomonas aeruginosa septicemia in three infants. Arch Pédiatrie 2015;22:616–20. [DOI] [PubMed] [Google Scholar]
- [13].Fang L-C, Peng C-C, Chi H, et al. Pseudomonas aeruginosa sepsis with ecthyma gangrenosum and pseudomembranous pharyngolaryngitis in a 5-month-old boy. J Microbiol Immunol Infect 2014;47:158–61. [DOI] [PubMed] [Google Scholar]
- [14].Befort P, Corne P, Riviere B, et al. Ecthyma gangrenosum et septicémie à Pseudomonas aeruginosa en réanimation. Ann Fr Anesth Réanimation 2011;30:80–2. [DOI] [PubMed] [Google Scholar]
- [15].Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 2015;34:633–9. [DOI] [PubMed] [Google Scholar]
- [16].Bouskraoui M, Belabes H, Zineddine A, et al. Septicémie communautaire à Pseudomonas aeruginosa chez le nourrisson. À propos de cinq observations. Médecine Mal Infect 1999;29:49–52. [Google Scholar]
- [17].Cohen N, Capua T, Bilavsky E, et al. Ecthyma gangrenosum skin lesions in previously healthy children. Acta Paediatr 2015;104:e134–8. [DOI] [PubMed] [Google Scholar]
- [18].Kang C-I, Kim S-H, Kim H-B, et al. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis Off Publ Infect Dis Soc Am 2003;37:745–51. [DOI] [PubMed] [Google Scholar]
- [19].Zhang Q, Smith JC, Zhu Q, et al. A five-year review of Pseudomonas aeruginosa bacteremia in children hospitalized at a single center in southern China. Int J Infect Dis 2012;16:e628–32. [DOI] [PubMed] [Google Scholar]
- [20].Bodey GP, Jadeja L, Elting L. Pseudomonas bacteremia: retrospective analysis of 410 episodes. Arch Intern Med 1985;145:1621–9. [DOI] [PubMed] [Google Scholar]
- [21].Chan YH, Chong CY, Puthucheary J, et al. Ecthyma gangrenosum: a manifestation of Pseudomonas sepsis in three paediatric patients. Singapore Med J 2006;47:1080–3. [PubMed] [Google Scholar]
- [22].Paul M, Leibovici L. Combination therapy for Pseudomonas aeruginosa bacteremia: where do we stand? Clin Infect Dis 2013;57:271–20. [DOI] [PubMed] [Google Scholar]
- [23].Bowers DR, Liew Y-X, Lye DC, et al. Outcomes of appropriate empiric combination versus monotherapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 2013;57:1270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hu Y, Li L, Li W, et al. Combination antibiotic therapy versus monotherapy for Pseudomonas aeruginosa bacteraemia: a meta-analysis of retrospective and prospective studies. Int J Antimicrob Agents 2013;42:492–6. [DOI] [PubMed] [Google Scholar]
- [25].Paul M, Lador A, Grozinsky-Glasberg S, et al. β Lactam antibiotic monotherapy versus β lactam–aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 2014;CD003344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tamma PD, Turnbull AE, Harris AD, et al. Less is more: combination antibiotic therapy for the treatment of gram-negative bacteremia in pediatric patients. JAMA Pediatr 2013;167:903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mota-Burgos A, Villa AV, Noguera-Julian A, et al. Fever and skin lesions in a healthy 6-month-old boy. Diagnosis: ecthyma gangrenosum. Pediatr Infect Dis J 2012;31:789–94. [DOI] [PubMed] [Google Scholar]


