Abstract
To determine risk factors related to recollapse of the augmented vertebrae after percutaneous vertebroplasty (PVP) for osteoporotic vertebral compression fractures (OVCFs) with intravertebral vacuum cleft (IVC).
Fifty-two patients treated with PVP for single OVCFs with the IVC were retrospectively reviewed. The follow-up period was at least 2 years. Vertebral height loss ≥15% or kyphotic angle ≥10° at the final follow-up in relation to the immediately postoperative values were adopted as a definition of recollapse of the augmented vertebrae. Correlation analysis and multiple logistic regression analyses were performed to elucidate the related clinical or radiological factors for recollapse of the augmented vertebrae including age, gender, bone mineral density, preoperative fracture severity, locations of IVC sign, distribution patterns of polymethylmethacrylate (PMMA), reduction rate, and reduction angle.
Assuming the increase of height loss more than 15% as a criterion of recollapse, only cleft filling pattern of PMMA in the IVC area was a significant risk factor for recollapse of the augmented vertebrae (P < 0.01). Assuming ≥10° progression of kyphotic angle as a criterion, cleft filling pattern of PMMA and higher values of reduction angle was as 2 significant risk factors for recollapse of the augmented vertebrae (P < 0.01). No significant difference was found in other clinical and radiological factors (P > 0.05).
Cleft filling pattern of PMMA and higher values of reduction angle may play an important role in inducing recollapse of the augmented vertebrae after PVP for OVCFs with the IVC. Careful observation of patients with these conditions is necessary to prevent deterioration of their clinical course.
Keywords: distribution patterns of PMMA, intravertebral vacuum cleft, locations of IVC sign, osteoporotic vertebral compression fractures, percutaneous vertebroplasty
1. Introduction
The intravertebral vacuum cleft (IVC) is not a rare phenomenon in osteoporotic vertebral compression fractures (OVCFs), having an incidence of 10% to 48%.[1,2] IVC is presented as an important risk factor for severe vertebral collapse, progressive kyphosis, intractable back pain, and even neurological deficit.[3,4] Hence, in order to restore spinal stability and obviate severe pain from the OVCFs with the IVC, percutaneous vertebroplasty (PVP) was widely recommended and had also achieved good outcomes at the initial follow-up.[5–7]
However, several studies[8–10] have reported a high incidence of recollapse of the augmented vertebrae after PVP for OVCFs with the IVC at long-term follow-up. To date, no critical factors related to recollapse of the augmented vertebrae after PVP have been clearly described. Some researchers[8–10] believed that the having had an IVC might be an important predisposing factor for recollapse. Moreover, in conjunction with previous reports,[11,12] the varied locations of an IVC include: adjacent to the superior endplate, or adjacent to the inferior endplate. Hence, we hypothesized that location of the IVC might have a significant factor in recollapse. Additionally, Li et al[13] reported that distribution patterns of polymethylmethacrylate (PMMA) in the IVC area might also have a significant effect on the stability of the augmented vertebrae, but they could not find a significant relationship with recollapse. Hence, the purpose of the present study was to determine factors related to recollapse of the augmented vertebrae after PVP for OVCFs with the IVC and evaluate their clinical significance.
2. Materials and methods
This study was designed to be performed retrospectively at our institute between January 2011 and December 2013.
2.1. Selection of patients
A total of 845 consecutive patients who underwent PVP to treat OVCFs were initially investigated during this interval. The inclusion criterion were as followed: a single-level osteoporotic vertebral fracture at the thoracolumbar region (T11-L1) and with no evidence of a previous adjacent osteoporotic vertebral fracture; magnetic resonance imaging (MRI) or computed tomography (CT) had been performed within 2 weeks (range, 0–14 days) before surgery; the affected vertebrae showed an IVC sign, which could be detected by CT or MRI; treatment with single-level PVP via bilateral portals; follow-up period of at least 2 year; no additional history of trauma after surgery; no complication after surgery, including leakage of PMMA into the spinal canal, or postoperative neurologic deficit; regular radiologic studies including preoperative, postoperative (immediately and at the final follow-up) images; and regular antiosteoporotic drug for 2 or more years (including calcitonin 600 mg/day, 1α-hydroxy vitamin D 0.5 μg/day and alendronate 70 mg weekly tablet). Exclusion criterions were severe trauma, known malignancies, neoplastic fractures, and spinal infections.[11] Finally, a total of 52 patients were enrolled in our study, with a female to male ratio of 40 to 12 males (mean, 75.21 years). All analyses were based on a retrospective study, no ethical approval and patient consent are required.
2.2. Operative procedure
All of PVP procedures were performed by 3 more than 10 years experienced spinal surgeons with standard trainings of PVP techniques. The PVP technique was adopted by using a transpedicular approach (bipedicular needle insertion) in an extended posture under local anesthesia (1% lidocaine). During the operation, 11 to 13-gauge bone biopsy needles were inserted parallel, or in a slightly descending course through the pedicle until the needle tip was optimally positioned in the IVC area. Then, the stylet was removed from the trocar and PMMA powder with sterile barium sulfate (Tianjin Synthetic Material Research Institute, Tianjin, China) was injected directly into the IVC area for complete filling of the cleft with maximizing stabilization of the fracture fragments.
2.3. Radiological assessment
All the radiological parameters would be measured twice by 2 more than10 years experienced spinal surgeons individually and independently to eliminate intra- and interobserver bias. In our study, for the measurement of all radiological parameters, the intra- and interobserver correlation coefficients was all excellent (correlation coefficients >0.82). However, 3 cases had a noticeable difference in preoperative and immediately postoperative vertebral height between both observers. In order to manage the bias, a 3rd more senile experienced evaluator was involved to have deciding vote. Additionally, the radiological report was also used to assist in the decision-making process if necessary. All radiologic measurements were checked digitally using the Picture Archiving and Communication System (PACS) and its related computer software at our department (M-viewTM, Marotech, Seoul, Korea).
2.3.1. An evaluation of the location of the IVC within the affected vertebrae
To evaluate the location of the IVC, preoperative magnetic resonance imaging (MRI) or computed tomography (CT) was performed. On CT, an IVC sign was identified by a linear, triangular, or irregular region of low density (gas) within a collapsed vertebral body. On MRI, an IVC usually shows as low signal intensity on T1-weighted images and high (fluid containing) or low signal (gas containing) on T2-weighted images. Additionally, a peripheral zone of hypointensity can be seen surrounding the hyperintensity on T-2 weighted images.[1,11] According to whether the IVC was adjacent to the superior or the inferior endplate, the IVC locations as: group 1, the IVC adjacent to the superior endplate; group 2, the IVC adjacent to the inferior endplate (Fig. 1).
Figure 1.
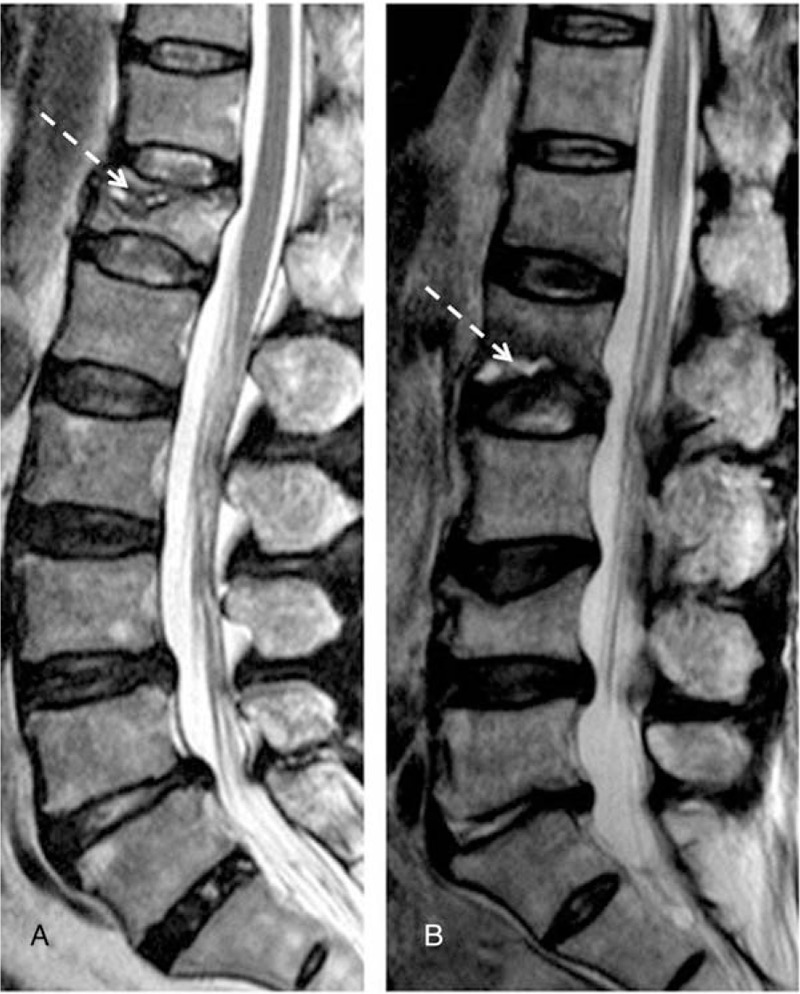
Two different locations of IVC sign on the sagittal T2-weighted image. (A) Adjacent to superior endplate; (B) adjacent to inferior endplate. IVC = intravertebral vacuum cleft.
2.3.2. An evaluation of distribution patterns of PMMA in the IVC area
The immediately postoperative radiological assessment on the sagittal CT was performed to evaluate the distribution patterns of PMMA within the affected vertebrae. According to whether PMMA was located only in the IVC area or infiltrated in the surrounding bone, PMMA distribution patterns in the IVC area as: group 1, the cleft filling pattern in which PMMA was located only in the IVC area and could not be sufficiently infiltrated into the surrounding cancellous bone; group 2, the interdigitated filling pattern in which PMMA could be sufficiently infiltrated into the surrounding cancellous bone in addition to be located in the IVC area (Fig. 2).
Figure 2.
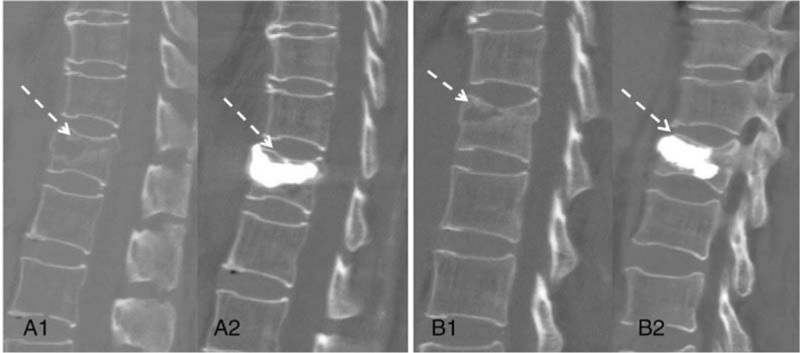
Two different distribution patterns of PMMA in IVC area on sagittal CT. (A1, A2) The local filled pattern of PMMA before and after PVP; (B1, B2) The interdigitated filled pattern of PMMA before and after PVP. CT = computed tomography, IVC = intravertebral vacuum cleft, PMMA = polymethylmethacrylate, PVP = percutaneous vertebroplasty.
2.3.3. An evaluation of fracture severity of the affected vertebrae
Based on the percentage of preoperative vertebrae collapse, fracture severity as: group 1, mild (<25% collapse); group 2, moderate (26%–40%), severe (>40%), respectively.[14,15]
2.3.4. An evaluation of preoperative T-score in bone mineral density (BMD)
On the 1st admission day, BMD scores of the lumbar vertebrae (L2–4) were determined using dual X-ray absorptiometry (DXA) (Hologic, Waltham, MA).
2.3.5. An evaluation of recollapse of the augmented vertebrae
To evaluate recollapse of the augmented vertebrae, we checked initial, immediately postoperative, and final follow-up plain radiograph. According to prior study reported by Ha and Kim,[14] 2 conditions were defined as progression of the augmented vertebrae: ≥15% progression of height loss between the immediately postoperative and last follow-up period; ≥10° progression of local kyphotic angle between the immediately postoperative and last follow-up period. Vertebral height and kyphotic angle was measured as previous literatures described by Linn et al[11] and Ha and Kim,[14] respectively. Vertebral height was measured at the point of maximal compression of the augmented vertebrae. The rate of vertebral compression at each follow-up period was measured as the rate of vertebral height of the augmented vertebrae to the mean vertebral height of the upper and lower vertebrae at the same site. Reduction rate was calculated using the difference between preoperative and immediately postoperative vertebral compression rate. The kyphotic angle would be measured using Cobb method between adjacent vertebrae, which was the angle between the upper endplate of the upper vertebral body and the lower endplate of the lower vertebrae. Reduction angle was calculated using the difference between the preoperative and immediately postoperative kyphotic angle.
2.4. Risk factors assessment
In total, 8 risk factors were evaluated including age, gender, preoperative T-score in BMD, preoperative fracture severity, the location of the IVC, distribution patterns of PMMA, reduction rate, and reduction angle in our study.
2.5. An assessment of clinical outcome
Clinical efficacy was assessed by 1 author (WBY) by using the visual analogue scale (VAS) scores for back pain evaluation (range, 0–10; 0, no pain at all; 10, worst pain imaginable) and the Oswestry disability index (ODI) for functional assessment. Preoperative and postoperative VAS and ODI scores were performed at the 1st admission day and 1st day after surgery, respectively; final follow-up VAS and ODI scores were performed at outpatient clinic.
2.6. Statistical analysis
SPSS 20.0 statistical software (SPSS, Inc., Chicago, IL) was used for analysis. Gender (male/female), fracture severity, the locations of the IVC, and distribution patterns of PMMA were modified to categorical variables. Qualitative characteristics of groups were expressed as the mean and standard deviation (SD) of data. The time to follow-up was reported as median and IQR. Age, T-score in BMD, reduction rate, and reduction angle were evaluated by the t test. VAS and ODI scores were compared between the 2 groups by means of Mann–Whitney U test preoperatively, postoperatively, and at last follow-up. Fisher exact test was performed for categorical variables. Univariate logistic regression analysis was 1st performed for each of 8 risk factors. Factors with a value of P < 0.2 and clinically significant variables (fracture level, the locations of the IVC, distribution patterns of PMMA, reduction rate, and reduction angle), regardless of their statistical significance, were included in the multivariate analysis. Odds ratios (ORs) for each condition of progressive recollapse and their 95% confidence intervals (CI) were calculated by multiple logistic regression test and backwald selection. A P < 0.05 was considered statistically significant.
3. Results
In total, 52 patients (M/F = 12:40) were reviewed. The average age of the patients was 75.21 ± 12.94 and the mean follow-up period ranged from 24 to 33 months (median, 26 months). During the follow-up period, conservative treatments were followed for all patients and no additional surgical intervention was needed for our included patients even in the recollapse group. The location of the IVC within the affected vertebrae was as follows: adjacent to the superior endplate in 41 patients, adjacent to the inferior endplate in 11 patients. The distribution patterns of PMMA in the IVC area was as follows: the cleft filling pattern in 9 patients, the interdigitated filling pattern in 43 patients.
3.1. ≥15% progression of height loss
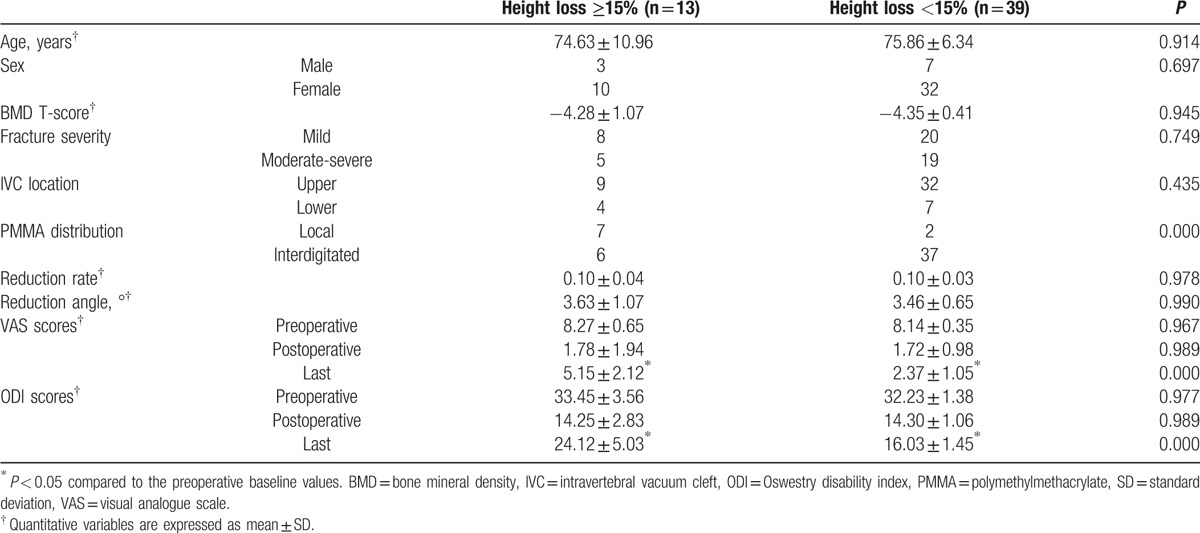
During the 2 years follow-up, 13 patients (26%, group Ah) were identified by ≥15% progression of height loss. Thirty-nine patients who showed <15% progression of height loss were assigned to group Bh. Patient characteristics for both groups were noted in Table 1. There was no significant difference regarding age, gender, BMD, fracture severity, and the location of the IVC between the 2 groups. However, Fisher exact test showed that distribution patterns of PMMA differed significantly between the 2 groups. At the immediately postoperative evaluation, there was no significant difference in reduction rate, reduction angle, the mean VAS, and ODI scores. However, at the final follow-up evaluation, the mean VAS and ODI scores in the group Ah were significantly higher than that in the group Bh; the mean VAS and ODI scores in both groups were still lower significantly than the preoperative baseline values.
Table 1.
Demographic data according to ≥15% progression of height loss.
Assuming the increase of height loss more than 15% compared to the immediately postoperative value as recollapse of the augmented vertebrae, univariate analysis showed that only distribution patterns of PMMA was significant (OR = 21.58, P = 0.001, Table 2). A multivariate regression analysis further revealed that distribution patterns of PMMA was as only a risk factor for recollapse of the augmented vertebrae (the cleft filling pattern, OR = 21.58, P = 0.001, Table 3). Progression of recollapse of the augmented vertebrae with the cleft filling pattern of PMMA in the IVC area was shown in Fig. 3 by analysis of radiological films from serial follow-ups.
Table 2.
Univariate logistic regression analysis for ≥15% progression of height loss.
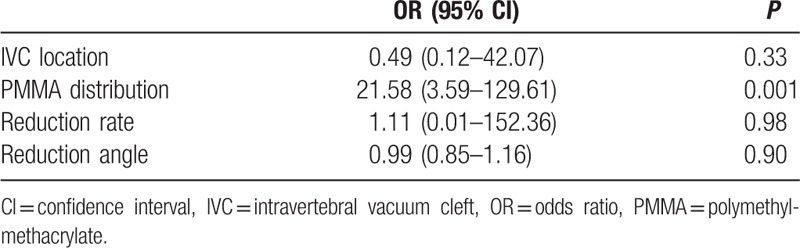
Table 3.
Outcome of multivariate logistic regression analysis.

Figure 3.
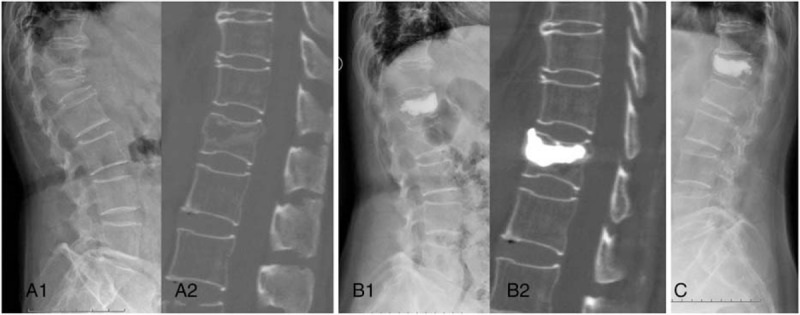
A 76-year-old female patient with an L1 osteoporosis vertebral compression fracture. (A1, A2) Preoperative sagittal X-ray and CT image demonstrated IVC sign was adjacent to inferior endplate; (B1, B2) immediately postoperative sagittal X-ray and CT image showed the local filled pattern of PMMA and reexpansion of compressed vertebrae; (C) a severe recollapse of the augmented vertebrae developed at last follow-up period. CT = computed tomography, IVC = intravertebral vacuum cleft, PMMA = polymethylmethacrylate.
3.2. ≥10% progression of kyphotic angle
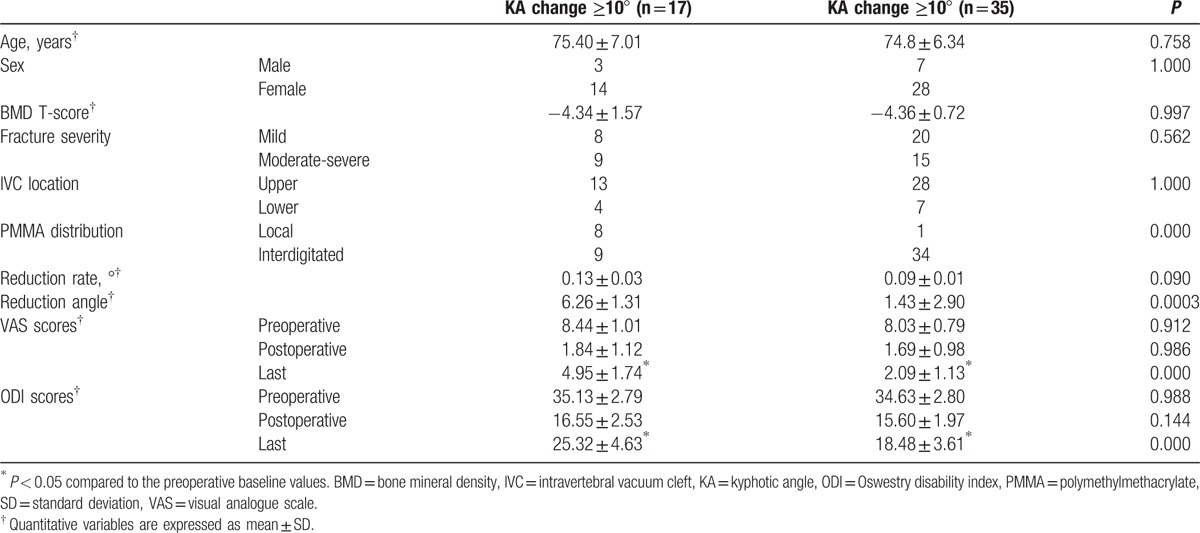
During the 2 years follow-up, 17 patients (32.7%, group Ak) were identified by ≥10% progression of kyphotic angle. Thirty-five patients who showed <10% progression of kyphotic angle were assigned to group Bk. Patient characteristics for both groups were noted in Table 4. There was no significant difference in age, gender, BMD, fracture severity, and the location of the IVC between the 2 groups. However, distribution patterns of PMMA showed a significant difference between the groups. At the immediately postoperative evaluation, there was no significant difference in reduction rate, VAS, and ODI scores. However, reduction angle was significantly higher in the group Ak and that in the group Bk (P = 0.0003). Additionally, at the final follow-up evaluation, the mean VAS and ODI scores were significantly higher in the group Ak than that in the group Bk; the mean VAS and ODI scores in both groups were still lower significantly than the preoperative baseline values.
Table 4.
Demographic data according to ≥10° progression of KA.
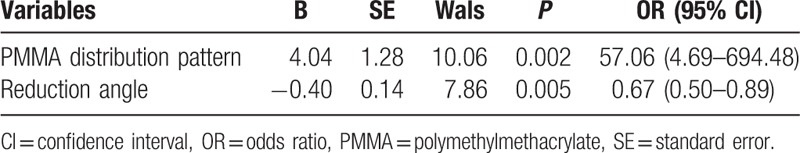
Assuming ≥10° progression of kyphotic angle compared to the immediately postoperative value as recollapse of the augmented vertebrae, univariate analysis showed distribution patterns of PMMA, reduction angle was significant (Table 5). A multivariate regression analysis also revealed significant difference in distribution patterns of PMMA (the cleft filling pattern, OR = 57.06, P = 0.002, Table 6) and reduction angle (higher values of reduction angle, OR = 0.67, P = 0.005, Table 6).
Table 5.
Univariate logistic regression analysis for ≥10° progression of kyphotic angle.
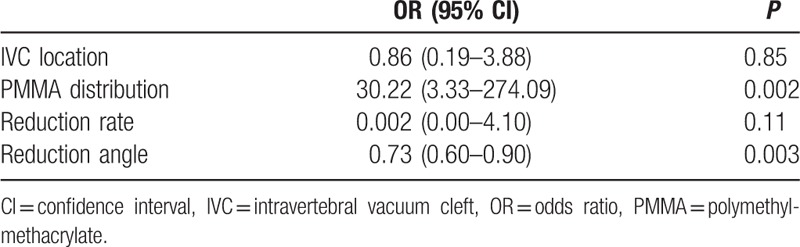
Table 6.
Outcome of multivariate logistic regression analysis.
4. Discussion
To date, the pathogenesis of IVC is unclear. According to previous radiological and histological studies,[12,15–17] it is mainly associated with osteonecrosis, nonunion, and pseudarthrosis after OVCFs. Based on dynamic mobility[18,19] in different body postures, the patients suffering from OVCFs with the IVC usually have severe back pain and do not respond to conservative treatments such as bed rest, medication, etc. Hence, it is necessary to be treated by surgical intervention with PVP to restore spinal stability and stop progressive collapse of the affected vertebrae.[5–7] Being consistent with previous studies, our study also showed that vertebral height and kyphotic angle were significantly corrected in both groups after PVP treatment. VAS and ODI scores were also significantly improved.
However, after 2 years follow-up, we found a high incidence of recollapse of the augmented vertebrae after PVP for OVCFs with the IVC (26% in ≥15% progression of height loss, 32.7% in ≥10% progression of kyphotic angle, respectively). Our results were compatible with prior studies.[8,10] Heo et al[8] reported that 6 out of 21 patients suffered a recollapse of the augmented vertebrae. Niu et al[10] also had a similar incidence (5 out of 15 patients). Meanwhile, similar with their studies, we found that the patients in the recollapse group (group Ah and group Ak) suffered from varying degrees of pain and dysfunction at the last follow-up, and the mean VAS and ODI scores were significantly higher than that in the nonrecollapse group (group Bh and group Bk). Hence, it was necessary to identity the relative risk factors for recollapse of the augmented vertebrae after PVP for OVCFs with the IVC in order to gain more clinical outcomes. Unfortunately, to the best of our knowledge, no critical factors have been clearly described.
According to our results, we demonstrated that that cleft filling pattern of PMMA was an important risk factor for recollapse of the augmented vertebrae by correlation analysis and multiple logistic regression analyses. Most patients in the cleft filling group suffered from a recollapse of the augmented vertebrae (7 out of 9 patients in ≥15% height loss, 8 out of 9 patients in ≥10% progression of kyphotic angle) after 2 years follow-up. We considered that the reason might be that the cleft filling pattern of PMMA may induce greater stress upon the already weaken surrounding cancellous bone, causing the significant recollapse of the “PMMA-nonsupported” area. Hence, in order to decrease recollapse of the augmented vertebral body, Niu et al[10] thought that cement injected should be sufficiently infiltrated into the surrounding cancellous bone in order to help improve the stability of the augmented vertebrae and reduce the stress upon the surrounding bone. Our result also supported their opinion. Our study found that rare patients in the interdigitated filling group had a recollapse (6 out of 43 patients in ≥15% height loss, 9 out of 43 patients in ≥10% progression of kyphotic angle).
In our study, another significant risk factor was higher values of reduction angle according to correlation analysis and multiple logistic regression analyses when assuming ≥10° progression of kyphotic angle for recollapse of the augmented vertebrae. This result was also consistent with previous opinion reported by Heo et al[20] and Kim et al.[21] They thought that too much restoration of kyphotic angle might cause increased paravertebral soft tissue tension, leading to increased mechanical loading on the augmented vertebrae or more instability in the fractured segment. Consequently, recollapse of the augmented vertebrae increased with a greater degree of kyphotic angle restoration. Other risk factors were found no significant association with recollapse of the augmented vertebrae in our study.
A major limitation of this study was that the number of cases was not large. We used strict criterion for patient selection and sought to evaluate results associated with the bony condition itself and to minimize extravertebral factors. Our efforts resulted in well-selected but small patient groups. Another limitation was that there is no gold standard to evaluate the recollapse of the augmented vertebrae. Although we took previous classification of Ha et al,[14] we believe that different classification criterion such as McKiernan method,[22] in which more than 4 mm anterior vertebral height loss was defined as a recollapse, might lead to different results for some cases. However, we believe that final results would not be significant. Another limitation was the inconsistency of last follow-up period for every subject in our study, wildly ranging from 24 to 37 months. As we know, the augmented vertebrae might have further recollapse if more follow-up time was performed. Finally, we did not examine any effect due to the filling material, because only PMMA was used. Well-designed and prospective studies using various filling materials would be helpful in terms of further evaluating recollapse of the augmented vertebrae after PVP.
5. Conclusion
According to our results, cleft filling pattern of PMMA and higher values of reduction angle may be 2 important factors related to inducing recollapse of the augmented vertebrae after PVP for OVCFs with the IVC. This recollapse may cause the patients to suffer from varying degrees of pain and dysfunction, and hence careful observation and follow-up of patients with these conditions is necessary to prevent deterioration of their clinical course.
Acknowledgments
The authors thank the Projects of The Health Ministry of China (NO.W2012ZT07, NO.W2014ZT256) and Guangdong Province Medical Science and Technology Research Program (NO.B2014175) for the support.
Footnotes
Abbreviations: BMD = bone mineral density, IVC = intravertebral vacuum cleft, ODI = Oswestry disability index, OR = odds ratio, OVCF = osteoporotic vertebral compression fracture, PMMA = polymethylmethacrylate, PVP = percutaneous vertebroplasty, VAS = visual analogue scale.
WY and DL contributed equally to this work.
Authorship: WY, DL, and XJ contributed to conceiving and designing the study. XJ, ZY, and WY performed the experiment. WY, LY, LY, and TQ analyzed the data. WY played main role in writing the manuscript.
Funding/support: This study was supported by Projects of The Health Ministry of China (NO.W2012ZT07, NO.W2014ZT256) and Guangdong Province Medical Science and Technology Research Program (NO.B2014175).
The authors have no conflicts of interest to disclose.
References
- [1].Libicher M, Appelt A, Berger I, et al. The intravertebral vacuum phenomen as specific sign of osteonecrosis in vertebral compression fractures: results from a radiological and histological study. Eur Radiol 2007;17:2248–52. [DOI] [PubMed] [Google Scholar]
- [2].Matzaroglou C, Georgiou CS, Wilke HJ, et al. Kummell's disease: is ischemic necrosis or vertebral “microcracking” the first step in the sequence? Med Hypotheses 2013;80:505. [DOI] [PubMed] [Google Scholar]
- [3].Sasaki Y, Aoki Y, Nakajima A, et al. Delayed neurologic deficit due to foraminal stenosis following osteoporotic late collapse of a lumbar spine vertebral body. Case Rep Orthop 2013;2013:682075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ito Y, Hasegawa Y, Toda K, et al. Pathogenesis and diagnosis of delayed vertebral collapse resulting from osteoporotic spinal fracture. Spine J 2002;2:101–6. [DOI] [PubMed] [Google Scholar]
- [5].Jang JS, Kim DY, Lee SH. Efficacy of percutaneous vertebroplasty in the treatment of intravertebral pseudarthrosis associated with noninfected avascular necrosis of the vertebral body. Spine (Phila Pa 1976) 2003;28:1588–92. [PubMed] [Google Scholar]
- [6].Becker S, Tuschel A, Chavanne A, et al. Balloon kyphoplasty for vertebra plana with or without osteonecrosis. J Orthop Surg (Hong Kong) 2008;16:14–9. [DOI] [PubMed] [Google Scholar]
- [7].Yang H, Gan M, Zou J, et al. Kyphoplasty for the treatment of Kummell's disease. Orthopedics 2010;33:479. [DOI] [PubMed] [Google Scholar]
- [8].Heo DH, Chin DK, Yoon YS, et al. Recollapse of previous vertebral compression fracture after percutaneous vertebroplasty. Osteoporos Int 2009;20:473–80. [DOI] [PubMed] [Google Scholar]
- [9].Fang X, Yu F, Fu S, et al. Intravertebral clefts in osteoporotic compression fractures of the spine: incidence, characteristics, and therapeutic efficacy. Int J Clin Exp Med 2015;8:16960–8. [PMC free article] [PubMed] [Google Scholar]
- [10].Niu J, Zhou H, Meng Q, et al. Factors affecting recompression of augmented vertebrae after successful percutaneous balloon kyphoplasty: a retrospective analysis. Acta Radiol 2015;56:1380–7. [DOI] [PubMed] [Google Scholar]
- [11].Linn J, Birkenmaier C, Hoffmann RT, et al. The intravertebral cleft in acute osteoporotic fractures: fluid in magnetic resonance imaging-vacuum in computed tomography? Spine (Phila Pa 1976) 2009;34:E88–93. [DOI] [PubMed] [Google Scholar]
- [12].Maldague BE, Noel HM, Malghem JJ. The intravertebral vacuum cleft: a sign of ischemic vertebral collapse. Radiology 1978;129:23–9. [DOI] [PubMed] [Google Scholar]
- [13].Li KC, Wong TU, Kung FC, et al. Staging of Kümmell's disease. J Musculoskel Res 2004;8:43–55. [Google Scholar]
- [14].Ha KY, Kim YH. Risk factors affecting progressive collapse of acute osteoporotic spinal fractures. Osteoporos Int 2013;24:1207–13. [DOI] [PubMed] [Google Scholar]
- [15].Zhu SY, Zhong ZM, Wu Q, et al. Risk factors for bone cement leakage in percutaneous vertebroplasty: a retrospective study of four hundred and eighty five patients. Int Orthop 2016. [DOI] [PubMed] [Google Scholar]
- [16].Sarli M, Perez Manghi FC, Gallo R, et al. The vacuum cleft sign: an uncommon radiological sign. Osteoporos Int 2005;16:1210–4. [DOI] [PubMed] [Google Scholar]
- [17].Dupuy DE, Palmer WE, Rosenthal DI. Vertebral fluid collection associated with vertebral collapse. AJR Am J Roentgenol 1996;167:1535–8. [DOI] [PubMed] [Google Scholar]
- [18].Kim DY, Lee SH, Jang JS, et al. Intravertebral vacuum phenomenon in osteoporotic compression fracture: report of 67 cases with quantitative evaluation of intravertebral instability. J Neurosurg 2004;100:24–31. [DOI] [PubMed] [Google Scholar]
- [19].McKiernan F, Jensen R, Faciszewski T. The dynamic mobility of vertebral compression fractures. J Bone Miner Res 2003;18:24–9. [DOI] [PubMed] [Google Scholar]
- [20].Heo DH, Chin DK, Yoon YS, et al. Refractures in cemented vertebrae after percutaneous vertebroplasty: a retrospective analysis. Eur Spine J 2008;17:592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim YJ, Lee JW, Kim KJ, et al. Percutaneous vertebroplasty for intravertebral cleft: analysis of therapeutic effects and outcome predictors. Skeletal Radio 2010;39:757–66. [DOI] [PubMed] [Google Scholar]
- [22].McKiernan F, Faciszewski T, Jensen R. Reporting height restoration in vertebral compression fractures. Spine 2003;28:2517–21. [DOI] [PubMed] [Google Scholar]


