Abstract
Rationale:
Neuromyelitis optica spectrum disorders (NMOSD) is considered as an immune-mediated disorder in the central nervous system (CNS). Numerous autoimmune diseases are frequently complicated with NMOSD and distinct clinical characteristics are noted in NMOSD patients with other autoimmune diseases. However, to our best knowledge, co-occurrence of NMOSD and thrombopenic purpura is rarely identified.
Patient concerns:
We presented a rare case of a 72-year-old female with 6-year history of thrombopenic purpura, and 1-month history of blurred vision as well as chest zonethesia. Anti-aquaporin-4 (AQP4) antibodies was positive in the serum of the patient.
Diagnoses:
With the addition of laboratory findings, iconography findings and physical examination results, the diagnosis of NMOSD was established according to the most recent diagnostic criteria.
Interventions and outcomes:
With the treatment of intravenous immunoglobulin (IVIg), the patient felt better at discharge without changing of expanded disability status scale (EDSS) score.
Lessons:
The case indicates that NMOSD could co-occur with thrombopenic purpura. The disturbance of immune system balance may explain this overlap. Further studies are warranted to reveal the mechanism and to explore whether patients with NMOSD with and without thrombopenic purpura have distinct clinical feature, drug responsiveness or prognosis.
Keywords: antiaquaporin-4 antibody, autoimmune disease, intravenous immunoglobulin, neuromyelitis optica spectrum disorders, thrombopenic purpura
1. Introduction
Neuromyelitis optica spectrum disorders (NMOSD) is considered as an immune-mediated disorder in central nervous system (CNS), mainly affecting the optic nerves and spinal cord. Aquaporin-4 (AQP4)-IgG is believed to be responsible in the pathogenesis of NMOSD, and the possible mechanisms includes complement-dependent cytotoxicity as well as antibody-dependent cellular cytotoxicity.[1] Cytokines, chemokines, and other molecular biomarkers of inflammation are also involved in the development of NMOSD.[2] The most recent diagnostic criteria of NMOSD is based on a combination of anti-AQP4 detection, clinical presentation, and MRI finding.[3] Expanded disability status scale (EDSS) is a widely used scale to evaluate the severity of multiple sclerosis,[4] however, it is applicable in NMOSD as well. According to the guideline proposed by the British Society of Haematology, the development of thrombopenic purpura is partly due to the platelet destruction mediated by autoantibodies targeting platelets.[5] The diagnosis of thrombopenic purpura is based on the medical history, results of physical examinations, complete blood count, and peripheral smear.[5] Although the mechanism remains unrevealed, approximately 25% of all NMOSD patients have other concomitant autoimmune disorders.[6,7] Nevertheless, the overlap of NMOSD and thrombopenic purpura has scarcely been reported.
2. Ethic statement
This study was approved by the ethics committee of the First Hospital of Jilin University, Changchun, China. Written informed consent was obtained from the patient.
3. Case presentation
We presented a case of a 72-year-old female who complained of reduced vision of right eye and severe chest zonesthesia for about 1 month. At the age of 66, the patient was diagnosed with thrombopenic purpura based on following findings: clinical findings (ecchymosis of left iliac skin, gum bleeding); blood routine examination findings (white blood cell count, 5.8 × 109/L, reference range: 4.0–10.0 × 109/L; red blood cell count, 4 × 1012/L, reference range: 3.5–5.0 × 1012/L; platelet count, 9 × 109/L, reference range: 100–300 × 109/L); myelogram findings (dysmaturity of macrophage, platelets were barely observed).
At present, she complained of reduced vision of right eye and severe chest zonesthesia for approximately 1 month. Physical examination was performed and her EDSS score was 6.5 (Table 1). The detection of anti-APQ4 IgG via a cell-based assay was performed (Euroimmun, Stockholm, Sweden). Briefly, the serum was incubated with the combinations of substrates. If the reaction was positive, antibodies of IgA, IgG, and IgM were binding to the antigens. In the next step, a fluorescence microscope was used to observe the attached antibodies, which were stained by fluorescein-labeled antibodies. The serum anti-APQ4 IgG of the patient was positive (Fig. 1A), and the negative control (without serum) is presented in Fig. 1B. The results of laboratory routine examinations including complete blood count, urine, coagulation, creatinine, electrolytes, liver function tests, fasting blood glucose, and serum lipid were normal. The level of serum folic acid, vitamin B12, tumor biomarkers, paraneoplastic biomarkers were within the reference range. The results of autoimmune diseases related blood examinations were normal as well. Valid evoking wave of right eye was absent on the pattern visual evoked potential examination, and unstable waves were observed of both eyes on flashed-elicited visual evoked potential examination. MRI of the spinal cord revealed a T2-hyperintense lesion (Fig. 2). As the lesion was not showed clearly on sagittal scans, only horizontal scans are presented in Fig. 2. No significant lesion was identified on brain MRI. The diagnosis of NMOSD could be established according to medical history, physical examination, laboratory examination, and MRI findings.[3] With the consideration of her medical history of bilateral necrosis of femoral heads and the unavailability of plasma exchange equipment in the hospital, intravenous immunoglobulin (IVIg) treatment at a dose of 0.4 g/kg daily was performed for 5 consecutive days. Though the EDSS score was not improved, the patient felt better at discharge.
Table 1.
Evaluation of expanded disability status scale score.
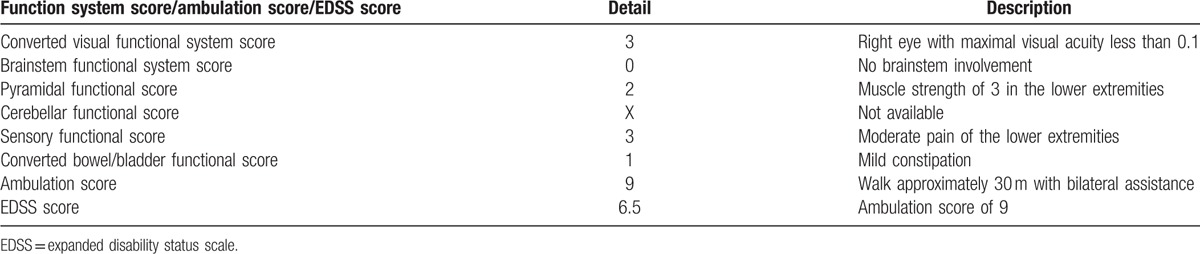
Figure 1.
Antiaquaporin-4 (AQP4) antibody detection. (A) The anti-AQP4 antibody was positive in the serum of the patient (red arrows). (B) No fluorescence was observed in negative control.
Figure 2.
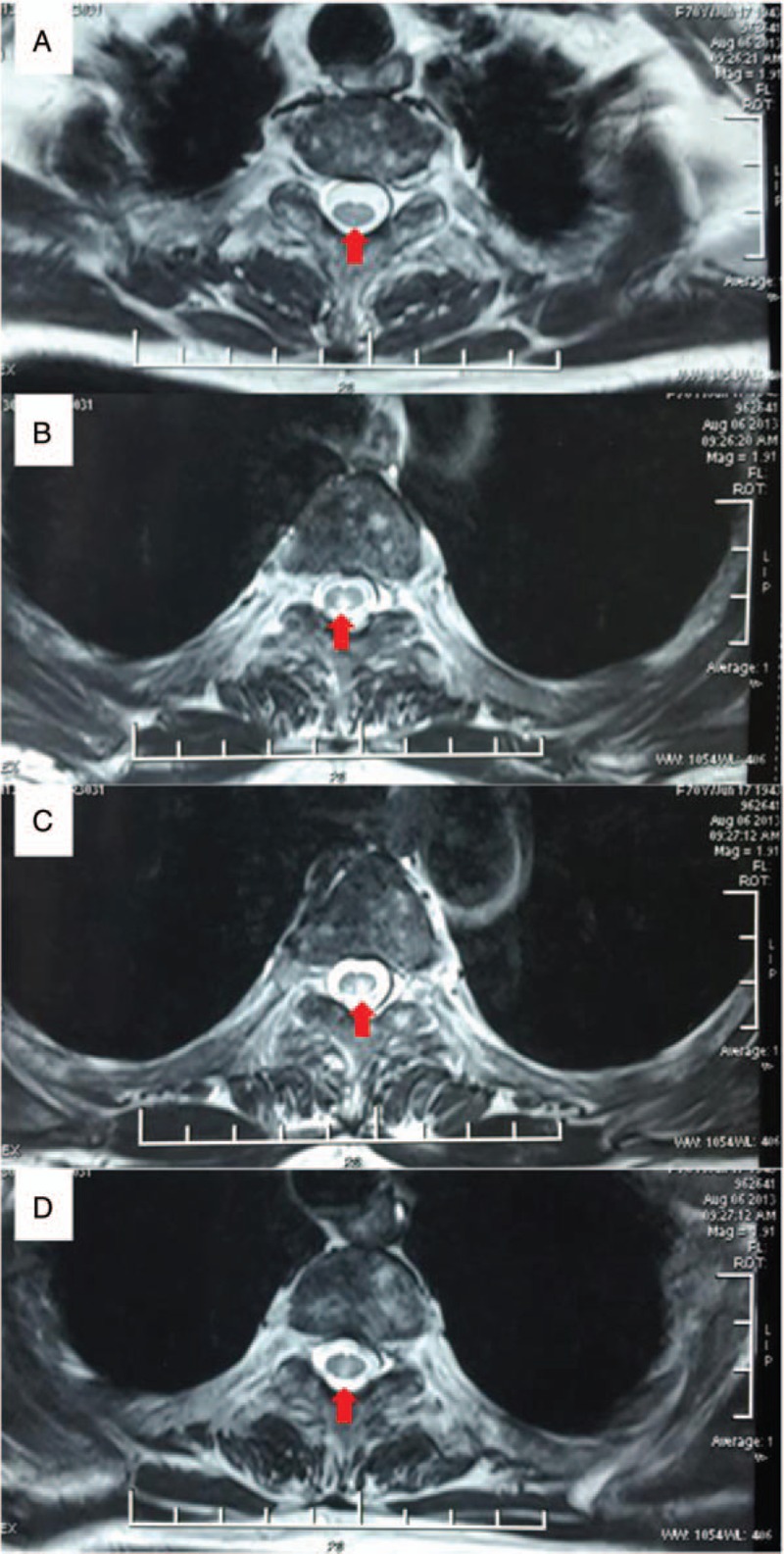
MRI of the spinal cord. MRI of spinal cord demonstrates abnormal signal intensity (red arrows) that extends from the level of the third to the sixth thoracic vertebrae in the horizontal plane.
4. Discussion
We have presented a rare case of anti-AQP4 positive NMOSD coexisting with thrombopenic purpura. With the combination of optic neuritis, acute myelitis, serum anti-AQP4 IgG positivity and exclusion of differential diagnosis, the diagnosis of NMOSD could be established according to the most recent diagnostic criteria.[3] A growing body of evidence demonstrates a close relationship between NMOSD and other autoimmune diseases.[6] Zhang et al[6] retrospectively compared the clinical features between NMOSD patients with and without autoimmune diseases, and concluded that NMOSD patients with coexisting autoimmune diseases had more brain lesions. Though multiple lines of evidence point out that NMOSD could complicate with various other autoimmune diseases and NMOSD with other autoimmune diseases may have distinct clinical features,[6,7] thrombopenic purpura has been rarely identified in NMOSD patients. Investigations to explain the association between NMOSD and other autoimmune diseases are not available currently; nevertheless, the disturbance of the immune system may be to blame. Both humoral and cell-mediated mechanisms contribute to the pathogenesis of thrombopenic purpura.[5] Antiplatelet antibody generation and Th0/Th1 cytokine profile imbalance play an important role in the development of thrombocytopenia.[5] Similarly, anti-AQP4 production and abnormal secretion of cytokines/chemokines are found responsible for the occurrence of NMOSD.[2] Thus, disorders of the immune system may serve as a bridge between NMOSD and thrombopenic purpura. Further studies are warranted to explain the association between NMOSD and thrombopenic purpura. Whether the NMOSD patients with and without thrombopenic purpura have distinct clinical features, drug responsiveness, or prognosis need to be further explored.
5. Summary
We presented a rare case of anti-AQP4 positive NMOSD coexisting with thrombopenic purpura. The disorders of immune system may be the mechanism of the overlap of thrombopenic purpura and NMOSD. Further studies are needed to explain this temporal association.
Footnotes
Abbreviations: AQP4 = aquaporin-4, CNS = central nervous system, EDSS = expanded disability status scale, IVIg = intravenous immunoglobulin, NMOSD = neuromyelitis optica spectrum disorders.
Funding: The work was supported by grants from the Elite PHD Program of Bethune Health Science Center, Jilin University (no. 470110000421).
The authors have no conflicts of interest to disclose.
References
- [1].Ratelade J, Verkman AS. Neuromyelitis optica: aquaporin-4 based pathogenesis mechanisms and new therapies. Int J Biochem Cell Biol 2012;44:1519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Melamed E, Levy M, Waters PJ, et al. Update on biomarkers in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm 2015;2:e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm 2015;85:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kurtzke JF. Natural history and clinical outcome measures for multiple sclerosis studies. Why at the present time does EDSS scale remain a preferred outcome measure to evaluate disease evolution? Neurol Sci 2000;21:339–41. [DOI] [PubMed] [Google Scholar]
- [5].Lo E, Deane S. Diagnosis and classification of immune-mediated thrombocytopenia. Autoimmun Rev 2014;13:577–83. [DOI] [PubMed] [Google Scholar]
- [6].Zhang BJ, Zhong Y, Wang YQ, et al. Neuromyelitis optica spectrum disorders without and with autoimmune disease. BMC Neurol 2014;14:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Freitas E, Guimaraes J. Neuromyelitis optica spectrum disorders associated with other autoimmune diseases. Rheumatol Int 2015;35:243–53. [DOI] [PubMed] [Google Scholar]


