Abstract
Colorectal neoplasm is considered to have a strong association with nonalcoholic fatty liver disease (NAFLD) and metabolic syndrome (MetS), respectively. The relationship among NAFLD, MetS, and colorectal neoplasm was assessed in 1793 participants. Participants were divided into 4 groups based on the status of NAFLD and MetS. Relative excess risks of interaction (RERI), attributable proportion (AP), and synergy index (SI) were applied to evaluate the additive interaction. NAFLD and MetS were significantly correlated with colorectal neoplasm and colorectal cancer (CRC), respectively. The incidence of CRC in NAFLD (+) MetS (+) group was significantly higher than other 3 groups. The result of RERI, AP, and SI indicated the significant additive interaction of NAFLD and MetS on the development of CRC. NAFLD and MetS are risk factors for colorectal neoplasm and CRC, respectively. And NAFLD and MetS have an additive effect on the development of CRC.
Keywords: colorectal neoplasm, metabolic syndrome, nonalcoholic fatty liver
1. Introduction
Colorectal cancer (CRC) is one of the most common cancers in the world, and studies suggested that the colorectal neoplasm in Asia has been increasingly prevalent over recent decades.[1] Moreover, CRC is one of the top leading causes of cancer-related deaths and also causes the low quality of life for survivors in the China.[2,3] It is widely accepted that colonoscopy is the first choice to diagnose and detect the CRC in common population,[4] which can significantly lower incidence and mortality of CRC.[5] However, people lacking the symptoms and risk factors usually do not undergo the examination because of the relatively high cost, especially in some developing countries. Thus, identifying a high-risk population has been an emergent event.
Nonalcoholic fatty liver disease (NAFLD) is defined as an accumulation of fat in the liver, and it is one of the most common chronic liver diseases in developed countries with certainly increasing speed.[6,7] NAFLD is a well known contributor to the development of the colorectal neoplasm.[8] Metabolic syndrome (MetS) is a disease composed of different metabolic derangements including central obesity, hypertension, hyperglycemia, dyslipidemia, and so on. In addition, MetS has also been proved to increase the risk of developing colorectal neoplasm.[8] There are several studies researching the association of NAFLD, MetS, and colorectal neoplasm, but few studies concentrate on the interactive effect of NAFLD and MetS on colorectal neoplasm. Therefore, the present study aims to investigate the combined effect of NAFLD and MetS on the development of colorectal neoplasm.
2. Patients and methods
The present study enrolled participants who accepted colonoscopy and other routine health status check up in our hospital from January 2011 to November 2015. Colorectal neoplasm includes benign neoplasm and malignant neoplasm, and the benign neoplasm included low- and high-grade adenomatoid polyp. Then the malignant neoplasm was defined as high-differentiated, middle-differentiated, and low-differentiated colorectal adenocarcinoma. At the beginning of the study, a total of 2241 participants were enrolled. Among these participants, 448 were excluded because of different reasons: 14 for incomplete colonoscopy, 13 for a history of polypectomy, 4 for inflammatory bowel disease, 30 for a history of cancer including CRC and other organs, 80 for viral hepatitis, and 307 for alcohol consumption >20 g/d. Finally, the study population consisted of 1793 participants. The present study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University, and informed consent was obtained from every subject.
2.1. Patients’ basic characteristics and laboratory measurements
Information on medical history, current use of medications, alcohol consumption, and family medical history were obtained by a standard questionnaire. Trained nurses measured the height and weight of all participants. Subject only wears a lightweight hospital gown and no shoes when measuring the height and weight. The body mass index (BMI) was calculated as kg/m2. Blood pressure was measured in rest state with a standard mercury sphygmomanometer.
Laboratory assay and measurements including alanine aminotransferase, aspartate aminotransferase, creatinine, platelet, hemoglobin, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and other related blood tests were performed for all the participants.
2.2. Colonoscopy
Colonoscopy was performed by experienced endoscopists with at least 5-year experience. Following 4 L polyethylene glycol lavage solution as bowel preparation, colonoscopy was performed on each subject. A complete examination was noted only if an endoscope reached the cecum, and otherwise it was regarded as incomplete examination. All the lesions were proven by biopsy. The differentiation of neoplasm was categorized as low-grade adenomatoid polyp, high-grade adenomatoid polyp, high-differentiated colorectal adenocarcinoma, middle-differentiated colorectal adenocarcinoma, and low-differentiated colorectal adenocarcinoma.[9]
2.3. Definition of NAFLD and MetS
All the participants routinely underwent hepatic ultrasonography scanning (Siemens; Munich, Germany) by experienced radiologists who were blinded to the result of colonoscopy. Exception of viral hepatitis, cirrhosis, liver cancer or other liver disease, and excess alcohol consumption, participants meeting specific ultrasonographic features including hepatomegaly, diffusely increased echogenicity of liver parenchyma, and blurring of vasculature were diagnosed as NAFLD.[10]
For MetS criteria, we utilized the guideline as proposed by the Diabetes Society of Chinese Medical Association in 2004. The MetS was defined as presence of equal or more than 3 of the following components: BMI ≥ 25 kg/m2, antihypertensive drug administration and (or) systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg, triglyceride ≥ 1.7 mmol/L and (or) HDL < 0.9 mmol/L (male), <1.0 mmol/L (female), and fasting plasma glucose ≥6.1 mmol/L or 2-hour postprandial glucose ≥7.8 mmol/L.
2.4. Statistical analysis
The statistical analysis was performed with the SPSS 19.0 (SPSS, Chicago, IL). The statistical results are presented as the mean ± standard deviation or percentages. Independent sample Student t test was used for continues variables and Chi-squared for categorical variables. The relationship of MetS and NAFLD with the presence of colorectal neoplasm or CRC was assessed, respectively, by multiple logistic regression analysis after adjustment for confounder variables, and the MetS-related factors including BMI, SBP, DBP, triglycerides, HDL, and fasting glucose were excluded even when they were significant in univariate analysis. Each odds ratio (OR) is presented together with its 95% confidence interval (CI). P < 0.05 was considered statistically significant.
In addition, logistic regression analysis was also performed to estimate the probability of the presence of colorectal neoplasm and the 95% CI for each risk factor category stratified by NAFLD and MetS, adjusting for age and sex. Meanwhile, the relative excess risk (RERI), attributable proportion (AP), and the synergy index (SI) were utilized to evaluate the interactive effect of NAFLD and MetS on the presence of colorectal neoplasm. The RERI evaluates the excess risk attributed to interaction relative to the risk without exposure. AP is used to measure the AP of the colorectal neoplasm, which was caused by an interactive effect in patients exposed to those 2 factors. In addition, SI represents the excess risk from exposure to those 2 factors when there is a biological interaction relative to the risk from exposure to both without interactive effect. When there is no additive interaction, RERI and AP include 0 or S includes 1. In addition, RERI > 0, AP > 0, or S > 1 is considered to have biological interaction.[11]
3. Result
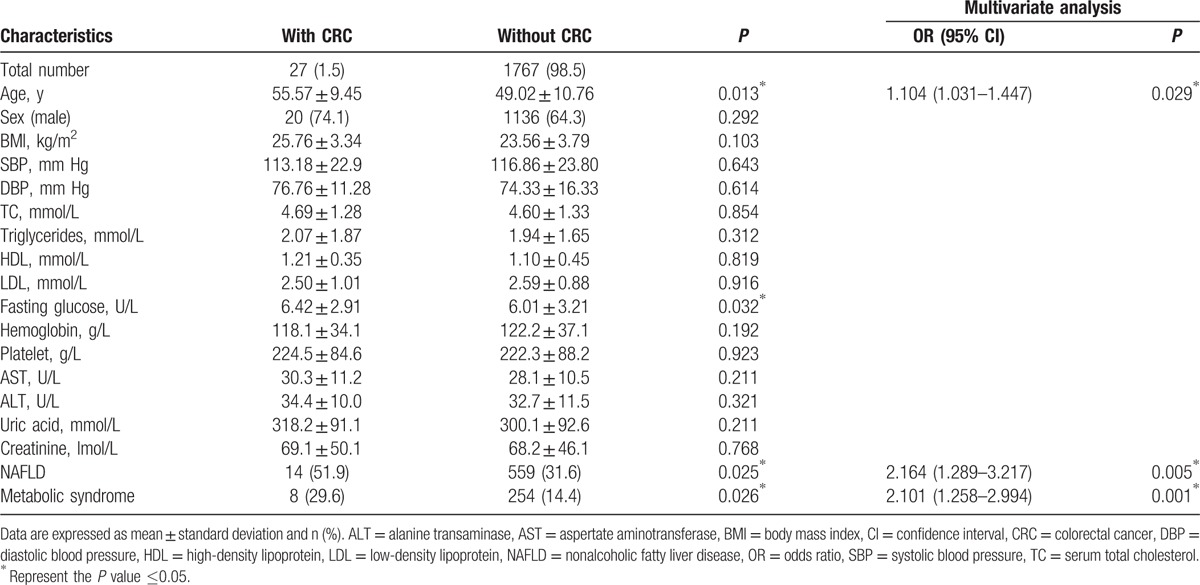
Among the 1793 subjects in the present study, 341 subjects (19.0%) were diagnosed as colorectal neoplasm, 27 subjects (1.5%) as CRC, 573 subjects (32.0%) as NAFLD, and 262 subjects (14.6%) as MetS. Compared to participants with or without colorectal neoplasm in Table 1, the subjects with colorectal neoplasm were more likely to be men and had higher BMI, SBP, triglyceride, fasting glucose, alanine transaminase (ALT), uric acid, and the presence of NAFLD and MetS. Then the multivariate analyses, adjusting with sex, age, ALT, and uric acid, were applied to further evaluate the relationship among NAFLD, MetS, and colorectal neoplasm. The result showed that NAFLD and MetS were still risk factors for colorectal neoplasm (95% CI, 1.352–2.871; OR, 2.113; P = 0.001 and 95% CI, 1.262–2.227; OR, 1.781; P = 0.001). Then we also evaluated the relationship among NAFLD, MetS, and CRC with univariate analysis, followed by multivariate analysis in Table 2. With the adjustment of age and sex, multivariate analysis indicated that NAFLD and MetS were also independent factors for CRC (95% CI, 1.289–3.217; OR, 2.164; P = 0.005 and 95% CI, 1.258–2.994; OR, 2.101; P = 0.001).
Table 1.
Univariate and multivariate analysis of the patients with or without colorectal neoplasm.
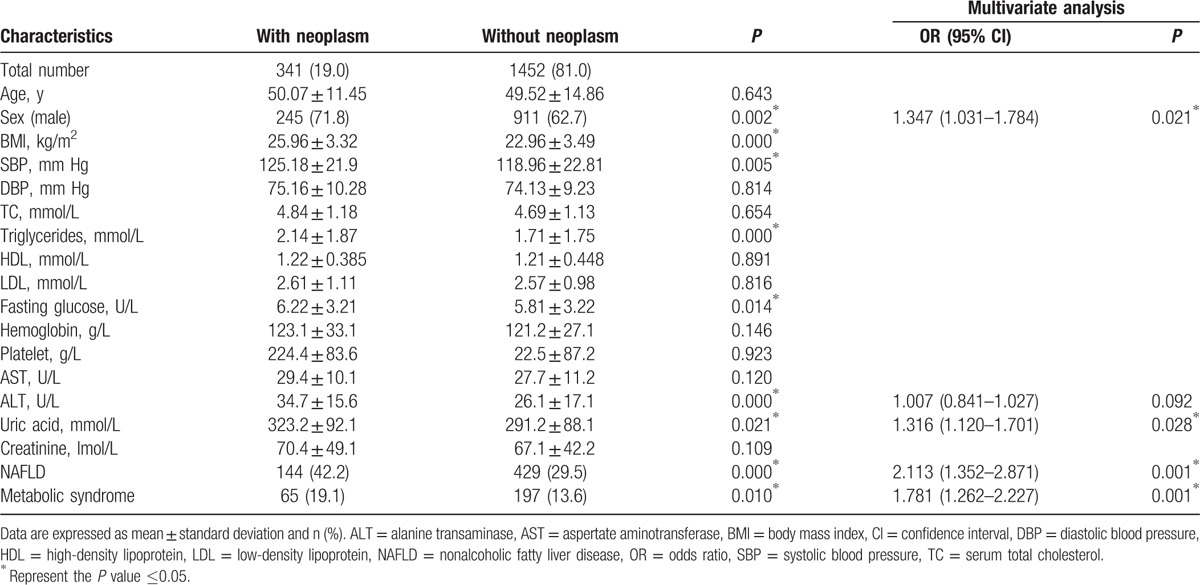
Table 2.
Univariate and multivariate analysis of the patients with or without CRC.
3.1. The potential interactive effect of NAFLD and MetS on colorectal neoplasm
The patients were divided into 4 groups basing on the status of NAFLD and MetS in Table 3, including NAFLD (−) MetS (−), NAFLD (−) MetS (+), NAFLD (+) MetS (−), and NAFLD (+) MetS (+) groups. The result showed that the probabilities of colorectal neoplasm in NAFLD (−) MetS (+), NAFLD (+) MetS (−), and NAFLD (+) MetS (+) groups were greater than that in NAFLD (−) MetS (−) group after adjusting for age and sex. However, the incidence of colorectal neoplasm had no difference among NAFLD (−) MetS (+), NAFLD (+) MetS (−), and NAFLD (+) MetS (+) groups (P = 0.796, 0.336, and 0.667). The similar preliminary result was observed among NAFLD, MetS, and CRC, and the probability of CRC in 3 groups was also higher compared with that in NAFLD (−) MetS (−) group. Furthermore, the incidence of CRC in NAFLD (+) MetS (+) group was significantly higher than NAFLD (−) MetS (+) and NAFLD (+) MetS (−) groups (P = 0.038 and 0.001).
Table 3.
Analysis of the risk for colorectal neoplasm and CRC by NAFLD and MetS status.
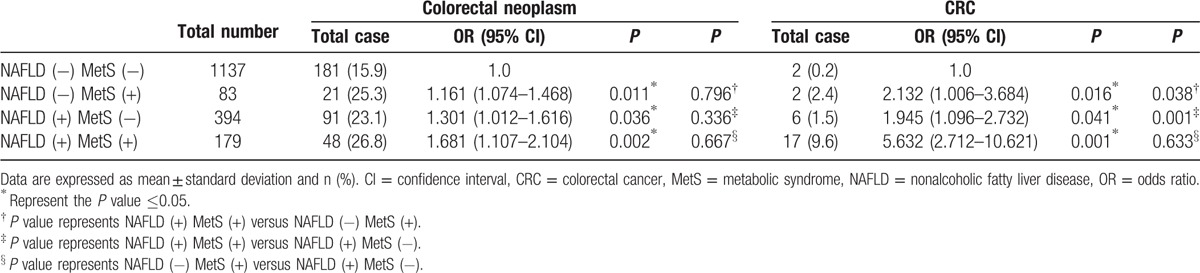
The RERI, AP, and SI for evaluation of the interaction between MetS and NAFLD for the risk of colorectal neoplasm were 0.123 (95% CI, −0.128 to 0.481), 0.019 (95% CI, −0.265 to 0.251), and 1.284 (95% CI, 0.922–3.187). There was no significant combined effect among NAFLD, MetS, and colorectal neoplasm, because RERI and AP included 0 or S included 1. In term of CRC, RERI was 3.313 (95% CI, 1.218–9.222), which indicated that there was a strong additive interaction between NAFLD and MetS on the presence of CRC, and there would be 3.313 RERI that was contributed by the additive interaction. AP was 0.713 (95% CI, 0.255–0.912), suggesting that 71.3% CRC exposed to the 2 risk factors was caused by the additive interaction of NAFLD and MetS. In addition, SI was 2.134 (95% CI, 1.122–7.897).
4. Discussion
This study aimed to investigate the potential interactive effect of NAFLD and MetS on the colorectal neoplasm. The influence of NAFLD or MetS on colorectal neoplasm was investigated, respectively, in the recent years. The NAFLD was found to have a strong association with colorectal neoplasm even with CRC.[12–14] Stadlmayr et al[15] collected data from 1382 consecutive Caucasians and found that NAFLD was an independent factor for early CRC. Lin et al[16] investigated 2858 patients and found that NAFLD was a risk factor for CRC. In term of MetS, previous studies also have reported that MetS was a risk factor for colorectal neoplasm.[12,17–19] Ahmed et al[20] investigated a large size of participants including 14,109 individuals and found that MetS had a positive association with incidence of CRC.[19] As there is a close association between NAFLD and the various component features of the MetS, including abdominal obesity, insulin resistance, dyslipidemia, and so on, NAFLD is often considered the hepatic manifestation of the MetS. Especially, insulin resistance was considered the critical linkage of NAFLD and MetS. However, it was found that NAFLD can occur independently of insulin resistance and the MetS.[21–23] Apart from this, there is also evidence for not only reciprocal causality between these 2 disease states, but also each acting as a perpetuating or exacerbating factor for the other, and therefore there is an apparent disconnection or dissociation between them.[24] In addition, there are other studies that have found the synergistic effect of NAFLD and MetS on the prostate gland and subclinical atherosclerosis.[25,26] Thus, the aim of the present study is to investigate the potential combined effect of NAFLD and MetS on colorectal neoplasm.[36–39]
The present study primarily found that NAFLD and MetS were independently associated with an increased frequency of both colorectal neoplasm and CRC with adjustment for confounders. Then the result showed that patients with NAFLD and MetS had the highest incidence of CRC with the greatest value of OR compared to other groups. The further results showed that there was a strong additive interaction of NAFLD and MetS on the presence of CRC. The patients with NAFLD and MetS were associated with a 3.313 times higher increased risk of CRC compared with those who had no NAFLD or MetS. Therefore, the increased risk of CRC prompted by the presence of NAFLD and MetS was significantly more than the interaction attributed to the either only NAFLD or MetS. Thus, this significant additive interaction indicates that MetS might attribute an extra risk of CRC for patients who also have NAFLD. To our knowledge, there is no prior study that focuses on the interactive effect of NAFLD and MetS on CRC. Hwang et al[12] suggested that NAFLD (+) MetS (+) group had a greater risk of colorectal adenomatous polyps than other groups because of the little higher OR value compared with other groups, but they did not research the CRC patients.
Currently, the mechanisms between the individual factor of NAFLD or MetS and colorectal neoplasms have not been clearly understood. It is well known that NAFLD represents a condition of profound insulin resistance, including increased insulin and insulin-like growth factor, which may promote the development of colorectal neoplasm via their proliferative and antiapoptotic effects.[27–30] It is widely accepted that NAFLD patients have adipocytokines metabolism disorders, which was considered to influence the development of the colorectal neoplasm.[28,29,31–34] In addition, inflammatory cytokines may also play a critical role in the NAFLD inducing colorectal neoplasm.[35] When it comes to the relationship between MetS and colorectal neoplasm, the reasonable explanation is that dysregulation of growth signals, inflammation cytokines, and vascular integrity factors may mediate the relationship and finally prompt tumorigenesis.[36] Our study found the additive effect of NAFLD and MetS on CRC, and we hypothesized the potential mechanism as follows. Inflammation is the important mechanism underlying the pathophysiology both for MetS and NAFLD, so it may be a critical role in the synergistic effect on CRC. Specifically, the mechanism may include the release of free fatty acids and other inflammatory mediators by the adipose tissue.[37] Furthermore, adipocytes also secrete monocyte chemotactic protein-1 that attracts macrophages and causes local inflammation as well as the release of further cytokines powering this system.[38] Inflammation has been proved as an important risk factor for the development of CRC.[39,40] Consequently, the presence of NAFLD and MetS in meantime may increase the risk of development of CRC. However, the mechanism by which the NAFLD and MetS cause local inflammation in colorectum needs to be further studied.
The present study also had several limitations. First, the cross-sectional study design may not provide evidence to explain the potential mechanism of the combined interaction of NAFLD and MetS on the presence of CRC. Second, it is a retrospective study, so there may have been a selection bias. Third, NAFLD in our study is based on ultrasound imaging examination that is the most prevalent method of diagnosing NAFLD in clinical practice. Then, the number of CRC patients is relatively small and the study is a single-center research, so the result needs to be further investigated in a multiple-center research with a large size of participants. Finally, this study does not evaluate the potential interactive effect of NAFLD and MetS on the prognosis of CRC.
5. Conclusion
The present study suggests that NAFLD and MetS are risk factors for increasing the risk of colorectal neoplasm and CRC, respectively. In addition, MetS may increase the risk of CRC in patients with NAFLD. Thus, patients with both NAFLD and MetS are recommended to undergo colonoscopy examination regularly.
Footnotes
Abbreviations: AP = attributable proportion, BMI = body mass index, CRC = colorectal cancer, DBP = diastolic blood pressure, HDL = high-density lipoprotein, MetS = metabolic syndrome, NAFLD = nonalcoholic fatty liver disease, RERI = relative excess risk, SBP = systolic blood pressure, SI = synergy index.
Funding/support: The study was supported by the Zhejiang Provincial Natural Science Foundation of China (LQ17H030004).
The authors have no conflicts of interest to disclose.
References
- [1].Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–9. [DOI] [PubMed] [Google Scholar]
- [2].Fang JY, Dong HL, Sang XJ, et al. Colorectal cancer mortality characteristics and predictions in China, 1991–2011. Asian Pac J Cancer Prev 2015;16:7991–5. [DOI] [PubMed] [Google Scholar]
- [3].Wang JW, Sun L, Ding N, et al. The association between comorbidities and the quality of life among colorectal cancer survivors in the People's Republic of China. Patient Prefer Adherence 2016;10:1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dominic OG, McGarrity T, Dignan M, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol 2009;104:2626–7. author reply 2628–29. [DOI] [PubMed] [Google Scholar]
- [6].Marignani M, Angeletti S. Nonalcoholic fatty liver disease. N Engl J Med 2002;347:768–9. author reply 768–9. [PubMed] [Google Scholar]
- [7].Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology 2003;37:1202–19. [DOI] [PubMed] [Google Scholar]
- [8].Muhidin SO, Magan AA, Osman KA, et al. The relationship between nonalcoholic fatty liver disease and colorectal cancer: the future challenges and outcomes of the metabolic syndrome. J Obes 2012;2012:637538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sung JJ, Lau JY, Young GP, et al. Asia Pacific consensus recommendations for colorectal cancer screening. Gut 2008;57:1166–76. [DOI] [PubMed] [Google Scholar]
- [10].Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res 2012;2012:145754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Knol MJ, VanderWeele TJ, Groenwold RH, et al. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol 2011;26:433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hwang ST, Cho YK, Park JH, et al. Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps. J Gastroenterol Hepatol 2010;25:562–7. [DOI] [PubMed] [Google Scholar]
- [13].Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut 2011;60:829–36. [DOI] [PubMed] [Google Scholar]
- [14].Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol 2012;27:91–5. [DOI] [PubMed] [Google Scholar]
- [15].Stadlmayr A, Aigner E, Steger B, et al. Nonalcoholic fatty liver disease: an independent risk factor for colorectal neoplasia. J Intern Med 2011;270:41–9. [DOI] [PubMed] [Google Scholar]
- [16].Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep 2014;41:2989–97. [DOI] [PubMed] [Google Scholar]
- [17].Fiori E, Lamazza A, De Masi E, et al. Association of liver steatosis with colorectal cancer and adenoma in patients with metabolic syndrome. Anticancer Res 2015;35:2211–4. [PubMed] [Google Scholar]
- [18].Trabulo D, Ribeiro S, Martins C, et al. Metabolic syndrome and colorectal neoplasms: an ominous association. World J Gastroenterol 2015;21:5320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kabat GC, Kim MY, Peters U, et al. A longitudinal study of the metabolic syndrome and risk of colorectal cancer in postmenopausal women. Eur J Cancer Prev 2012;21:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ahmed RL, Schmitz KH, Anderson KE, et al. The metabolic syndrome and risk of incident colorectal cancer. Cancer 2006;107:28–36. [DOI] [PubMed] [Google Scholar]
- [21].Niebergall LJ, Jacobs RL, Chaba T, et al. Phosphatidylcholine protects against steatosis in mice but not non-alcoholic steatohepatitis. Biochim Biophys Acta 2011;1811:1177–85. [DOI] [PubMed] [Google Scholar]
- [22].Jacobs RL, Zhao Y, Koonen DP, et al. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J Biol Chem 2010;285:22403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shen J, Wong GL, Chan HL, et al. PNPLA3 gene polymorphism accounts for fatty liver in community subjects without metabolic syndrome. Aliment Pharmacol Ther 2014;39:532–9. [DOI] [PubMed] [Google Scholar]
- [24].Wainwright P, Byrne CD. Bidirectional relationships and disconnects between NAFLD and features of the metabolic syndrome. Int J Mol Sci 2016;17:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Russo GI, Cimino S, Castelli T, et al. Benign prostatic hyperplasia, metabolic syndrome and non-alcoholic fatty liver disease: is metaflammation the link? Prostate 2016;76:1528–35. [DOI] [PubMed] [Google Scholar]
- [26].Hong HC, Hwang SY, Ryu JY, et al. The synergistic impact of nonalcoholic fatty liver disease and metabolic syndrome on subclinical atherosclerosis. Clin Endocrinol (Oxf) 2015;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [27].Wong VW, Hui AY, Tsang SW, et al. Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2006;4:1154–61. [DOI] [PubMed] [Google Scholar]
- [28].Wong VW, Wong GL, Tsang SW, et al. Genetic polymorphisms of adiponectin and tumor necrosis factor-alpha and nonalcoholic fatty liver disease in Chinese people. J Gastroenterol Hepatol 2008;23:914–21. [DOI] [PubMed] [Google Scholar]
- [29].Hui JM, Hodge A, Farrell GC, et al. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004;40:46–54. [DOI] [PubMed] [Google Scholar]
- [30].Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr 2001;131(11 suppl):3109S–20S. [DOI] [PubMed] [Google Scholar]
- [31].Ogunwobi OO, Beales IL. The anti-apoptotic and growth stimulatory actions of leptin in human colon cancer cells involves activation of JNK mitogen activated protein kinase, JAK2 and PI3 kinase/Akt. Int J Colorectal Dis 2007;22:401–9. [DOI] [PubMed] [Google Scholar]
- [32].Saxena A, Chumanevich A, Fletcher E, et al. Adiponectin deficiency: role in chronic inflammation induced colon cancer. Biochim Biophys Acta 2012;1822:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Procaccini C, Galgani M, De Rosa V, et al. Leptin: the prototypic adipocytokine and its role in NAFLD. Curr Pharm Des 2010;16:1902–12. [DOI] [PubMed] [Google Scholar]
- [34].Tilg H, Diehl AM. NAFLD and extrahepatic cancers: have a look at the colon. Gut 2011;60:745–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004;92:347–55. [DOI] [PubMed] [Google Scholar]
- [36].Hursting SD, Hursting MJ. Growth signals, inflammation, and vascular perturbations: mechanistic links between obesity, metabolic syndrome, and cancer. Arterioscler Thromb Vasc Biol 2012;32:1766–70. [DOI] [PubMed] [Google Scholar]
- [37].Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 2014;59:1174–97. [DOI] [PubMed] [Google Scholar]
- [38].Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl Res 2016;167:257–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Erreni M, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) and inflammation in colorectal cancer. Cancer Microenviron 2011;4:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol 2013;35:229–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


