Abstract
A total of 184 cases of surgically treated male prolactinoma were analyzed retrospectively to summarize the outcome of this surgical intervention. We analyzed the general characteristics, clinical manifestations, hormone levels, imaging features, preoperative treatments, surgical outcomes, pathology results, and follow-up records for all included patients. The most common clinical manifestations included sexual dysfunction (47.4%), headache (55.9%), and visual disturbance (46.7%). Serum prolactin levels ranged from 150 to 204,952 ng/mL. Tumor size varied from 6 to 70 mm. Pituitary adenomas grew in a parasellar pattern with visual deficits occurring 40.7% of the time. After surgical therapy, 88.6% of patients achieved symptom relief, and 98.4% experienced an immediate postoperative decline in prolactin level. Fifty-seven patients (31.0%) achieved initial remission, and 26 patients (45.6%) experienced recurrence. Hence, our results suggest that in male prolactinoma characterized by a large pituitary diameter and high serum prolactin level, tumor size predicts the degree of gross resection. The prognostic predictors included preoperative tumor growth pattern and Ki-67 index.
Citation: Yi-jun S, Mei-ting C, Wei L, Bing X, Yong Y, Ming F, Ren-zhi W. (2016) Surgical treatment for male prolactinoma: a retrospective study of 184 cases
Keywords: male, prolactinoma, surgery procedures, treatment outcome
1. Introduction
Pituitary adenoma is one of the most common benign intracranial tumors and has had increasing incidence rates in recent years. Approximately 40% to 50% of functional hypophysis adenomas are prolactinomas.[1] Recent epidemiology research found the incidence of prolactinoma to be 35–50/100,000. Most prolactinomas are found in women aged 20 to 50 years,[1] and its unique clinical manifestations and tumor characteristics are less commonly seen in males. Dopamine agonists have been the 1st-line treatment for prolactinoma since the 1970s.[1–3] Transsphenoidal surgery is accepted as the 2nd-line treatment for dopamine agonists-intolerant patients or patients with severe symptoms, such as rapid vision loss.[3] Therefore, we retrospectively analyzed the clinical manifestations and tumor characteristics of prolactinomas in male patients in Peking Union Medical College Hospital (PUMCH) to describe the treatment outcomes and prognostic factors of surgical therapy.
2. Subjects and methods
2.1. Preoperative information
The Institutional Review Board (IRB) of PUMCH has reviewed our protocol and approved our study. We summarized the general characteristics, clinical manifestations, hormone levels, imaging features, preoperative treatments, surgical outcomes, pathology results, and follow-up records of 184 cases of surgically treated prolactinoma in males in PUMCH between January 1990 and December 2014 retrospectively by record review from January to May 2016. The diagnosis of prolactinoma was postoperatively confirmed by pathological and immunohistochemical analyses of the tumor.
Baseline levels of prolactin (PRL), cortisol, 24-hour urinary-free cortisol, adrenocorticotropic hormone (ACTH), growth hormone (GH), testosterone, thyroid hormone, and thyroid-stimulating hormone were measured in all patients. Elevation of GH was defined as GH ≥ 2 ng/mL. Hyperprolactinemia was defined as PRL higher than 200 ng/mL.
We recorded microadenoma (maximum diameter <10 mm), macroadenoma (>10 mm), and giant adenoma (>40 mm) from magnetic resonance imaging (MRI) reports. Invasive adenoma was defined as Knosp grade III or IV. Tumors were also sorted based on growth pattern.
Bromocriptine sensitivity tests were conducted for all patients. Bromocriptine (2.5 mg) was orally administrated. The venous blood was collected to measure PRL level before bromocriptine administration and at internals of 1 hour for the next 5 hours after bromocriptine administration. Bromocriptine sensitivity was determined by PRL level after 300 minutes.[4] Patients with decline of PRL level higher than 50% was indicated to be responders for bromocriptine and was suggested for medical treatment while others were insensitive to bromocriptine and may consider 2nd line therapy.
Indications for surgery included insensitivity for bromocriptine and intolerance for medical therapy. Some patients urged for surgery for poor adherence of medical therapy. The treatment algorithm for both initial and recurrent prolactinoma was summarized in Figs. 1 and 2.
Figure 1.
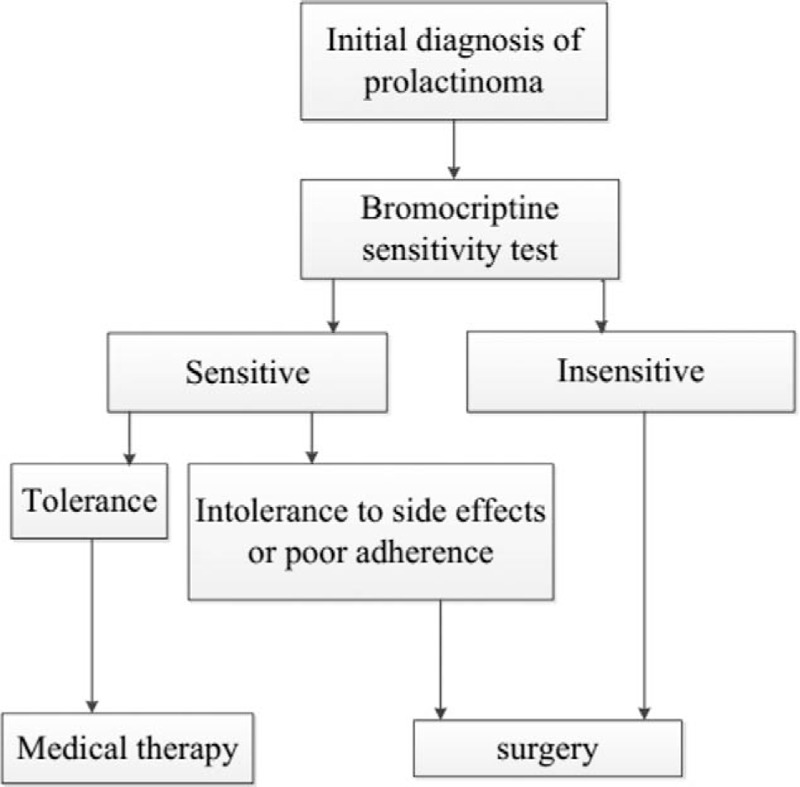
Treatment algorithm for initial prolactinoma.
Figure 2.
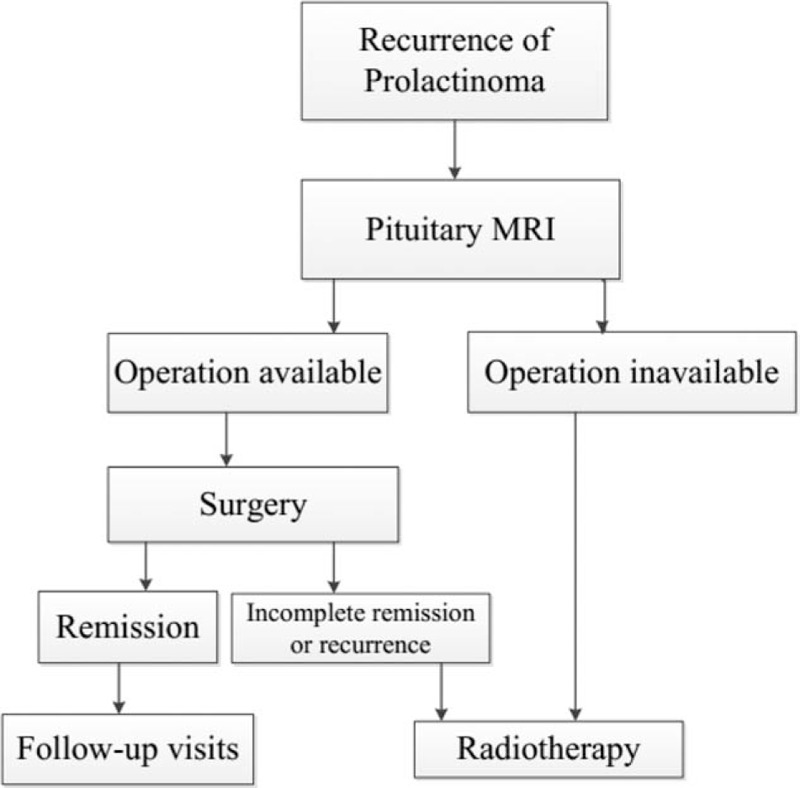
Treatment algorithm for recurrent prolactinoma.
2.2. Surgical outcomes and follow-ups
We measured PRL levels and performed enhanced pituitary MRI immediately, 3 months, 6 months, and 1 year after surgery. The symptoms and extent of tumor remission were observed and recorded in the clinic. Symptom improvement was defined as an improvement in more than 1 symptom. Initial remission was defined as a measured PRL level that had returned to normal (<13 ng/mL) immediately after surgery with the disappearance of all symptoms after total adenoma excision. Follow-up remission was defined as a PRL level within normal limits and total excision confirmed by MRI 1 year after surgery. Recurrence was defined as symptom relapse or tumor reappearance confirmed by MRI after initial remission.
2.3. Statistical analysis
Student t tests, chi-squared tests, and logistic regression and correlation analyses were performed to compare continuous and discrete variables using the statistical software SPSS 19.0. We performed t test for hypothesis that gross resection was more likely to perform for microadenoma as well as lower PRL level. Chi-squared test was performed for the relationship of tumor growth pattern and recurrence. For the relation of Ki-67 index, tumor size, and recurrence, we also performed chi-squared test hypothesized that the higher Ki-67 index and larger tumor size, the higher risk for recurrence. We wondered whether larger tumor size may present higher PRL level, thus we analyzed the relationship of tumor size and preoperative PRL level by correlation analysis. For follow-up visits, we analyzed the relation of postsurgical PRL concentration and recurrence time by t test.
3. Results
3.1. Baseline information
The mean age of patients was 36.3 years old and ranged from 11 to 72 years old. The mean disease duration was 41.9 months and ranged from 5 days to 312 months.
3.2. Clinical manifestations
Prolactinoma patients present with the clinical manifestations of hyperprolactinemia and mass effects on neighboring structures due to macroadenoma. For hyperprolactinemia in males, 88 (47.4%) men presented with libido reduction, and 43 (23.4%) men complained of loss of facial, pubic, and axillary hair. Galactorrhea and gynecomastia were uncommon in these cases of male prolactinoma. Our research shows that only 17 (9.2%) men suffered from galactorrhea, and 16 (8.7%) men presented with gynecomastia. In addition, 4 patients had erectile dysfunction, 2 patients presented with delayed puberty, 1 patient had osteoporosis, and 1 patient suffered from infertility. As for compressive effects, headache was the most common symptom, present in 55.9% (103/184) of patients. Eighty-six (46.7%) patients complained of visual impairment. Thirty-five patients presented with visual field defects. Seventeen patients presented with temporal hemianopsia on 1 side, and 17 presented with bitemporal hemianopsia. Only 1 patient suffered from nasal hemianopsia on 1 side. Spontaneous cerebrospinal fluid (CSF) rhinorrhea was reported in 5 patients. Other symptoms due to mixed prolactinoma included acromegaly or gigantism (9.2%, n = 17), hypothyroidism (1.1%, n = 2), hyperthyroidism (2.2%, n = 4), and Cushing syndrome (0.5%, n = 1).
3.3. Hormone evaluation
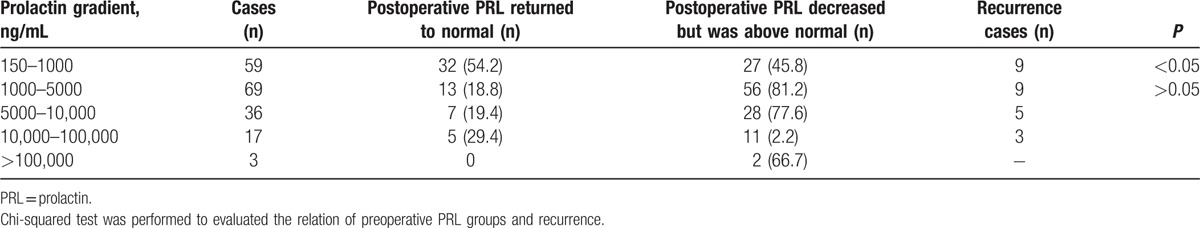
The mean PRL level was 6735.2 ng/mL, ranging from 150 to 204,952 ng/mL. PRL levels were sorted into different ranges, as shown in Table 1. Three patients presented elevated PRL level after surgery. GH was elevated in 17 patients, hyperthyroidism was found in 5 patients, and hypothyroidism was found in 4 patients. Serum cortisol levels were increased in 7 patients, 24-hour urinary-free cortisol was elevated in 6 patients, and ACTH was elevated in 2 patients. Testosterone was decreased in 106 cases.
Table 1.
Preoperative prolactin and recurrence in male prolactinoma, n (%).
3.4. Imaging evaluations
Among 184 cases of male prolactinoma, the mean maximum tumor diameter was 26.3 mm, ranging from 6 to 70 mm. Microadenoma accounted for 3.3% (6 cases), while 152 patients presented with macroadenoma, and 26 patients had giant adenoma. Invasive adenoma was accounted for 33.1% (61 cases). Fifteen patients had cystic degeneration, and 16 patients suffered from pituitary apoplexy. Moreover, tumors were sorted based on growth pattern. Forty tumors were intrasellar, 17 tumors were suprasellar without visual deficits, and 75 tumors were suprasellar with visual deficits. Twenty tumors grew in a parasellar or sphenoidal pattern. Thirty-two tumors were giant tumors or extended to both intrasellar and parasellar regions.
3.5. Preoperative treatments
Sixty-two patients were found to be insensitive to bromocriptine therapy. Among bromocriptine responders, 79 patients were treated irregularly, while 38 patients accepted bromocriptine treatment for a mean course of 5.0 months. Course duration ranged from 3 weeks to 36 months, and dose varied from 2.5 to 20 mg per day. Although PRL decreased to a normal level in 6 patients, these patients still opted for surgery rather than undergo a long course of medical treatment. Twenty-two patients were insensitive to bromocriptine, 1 patient relapsed, and 2 patients discontinued treatment because of adverse side effects. Five patients received carbergoline with courses ranging from 1 week to 9 months and doses ranging from 0.25 to 1 mg 2 to 3 times per week. In all 5 cases, PRL did not return to normal. Six patients received radiotherapy, and 1 patient exhibited central diabetes insipidus after radiotherapy.
3.6. Surgery and complications
The 184 transsphenoidal microsurgeries were performed by experienced surgeons. Of these cases, 149 patients succeeded in total removal of the adenoma, while 35 excisions were partial. Intraoperatively, 24 patients exhibited cystic degeneration or apoplexy, and 16 patients suffered from CSF leakage requiring immediate repair. Sixteen patients experienced postsurgical complications, including 6 cases of CSF rhinorrhea, 2 cases of central nerve system infection, 3 cases of hypopituitarism, 3 cases of oculomotor nerve palsy and blindness, 10 cases of central diabetes insipidus, and 2 cases of sellar hematoma.
3.7. Tumor characteristics
Most prolactinomas in males were macroadenomas, accounting for 82.6% (n = 152); 14.1% (n = 26) of cases were giant adenomas. Preoperative PRL levels were not correlated with tumor size (r = 0.203, P > 0.05), as indicated by logistic regression. As for histopathological results, out of 99 cases of mixed prolactinoma, GH-prolactinoma accounted for 26.1% (n = 48), but only 17 exhibited clinical manifestations of acromegaly or gigantism and serum GH elevation. GH decreased to within normal limits in 10 patients after surgery. Only 1 case of ACTH-prolactinoma and 3 cases of thyroid-stimulating hormone-prolactinoma had consistent corresponding clinical and hormonal manifestations; there was no clinical consistency in the FSH-prolactinoma and LH-prolactinoma cases. Table 2 shows the relationship between Ki-67 index and prolactinoma recurrence. The most favorable recurrence rate, which increased with increasing Ki-67 index (P < 0.001), was detected in the subgroup with a Ki-67 index <1%. Moreover, a distinct difference in recurrence rate was found between the subcategory of Ki-67 index <3% and those greater than 3% (P < 0.001).
Table 2.
Relation of Ki-67 index and recurrence in male prolactinoma, n (%).

3.8. Prediction for gross resection
All cases of microadenoma, 84.9% (n = 129) of macroadenoma cases and 53.8% of the 26 giant adenomas were resected completely. We concluded that the likelihood of total resection decreases with increasing tumor size (P < 0.05). However, preoperative PRL levels had no relation to gross resection (P > 0.05).
3.9. Prognosis
Table 1 shows the change in PRL level and the recurrence rate for each range of PRL. Lower preoperative PRL subgroups (150–1000 ng/mL) were more likely to achieve normal postoperative PRL levels as well as initial remission (P < 0.05). However, no significant difference in recurrence rate was found between the 1000 and 5000 ng/mL preoperative PRL subgroup and the >5000 ng/mL preoperative PRL subgroup (P > 0.05).
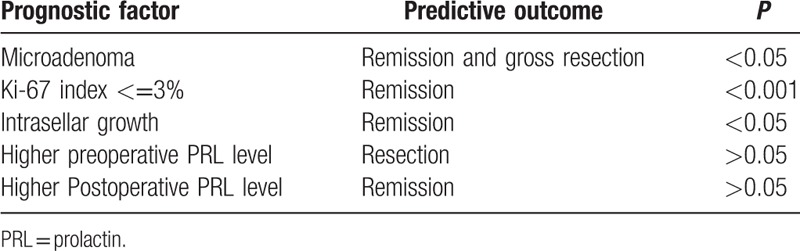
Symptoms were classified by 3 major manifestations, including headache, visual deficits, and libido reduction. Symptoms improved in 163 patients but remained the same or worsened in 21 patients, as shown in Table 3. However, no significant relation was discovered between immediate postoperative PRL level and specific symptom improvement (P > 0.05). All symptoms of the 6 patients with microadenoma improved, 92.1% of patients with macroadenoma experienced symptom improvement, and 65.4% of patients with giant adenoma experienced symptom improvement. It was concluded that symptom improvement was less likely to occur with larger adenomas (P < 0.05). Among 149 patients who underwent total adenoma excision, 57 exhibited initial remission, 26 patients experienced recurrence, and 31 patients exhibited follow-up remission. The relations between growth pattern and both remission and recurrence are shown in Table 4. For intrasellar adenomas, the initial remission rate was significantly higher than the others (P < 0.001), and likelihood for relapse was significantly lower (P < 0.01). No relation was found between recurrence time and growth pattern (P > 0.05). Table 5 illustrates the relationship between postoperative PRL level and recurrence, indicating that there was no significant difference between PRL level and recurrence rate (P > 0.01). We summarized prognosis factors for male prolactinoma in Table 6.
Table 3.
Relation between immediate postoperative prolactin level and symptom relief, n (%).
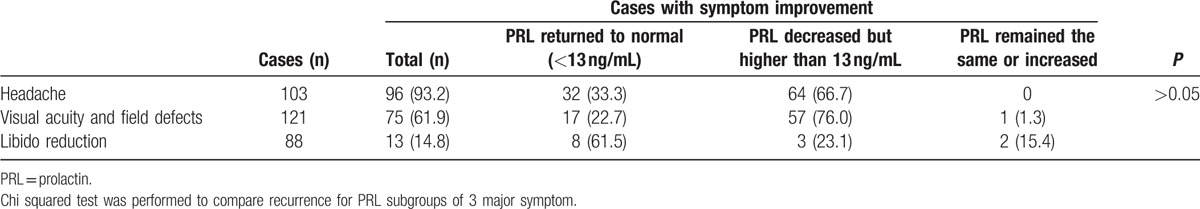
Table 4.
Relation between tumor growth pattern and recurrence in male prolactinoma, n (%).
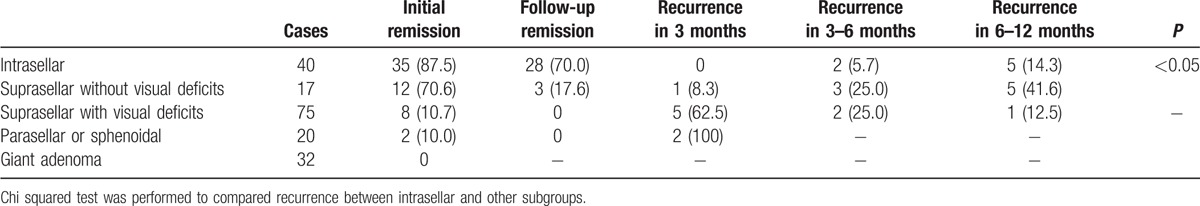
Table 5.
Relation between immediate postoperative PRL level and recurrence in male prolactinoma, n (%).
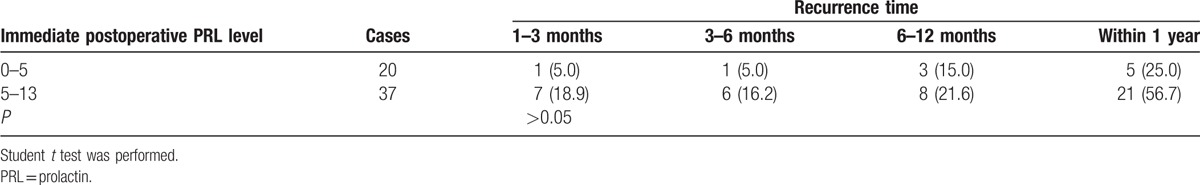
Table 6.
Summary for prognostic factors.
4. Discussion
The clinical manifestations of prolactinoma vary with gender. Although the main manifestations for female patients include oligomenorrhea and galactorrhea, most male patients complain of osteoporosis and libido reduction in the form of impotence, oligospermia, and erectile dysfunction. Male patients predominantly present with clinical manifestations resulting from mass effects and pituitary apoplexy.[1,2] In our research, the major manifestations triggered by hyperprolactinemia out of the 184 patients included libido reduction, headache, and visual defects.
Currently, the 1st-line treatment for prolactinoma is a dopaminergic agonist, such as bromocriptine or cabergoline, which bind dopamine 2 receptors on lactotroph cells and inhibit the secretion of PRL. This leads to decreased tumor size and the functional recovery of sexual glands.[3] The PRL levels in 59% of patients returned to normal after bromocriptine treatment,[5] while 12% of patients could not tolerate its side effects.[6] Moreover, the efficacy of cabergoline, a long-acting dopaminergic agonist, reaches up to 83%,[3] and cabergoline has fewer side effects than bromocriptine. However, Colao et al[7] demonstrated that the 5-year recurrence rate in microadenoma and macroadenoma patients after cabergoline treatment was 26% and 33%, respectively. MRI revealed that the recurrence rate of macroadenoma patients after drug withdrawal was as high as 78%. In our study, 43 patients accepted regular dopaminergic agonist therapy before surgery, but more than 80% were either resistant or intolerant.
Therefore, 2nd-line treatment, that is, neurosurgery, is commonly required for many prolactinoma patients. Indications for neurosurgery include resistance to bromocriptine or cabergoline treatment, intolerability of side effects, leakage of CSF due to the shrinking tumor, rapid diminution of vision, cranial nerve palsy caused by tumor bleeding and pituitary apoplexy, and a strong patient preference.[1–3]
Microadenoma is most commonly found in females, while macroadenoma is most commonly found in males.[8] Our research illustrates that macroadenoma accounted for 82.6% of prolactinomas in male patients. This may be because of challenges in the early detection and treatment of libido dysfunction. In females, menstrual disorders are much easier to detect and are thus observed earlier. Consequently, the course of this disease is longer and the tumors tend to be larger in males. The relationship between tumor size and serum PRL level remains controversial.[9] It has been demonstrated that the preoperative PRL level does not correlate with tumor size. Lactotropes and somatotrophs share a common precursor cell;[10] therefore, the overgrowth of precursor cells may lead to GH-PRL mixed adenomas.[11] The postoperative pathological immunohistochemical results indicated that GH-PRL-co-expressing adenomas accounted for approximately half of the mixed adenomas. However, when considering clinical manifestations and hormone evaluations, only 17 cases of GH-PRL adenomas were consistent with its pathological expression. Thus, clinical manifestations, hormone levels, and pathological results should be integrated together to diagnose mixed prolactinoma. Atypical pituitary adenoma refers to tumors with a Ki-67 index greater than 3% and with atypical hyperplasia or nuclear fission, as determined by microscopy. Tortosa and Webb[12] found that the recurrence rate of 220 patients with atypical pituitary adenoma was 57.1%. Our research showed that a higher Ki-67 index indicates a higher recurrence rate. Conversely, a Ki-67 index <3% was predictive of a favorable prognosis. Therefore, we suggest that patients with Ki-67 indexes higher than 5% should undergo adjuvant radiotherapy.
Recent reports have shown that the cure rate of macroadenoma is less than 50%.[1,3,13] Tumor size and preoperative PRL level were predictive factors for gross resection.[3,13] Our research demonstrated that the likelihood of total gross resection decreased as tumor size increased, but preoperative PRL had no significant relation with gross resection. We suggest that PRL secretion is related to the number of endocrine granules in lactotroph cells, which may explain why the PRL level was lower than expected in some macroadenomas.
Through the analysis of tumor size, growth pattern, and preoperative and immediate postoperative PRL levels, we found that the rate of symptom improvement decreased as tumor size increased. This may be because it is too difficult to completely resect a macroadenoma; therefore, the goal of surgical intervention was mainly to relieve symptoms caused by mass effects, such as visual deficits and headache. The lower the preoperative PRL level, the more likely it was to return to normal after surgery, possibly due to the slow decrease in postoperative PRL. Kreutzer et al[14] concluded that remission rate is related to pituitary adenoma growth pattern in a study of 212 patients. The remission rate of intrasellar tumors was 72.5%, while that of suprasellar tumors with or without visual deficits was 12.5%.[14] Our research indicated that for intrasellar adenomas, the initial remission rate was remarkably higher and the recurrence rate was lower than for suprasellar tumors, parasellar tumors, and giant adenomas. No significant relation was found between growth pattern and recurrence time. For patients with high risk factors for recurrence, adjuvant therapy should take into consideration. Currently, it is controversial whether the immediate postoperative PRL level can predict prognosis.[15] In our research, the immediate postoperative PRL level was not significantly correlated with symptom improvement, recurrence rate, or time of recurrence.
Compared to similar articles in recent years, our research had a larger sample size and clear definitions of recurrence, complete remission, and symptom relief. In addition, multiple prognostic factors were analyzed, such as preoperative PRL level, tumor size, Ki-67 index, tumor growth pattern, and postoperative PRL level. Xu et al[16] found no significant association between tumor size and remission in 103 cases of macroprolactinoma in males. Thus, they recommended medical therapy as the 1st choice for macroadenoma. However, remission was defined as PRL returning to normal with the disappearance of the tumor, as confirmed by MRI, and the duration of remission was not required. In our research, most of the target population complained of optic chiasm suppression, and the majority of tumors grew in a suprasellar pattern. In Xu Jian study, most cases presented with an intrasellar growth pattern, which may explain the different prognosis results. We defined remission in a more detailed and stringent way, which lowered our remission rates. Qu et al[17] demonstrated that the preoperative PRL level could predict recurrence in 87 cases of transsphenoidal surgery for prolactinoma in males. Surgery followed by adjuvant therapy was required, especially for suprasellar adenomas. In addition, the sample size was small, and only the preoperative PRL level was analyzed. In summary, the use of the preoperative PRL level for prognosis prediction remains controversial, and further multicenter studies of prolactinoma should be performed.
In turns of limitation of our research, it was that 1-year follow-up was not long enough. But it was difficult for longer visit because our patients came from all over the country and they might be back to other hospitals after remission. To date, we are persevering in following up more patients, collecting data, and enriching further research.
5. Conclusion
In this study, prolactinomas in males were typically macroadenomas characterized by high serum PRL levels. The main clinical manifestations were sexual hypoactivity, headache, and visual defects. Most prolactinomas grew in a suprasellar pattern with optic chiasm elevation. In most cases, the Ki-67 index was lower than 1%. Tumor size was not connected with serum PRL level. The probability of gross resection decreased with increasing tumor size. However, the preoperative PRL level was not significantly correlated with the likelihood of gross resection. Microadenomas, intrasellar adenomas, and a Ki-67 index <3% were predictive factors of a favorable prognosis. The immediate postoperative PRL level was not significantly correlated with either symptom improvement or recurrence.
Acknowledgments
The authors thank the Department of Neurosurgery, Peking Union Medical College Hospital, whose faculty performed successful surgeries and guided our research.
Footnotes
Abbreviations: ACTH = adrenocorticotropic hormone, GH = growth hormone, MRI = magnetic resonance imaging, PRL = prolactin, PUMCH = Peking Union Medical College Hospital.
Y-jS and M-tC contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Salazar-Lopez-Ortiz C, Hernandez-Bueno J, Gonzalez-Barcena D, et al. Clinical practice guideline for the diagnosis and treatment of hyperprolactinemia. Ginecol Obstet Mex 2014;82:123–42. [PubMed] [Google Scholar]
- [2].Wong A, Eloy JA, Couldwell WT, et al. Update on prolactinomas. Part 1: clinical manifestations and diagnostic challenges. J Clin Neurosci 2015;22:1562–7. [DOI] [PubMed] [Google Scholar]
- [3].Wong A, Eloy JA, Couldwell WT, et al. Update on prolactinomas. Part 2: treatment and management strategies. J Clin Neurosci 2015;22:1568–74. [DOI] [PubMed] [Google Scholar]
- [4].Panza N, Rambaldi PF, Battista C, et al. Receptor imaging with 111In-pentreotide and 123I-methoxybenzamide, and inhibition tests with octreotide and bromocriptine of mixed growth hormone/prolactin-secreting pituitary tumors. Biomed Pharmacother 1999;53:319–22. [DOI] [PubMed] [Google Scholar]
- [5].Webster J, Piscitelli G, Polli A, et al. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. N Engl J Med 1994;331:904–9. [DOI] [PubMed] [Google Scholar]
- [6].Mancini T, Casanueva FF, Giustina A. Hyperprolactinemia and prolactinomas. Endocrinol Metab Clin North Am 2008;37:67–99. [DOI] [PubMed] [Google Scholar]
- [7].Colao A, di Sarno A, Cappabianca P, et al. Withdrawal of long-term cabergoline therapy for tumoral and nontumoral hyperprolactinemia. N Engl J Med 2003;349:2023–33. [DOI] [PubMed] [Google Scholar]
- [8].Agustsson TT, Baldvinsdottir T, Jonasson JG, et al. The epidemiology of pituitary adenomas in Iceland, 1955-2012: a nationwide population-based study. Eur J Endocrinol 2015;173:655–64. [DOI] [PubMed] [Google Scholar]
- [9].Alkabbani AG, Mon SY, Hatipoglu B, et al. Is a stable or decreasing prolactin level in a patient with prolactinoma a surrogate marker for lack of tumor growth? Pituitary 2014;17:97–102. [DOI] [PubMed] [Google Scholar]
- [10].Ferraris J, Zarate S, Jaita G, et al. Prolactin induces apoptosis of lactotropes in female rodents. PLoS One 2014;9:e97383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li J, Stefaneanu L, Kovacs K, et al. Growth hormone (GH) and prolactin (PRL) gene expression and immunoreactivity in GH- and PRL-producing human pituitary adenomas. Virchows Arch A Pathol Anat Histopathol 1993;422:193–201. [DOI] [PubMed] [Google Scholar]
- [12].Tortosa F, Webb SM. Atypical pituitary adenomas: 10 years of experience in a reference centre in Portugal. Neurologia 2016;31:97–105. [DOI] [PubMed] [Google Scholar]
- [13].Buchfelder M, Schlaffer S. Surgical treatment of pituitary tumours. Best Pract Res Clin Endocrinol Metab 2009;23:677–92. [DOI] [PubMed] [Google Scholar]
- [14].Kreutzer J, Buslei R, Wallaschofski H, et al. Operative treatment of prolactinomas: indications and results in a current consecutive series of 212 patients. Eur J Endocrinol 2008;158:11–8. [DOI] [PubMed] [Google Scholar]
- [15].Amar AP, Couldwell WT, Chen JC, et al. Predictive value of serum prolactin levels measured immediately after transsphenoidal surgery. J Neurosurg 2002;97:307–14. [DOI] [PubMed] [Google Scholar]
- [16].Xu J, Jin ZM, Deng JY, et al. A clinical analysis of 103 male patients with macroprolactinoma. Zhonghua Nei Ke Za Zhi 2005;44:356–9. [PubMed] [Google Scholar]
- [17].Qu X, Wang M, Wang G, et al. Surgical outcomes and prognostic factors of transsphenoidal surgery for prolactinoma in men: a single-center experience with 87 consecutive cases. Eur J Endocrinol 2011;164:499–504. [DOI] [PubMed] [Google Scholar]


