Abstract
Introduction:
Febuxostat, a nonpurine xanthine oxidase inhibitor, is approved as the first-line urate-lowering therapy in gout patients with normal renal function or mild to moderate renal impairment. The most common adverse effects of febuxostat are liver function test abnormalities, diarrhea, and skin rash. However, there is insufficient data in patients with severe renal impairment and end-stage renal disease (ESRD). We report the first case, to our knowledge, in which agranulocytosis developed after febuxostat treatment in an ESRD patient.
Clinical presentation:
A 67-year-old woman with gout and ESRD received febuxostat 40 mg a day for 2.5 months. She subsequently complicated with febrile neutropenia and the absolute neutrophil count was only 14/μL. After broad-spectrum antibiotics treatment and no more exposure to febuxostat for 17 days, her infection and neutrophil count recovered. Bone marrow study during neutropenic period showed myeloid hypoplasia without evidence of hematologic neoplasms.
Conclusion:
As febuxostat use may become more common in the population of advanced renal failure, clinicians should be aware of this rare but potentially life-threatening adverse effect. Based on our experience, close monitoring hemogram and immediate discontinuation of this medication may prevent serious consequences.
Keywords: agranulocytosis, end-stage renal disease, febuxostat, hemodialysis
1. Introduction
Gout is the most common inflammatory arthritis, and the clinical presentation is characterized by acute episodic flares of intense joint pain, caused by the deposition of monosodium urate crystals. Hyperuricemia is the central risk factor for gout and the pharmacologic therapeutic goal of gout is to lower serum uric acid level below 6 mg/dL, the saturation point for monosodium urate.[1,2] In patients with chronic kidney disease (CKD), their urinary uric acid secretion is decreased, and the prevalence of hyperuricemia is higher than those with normal renal function.[3] On the other hand, elevated serum uric acid has been proposed to attribute to CKD progression.[3] For CKD patients with gout, aggressive control of serum uric acid level is crucial for renal protection and gout therapy.
Febuxostat, a nonpurine xanthine oxidase inhibitor, has been approved by United States Food and Drug Administration since 2009 for chronic management of hyperuricemia in patients with gout. When comparing with the traditional xanthine oxidase inhibitor, allopurinol, febuxostat has shown its superiority in greater urate-lowering efficacy.[4] The most common adverse effects of febuxostat include liver function abnormalities, diarrhea, and skin rash. According to the American College of Rheumatology guidelines,[1] febuxostat is recommended as the first-line pharmacologic urate-lowering therapy approach in gout. The recommended starting dose is 40 mg once daily. In patients with mild to moderate renal impairment (creatinine clearance, CrCl 30–89 mL/min), the starting dosage is the same as for patients with normal renal function.[1] Nevertheless, early clinical trials exclude patients with CrCl less than 30 mL/min.[5] There is still insufficient experience for febuxostat usage in patients with severe renal impairment and end-stage renal disease (ESRD), and the safety is still a concern. Here, we report an ESRD patient developing agranulocytosis after febuxostat treatment.
2. Case report
A 67-year-old woman was sent to emergency department because of fever and sore throat. Her medical history included ESRD with regular hemodialysis 3 times per week for 15 years, hyperuricemia and gout. She also had comorbidity such as dyslipidemia, hypertension, secondary hyperparathyroidism, and diabetes. The hemogram revealed a white blood cell count (WBC) of 700/μL, with 2% neutrophils, 94% lymphocytes and 2% monocytes, hemoglobin 11.1 g/dL, and platelet count, 131,000/μL. Under the impression of febrile neutropenia and acute pharyngitis, she was admitted to our hematologic ward for further survey and management. Broad-spectrum antibiotics with piperacillin 2 g and tazobactam 0.25 gm i.v. q8h had been administered and her infection sign resolved gradually. Upon admission, we reviewed her oral medication: glipizide 5 mg tid, saxagliptin 2.5 mg qd, fenofibrate 600 mg qd, aluminum hydroxide 324 mg tid, folic acid 5 mg qd, calcium carbonate 1000 mg tid, and febuxostat 40 mg qd. Besides, she also received epoetin-beta 2000 iu i.v. tiw. Except for febuxostat, all the other drugs had been used for more than 1 year. Febuxostat was administered 2½ months before admission for inadequate serum uric acid control by allopurinol 50 mg qd. Two weeks before febuxostat exposure, routine laboratory test revealed WBC 6000/μL and serum uric acid level 9.8 mg/dL. Febuxostat was discontinued thereafter due to the causal relationship of agranulocytosis cannot be excluded. Besides, we also surveyed viral infection and autoimmune disorder. There were no clinical or laboratory evidence of Epstein–Barr virus, cytomegalovirus, or human immunodeficiency virus infections; antinuclear antibody (ANA) and antiextractable nuclear antigen (anti-ENA) were both negative. Bone marrow examination during hospitalization showed hypocellular marrow with a marked decrease in myeloid component but no evidence of hematologic neoplasms. Chromosome analysis of bone marrow was normal karyotype. The patient denied history of radiation or chemicals exposure. After stopping febuxostat for 17 days, her neutropenia improved significantly (WBC 2100/μL, and neutrophil 66%), without any granulocyte colony-stimulating factor (G-CSF) support. After discharge, her WBC and differential count was completely normal during follow-up (Fig. 1). This study was approved by our institutional review board.
Figure 1.
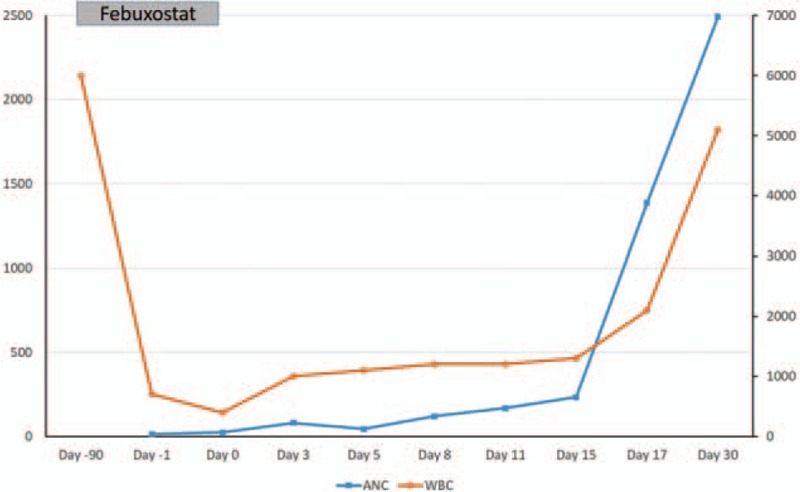
The time course of drug administration, total WBC count (WBC × 1000/μL) and absolute neutrophil count (ANC).
3. Discussion
Agranulocytosis is characterized by a decrease in peripheral neutrophil count less than 500/μL and most cases in adults result from adverse effects of drugs.[6,7] Except for chemotherapy, the most well-known medication to induce agranulocytosis is antithyroid drugs.[6] The clinical presentation is usually fever due to infection, so-called febrile neutropenia, and upper aero-digestive tract infection is the most common infection source just as our patient.[8,9] The most important managements of drug-induced agranulocytosis are to identify the culprit drug, to withdraw this agent, and to give broad-spectrum antibiotics for those with infection.[6,10] In our case, before the initiation of febuxostat, her WBC was normal and agranulocytosis developed 2.5 months after taking febuxostat. Other medications that the patient had taken for her underlying chronic diseases were considered to be unlikely culprits, because she had been receiving them for several years and continued after this episode. Besides, we excluded the possibility of viral infection and autoimmune diseases. Bone marrow examination also excluded hematologic disorder. We stopped febuxostat immediately when we suspected the causality of this offending drug to agranulocytosis. Her neutrophil count recovered in 17 days without other intervention such as steroids or G-CSF. Due to the safety concern, we did not rechallenge febuxostat thereafter. Using Naranjo probability scale, we determined the occurrence of agranulocytosis related to febuxostat treatment to be probable.[11]
Febuxostat has been approved in the USA for the chronic management of hyperuricemia in patients with gout, and in EU for the treatment of chronic hyperuricemia in conditions where urate deposition has already occurred, and in Japan for the treatment of hyperuricemia in patients with or without gout.[12] The recommended starting dose range from 40 to 80 mg daily in different countries and no dose adjustment is required for those with mild to moderate renal impairment (CrCl >30 mL/min). However, the efficacy and safety have not been fully evaluated in patients with severe renal impairment (CrCl <30 mL/min) and ESRD, and febuxostat has not been approved in this population. In a small series of 17 hemodialysis patients with asymptomatic hyperuricemia, febuxostat 10 mg once daily has demonstrated significant urate-lowering efficacy and no specific adverse effect was observed.[13] Another series of 5 male cases with chronic hemodialysis, febuxostat 10 to 20 mg daily were administered without apparent adverse effects.[14]
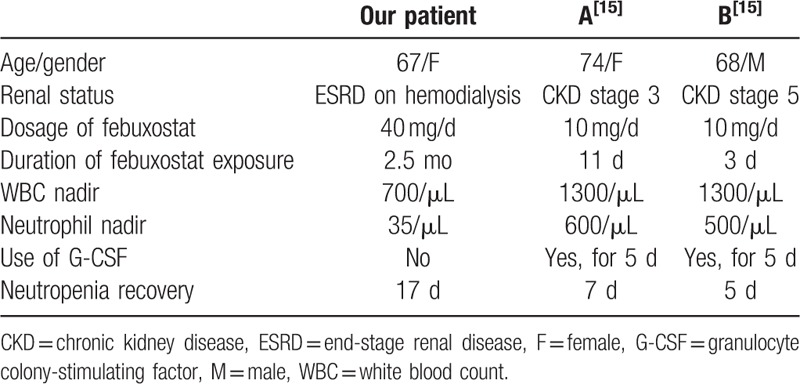
Regarding agranulocytosis induced by febuxostat, 2 patients with CKD have been reported.[15] A 74-year-old woman with liver cirrhosis and CKD stage 3 was treated with febuxostat 10 mg per day for hyperuricemia during hospitalization. Eleven days after febuxostat administration, she developed neutropenia with absolute neutrophil count only 600/μL. Another 68-year-old man with CKD stage 5 due to diabetic nephropathy was administered with febuxostat 10 mg per day for inadequate uric acid control during admission. After 3 days of febuxostat treatment, his neutrophil count dropped from 5700 to 500/μL. In these 2 patients, neutrophil count increased within 5 to 7 days after febuxostat discontinuation and with G-CSF support.[15] Apparently, our patient had more advanced renal disease and exposed to higher dose of febuxostat for longer duration (40 mg qd for 2.5 months), and then had lower neutropenic nadir and exhibited longer neutropenic period of time. Table 1 shows comparison of clinical presentation of reported cases of febuxostat-induced agranulocytosis.
Table 1.
Clinical presentation of reported cases of febuxostat-induced agranulocytosis.
As for patients with ESRD and hemodialysis, febuxostat could improve endothelial dysfunction and reduce serum uric acid levels and oxidative stress.[13,16] With these study results, it is expected that more and more patients with CrCl less than 30 mL/min will receive this drug and some other patients may experience this uncommon adverse effect, agranulocytosis, which is seldom reported in patients without advanced renal failure. Two possible mechanisms have ever been proposed in drug-induced agranulocytosis. Immune-mediated destruction of circulating neutrophils suggests that a reactive metabolite of febuxostat may irreversibly bind to the membrane of mature neutrophils. Another possible mechanism is the metabolite may directly damage myeloid precursors.[17,18] However, these speculations need to be tested in future studies.
4. Conclusion
We report an ESRD patient experiencing febuxostat-induced agranulocytosis, a rare but severe complication. When prescribing febuxostat to patients with severe CKD or ESRD, clinicians should be aware of this potentially life-threatening adverse effect and a regular hemogram monitoring during the initial treatment of febuxostat is recommended.
Footnotes
Abbreviations: ANA = antinuclear antibody, anti-ENA = antiextractable nuclear antigen, CKD = chronic kidney disease, CrCl = creatinine clearance, ESRD = end-stage renal disease, G-CSF = granulocyte colony-stimulating factor, WBC = white blood count.
The authors have no conflicts of interest to disclose.
References
- [1].Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;10:1431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2006;10:1312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 2010;8:1388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Becker MA, Schumacher HR, Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;23:2450–61. [DOI] [PubMed] [Google Scholar]
- [5].Febuxostat prescribing information, FDA approval at 2009, revised at 2012. 2012. [Google Scholar]
- [6].Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007;9:657–65. [DOI] [PubMed] [Google Scholar]
- [7].Pisciotta AV. Drug-induced agranulocytosis. Peripheral destruction of polymorphonuclear leukocytes and their marrow precursors. Blood Rev 1990;4:226–37. [DOI] [PubMed] [Google Scholar]
- [8].Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011;4:e56–93. [DOI] [PubMed] [Google Scholar]
- [9].Pei S-N, Wang M-C, Huang E-Y, et al. Febrile neutropenia in patients with acute leukemia following chemotherapy: a retrospective review. J Intern Med Taiwan 2006;17:106–13. [Google Scholar]
- [10].Heit W, Heimpel H, Fischer A, et al. Drug-induced agranulocytosis: evidence for the commitment of bone marrow haematopoiesis. Scand J Haematol 1985;5:459–68. [DOI] [PubMed] [Google Scholar]
- [11].Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;2:239–45. [DOI] [PubMed] [Google Scholar]
- [12].Frampton JE. Febuxostat: a review of its use in the treatment of hyperuricaemia in patients with gout. Drugs 2015;4:427–38. [DOI] [PubMed] [Google Scholar]
- [13].Akimoto T, Morishita Y, Ito C, et al. Febuxostat for hyperuricemia in patients with advanced chronic kidney disease. Drug Target Insights 2014;39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Horikoshi R, Akimoto T, Inoue M, et al. Febuxostat for hyperuricemia: experience with patients on chronic hemodialysis treatment. Clin Exp Nephrol 2013;1:149–50. [DOI] [PubMed] [Google Scholar]
- [15].Kobayashi S, Ogura M, Hosoya T. Acute neutropenia associated with initiation of febuxostat therapy for hyperuricaemia in patients with chronic kidney disease. J Clin Pharm Ther 2013;3:258–61. [DOI] [PubMed] [Google Scholar]
- [16].Tsuruta Y, Kikuchi K, Tsuruta Y, et al. Febuxostat improves endothelial function in hemodialysis patients with hyperuricemia: a randomized controlled study. Hemodial Int 2015;4:514–20. [DOI] [PubMed] [Google Scholar]
- [17].Watts RG. Neutropenia. Wintrobe's Clinical Hematology. 1999;USA:Lippincott Williams and Wilkins, 1862–1888. [Google Scholar]
- [18].Drug-induced neutropenia and agranulocytosis, Up to date 2016. Accessed on December 7, 2016. [Google Scholar]


