Abstract
Improving healthspan, defined as the period where organisms live without frailty and/or disease, is a major goal of biomedical research. While healthspan measures in people are relatively easy to identify, developing robust markers of healthspan in model organisms has proven challenging. Studies using the nematode Caenorhabditis elegans have provided vital information on the basic mechanisms of aging; however, worm health is difficult to define, and the impact of interventions that increase lifespan on worm healthspan has been controversial. Here, we describe a marker of population healthspan in C. elegans that we term age-associated vulval integrity defects, or Avid, frequently described elsewhere as rupture or exploding. We connect the presence of this phenotype with temperature, reproduction, diet, and longevity. Our results show that Avid occurs in post-reproductive worms under common laboratory conditions at a frequency that correlates negatively with temperature; Avid is rare in worms kept at 25 °C and more frequent in worms kept at 15 °C. We describe the kinetics of Avid, link the phenotype to oocyte production, and describe how Avid involves the ejection of worm proteins and/or internal organ(s) from the vulva. Finally, we find that Avid is preventable by removing worms from food, suggesting that Avid results from the intake, digestion, and/or absorption of food. Our results show that Avid is a significant cause of death in worm populations maintained under laboratory conditions and that its prevention often correlates with worm longevity. We propose that Avid is a powerful marker of worm healthspan whose underlying molecular mechanisms may be conserved.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-016-9936-8) contains supplementary material, which is available to authorized users.
Keywords: C. elegans, Healthspan, Temperature, Rupture, Longevity, Reproduction
Introduction
The nematode Caenorhabditis elegans has played a crucial role in our understanding of many types of conserved biological processes, including development, genetics, innate immunity, and aging (Corsi et al. 2015). Worms were among the first important organisms used to study longevity (Klass 1977), with early studies identifying the first genes to directly affect aging in any organism (Klass 1983; Friedman and Johnson 1988). Because of this history, more is known about worm aging than perhaps any other organism, and worms are regularly used to screen for and elucidate the molecular mechanisms of aging (Lee et al. 2003; Hamilton et al. 2005; Petrascheck et al. 2009). Despite our relatively sophisticated understanding of aging in worms, there is a considerable gap in our knowledge of specific causes of worm death. Previous studies have identified a number of plausible mechanisms and descriptions of death in worms, including bacterial pathogenesis (Garigan et al. 2002), death of muscle cells and/or neuromuscular junctions (Herndon et al. 2002), loss of intestinal integrity (McGee et al. 2011), and necrotic cell death/spreading of anthranilic acid (Coburn et al. 2013). What is clear from each of these studies is that while there are many possible mechanisms mediating specific causes of death in C. elegans, defining the direct cause of death for an individual worm remains challenging.
A major challenge of research in model organisms is to define the health status of the animal and translate it to people. Because the goal of longevity interventions is to increase healthy life rather than to stretch out the difficult late-life period people often face, healthspan has become a focus of many studies (Martin et al. 2007; Kirkland and Peterson 2009). A recent paper from the Tissenbaum group (Bansal et al. 2015) examined healthspan in C. elegans and suggested that many longevity interventions may not increase the percentage of life nematodes spend in good health. They measured healthspan by monitoring worm movement, eating, and stress resistance over time. This is in contrast to prior studies which have reported improvements in nematode healthspan resulting from the same and other longevity-enhancing interventions (Johnson et al. 2000; Herndon et al. 2002; Huang et al. 2004; Kaeberlein et al. 2006; Mehta et al. 2009; Sutphin et al. 2012). A major challenge for these types of studies in nematodes is that healthspan (and health itself) is difficult to define in worms, and each lab uses their own, somewhat arbitrary, healthspan measurements. However, while it is difficult to agree how best to measure healthspan in model organisms such as C. elegans, increasing healthspan is widely accepted as a necessary goal of longevity studies (Burch et al. 2014).
It is surprising, therefore, that the nematode aging field to date has paid little attention to a common phenotype that, at first glance, would appear to represent an obvious marker of poor healthspan and a potential cause of death. Age-associated vulval integrity defects (Avid), sometimes referred to as herniated (Pvl), ruptured (Rup), or exploded worms, are often censored in worm aging experiments (Greer et al. 2007; Hansen et al. 2007; Chen et al. 2009; Zhang et al. 2009). Avid is described in detail below and can be simply defined as a loss of vulval integrity that leads to ejection of internal material and premature death in C. elegans. Unlike bagging, a phenotype that occurs when food is scarce in which eggs are retained and hatch inside the worm, allowing the resulting larva to devour their mother (Chen and Caswell-Chen 2004), there is no clear evolutionary selection for Avid. Similar to many diseases in humans, Avid does not occur in every individual worm, and thus is not a marker of individual healthspan. Rather, like common human diseases, Avid is a useful measure of healthspan in worm populations that is easily observed and clearly detrimental to short- and long-term health.
Avid is a term coined several years ago by our lab (Leiser et al. 2011), but we were hardly the first group to note the phenotype. It is difficult to ascertain the first group to report Avid, because most labs do not differentiate between the loss of vulval integrity observed during aging and visually similar phenotypes observed in mutants defective for proper vulval development (Kamath et al. 2003). Regardless, Avid is relatively common in aging C. elegans (>20 % at 20 °C in wild-type worms, see below), and many labs include some description of Avid in the methods section of their aging studies specifically in order to note that Avid worms were censored from survival experiments (Greer et al. 2007; Hansen et al. 2007; Chen et al. 2009; Zhang et al. 2009). The assumption that worms exhibiting loss of healthspan due to Avid die of “unnatural” causes, to our knowledge, is neither supported nor refuted by any studies. Additionally, it is unclear whether the Avid phenotype itself might mark a substantial phenotype that, while obviously worm-specific, may have evolutionarily conserved molecular mechanisms.
To better understand Avid and its role in nematode healthspan, we have carefully examined this phenotype in the context of several genetic and environmental conditions known to modify worm longevity. These studies describe an age-associated marker of healthspan whose frequency and development depend on time, temperature, reproduction, genotype, and diet. The results suggest that Avid is a definitive cause of healthspan loss in a subset of the population, that it is associated with early death, and that more detailed studies are warranted to understand the mechanisms that cause Avid and their role in modulating healthy aging.
Results
Avid is a time- and temperature-dependent phenotype
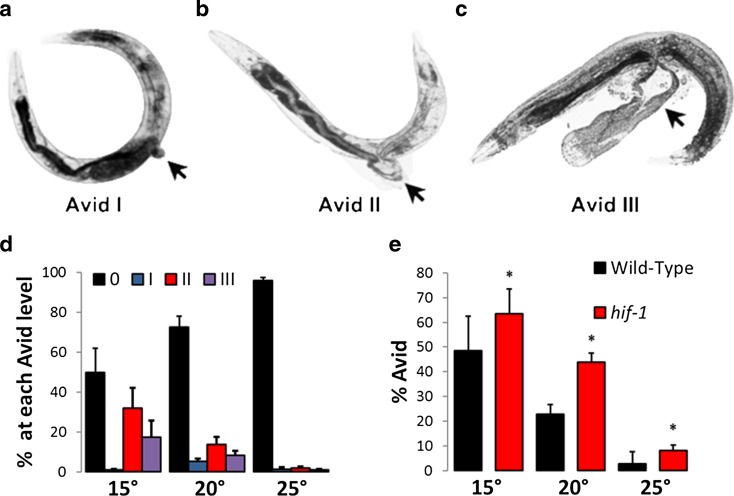
Avid consists of a loss of integrity of the vulva resulting in a visible phenotype occurring after development. To better understand the phenomenon of Avid, we first visualized and further defined the phenotype. While performing more than 25 experiments at three temperatures following worms over their lifetime, we classified Avid in three stages (Fig. 1a–c, Table S1). In the first stage, worms show a small protrusion at the vulva, similar to a hernia (Pvl), sometimes with slight leakage in the area (Fig. 1a). We noticed that in many cases, one side of the intestine is pale in pigmentation compared to the other half. This can happen either in the posterior section of the intestine or in the anterior section and may suggest the loss of intestinal material or integrity. This stage is usually a precursor to stage II or III, as worms very rarely die in stage I (Fig. 1d). We define stage II as a tearing of the vulva with visible excretion of material (Fig. 1b), and stage III as the ejection of bodily tissue(s), generally the intestine (Fig. 1c). We note that Avid rates increase significantly as temperature decreases; Avid levels average 3 % at 25 °C, 25 % at 20 °C, and 48 % at 15 °C (Fig. 1d, e, Table S2).
Fig. 1.
Characterization of the Avid phenotype. a–c Worms exhibiting different levels of Avid, including level I (herniated vulva, a) level II (tearing/ruptured vulva, b) and level III (exploded vulva, c). d Quantification of the total level of Avid observed in lifetimes of populations of wild-type (N2) worms at 15, 20, and 25 °C. e Total Avid (all levels) observed in lifetimes of populations of wild-type N2 and hif-1(ia4) mutant worms at 15, 20, and 25 °C. The bars represent the average of all experiments at the given temperature. All error bars represent standard error of the mean, asterisk represents p value <0.05 by paired t test
We next compared two strains known to differ in their Avid rates, wild-type N2 worms, and worms lacking the hypoxic response transcription factor, hif-1(ia4) (Leiser et al. 2011). Our results recapitulate and extend previous results, showing that the hif-1 mutant worms have increased Avid at all temperatures and that both strains have an increase in Avid as temperature decreases (Fig. 1e). We note that while there is rarely more than 5 % Avid in worms grown at 25 °C, there is considerable variability in the amount of Avid from experiment to experiment at lower temperatures, despite best efforts to maintain identical environmental conditions. These results suggest that small fluctuations in temperature, other subtle environmental differences, or stochastic variation may have large effects on the development of Avid at lower temperatures.
Avid occurs in post-reproductive “mid-life” stages
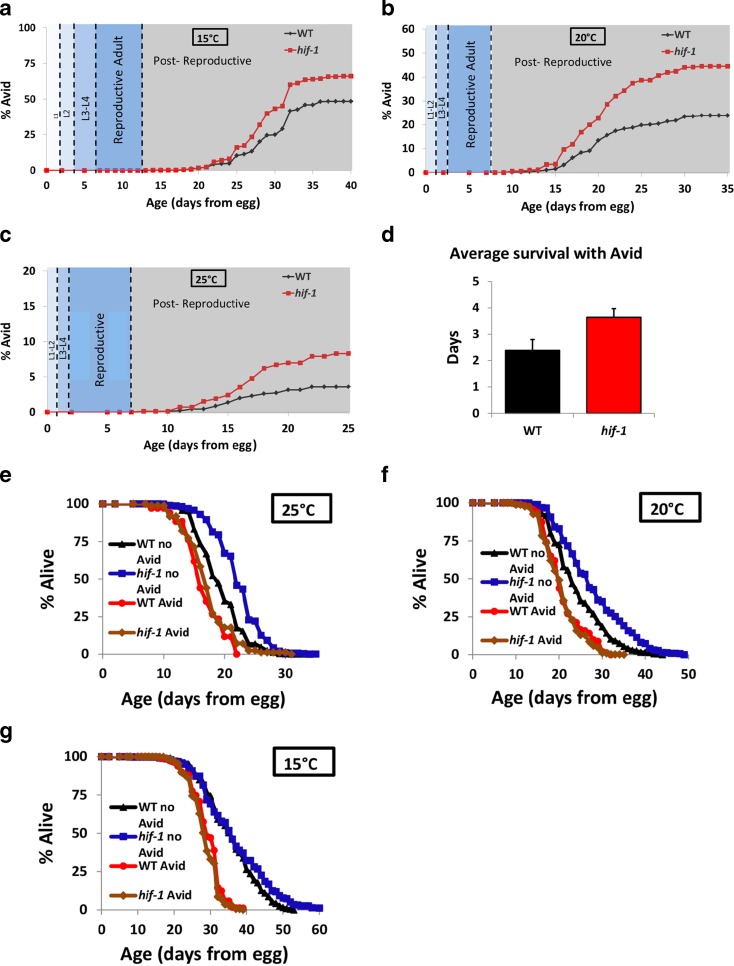
We next asked when Avid develops and whether the timing is dependent on temperature relative to specific stages of life. First, we measured the formation of any Avid stage over the worms’ lifespan (Fig. 2a–c, Table S2). We found that at each temperature, the Avid phenotype did not become apparent until “middle age,” after both development and reproductive lifespan are completed (note that the graphs show Avid at death, when worms were removed from plates, but that subtracting for the time worms live with Avid (Fig. 2d) does not change the result). This is an important distinction from the Pvl and Rup phenotypes described by previous researchers (Kamath et al. 2003) and found in Wormbase annotations (www.wormbase.org), which refer to defective development of the vulva and are exhibited during or shortly after the transition to adulthood. The Avid phenotype is, by definition, never developmentally exhibited, and additionally, is rarely observed during the reproductive period of life. We also note that the incidence of Avid does not immediately cause death of the worms and that worms can live after loss of vulval integrity for several days (Fig. 2d). We do observe that the worms displaying Avid have a significantly shorter lifespan than worms that do not develop Avid; however, indicating that Avid represents a loss of healthspan associated with early mortality in a sub-population of the cohort (Fig. 2e–g, Table S3).
Fig. 2.
Timeline of Avid development. a–c Quantification of observed Avid at death over the life of wild-type and hif-1 worms at 15 °C (a), 20 °C (b), and 25 °C (c). d Average time between onset of Avid and death at 20 °C. e–g Lifespans of wild-type and hif-1(ia4) Avid and healthy (no Avid/censored) worms at 15 °C (e) 20 °C (f), and 25 °C (g)
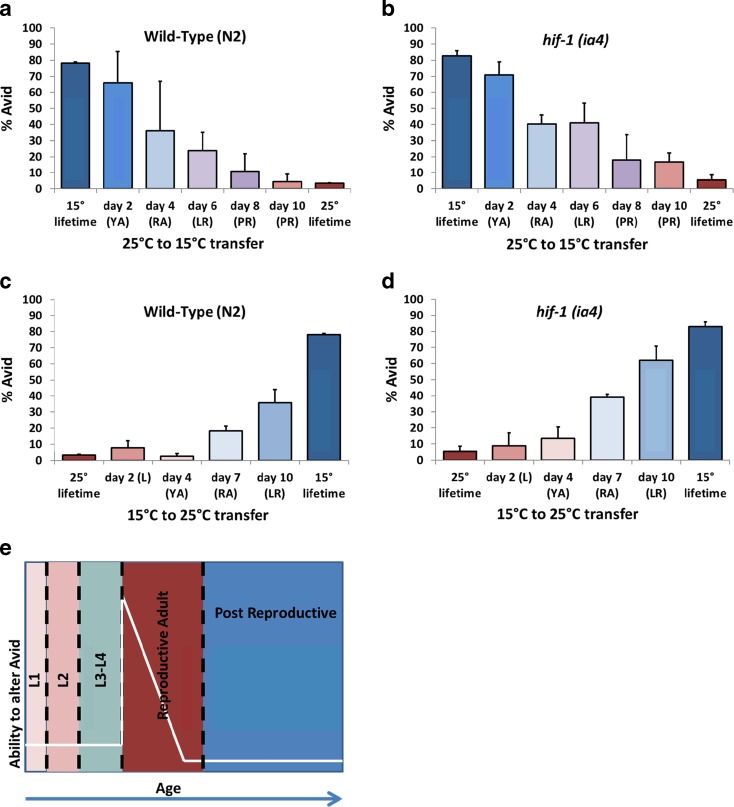
To further understand the temperature dependence of Avid, we also asked at what stage of life temperature can affect the frequency of Avid. We compared worms grown at 25 and 15 °C during their entire lives with worms that began at one temperature and were later switched to a different temperature. We switched the worms from warm (25 °C) to cold (15 °C) and vice versa at time points including during development (day 2 from hatching), after development (day 4), during reproduction (days 6 and 8), and after reproduction (day 10). The results (Fig. 3a–d, Table S2) show that the temperature where the worms spend their reproductive life is most important for the rate of Avid; worms at low temperature during egg-laying have more Avid compared to worms at high temperature. Interestingly, the temperature during development (days 1–2 at 25 °C and 1–4 at 15 °C) has less effect on the rate of Avid, as does the temperature after reproductive life (Fig. 3a–d). This suggests that there is a defined window of time where temperature is important for the development of Avid (Fig. 3e).
Fig. 3.
Temporal relationship between Avid and temperature. a–b Total Avid observed in wild-type (a) and hif-1 (b) worms who started their lifespans at 25 °C and were transferred to 15 °C at progressively later times during their life. c–d Avid observed in wild-type (c) and hif-1 (d) worms who started their lifespans at 15 °C and were transferred to 25 °C at progressively later times during their life. e Model for the time frame when Avid levels can be modified by changing temperature. For a–d, the stage of life where temperature was changed is noted (L = larval, YA = young adult, RA = reproductive adult, LR = late reproductive adult, PR = post-reproductive adult)
Avid is linked to reproduction.
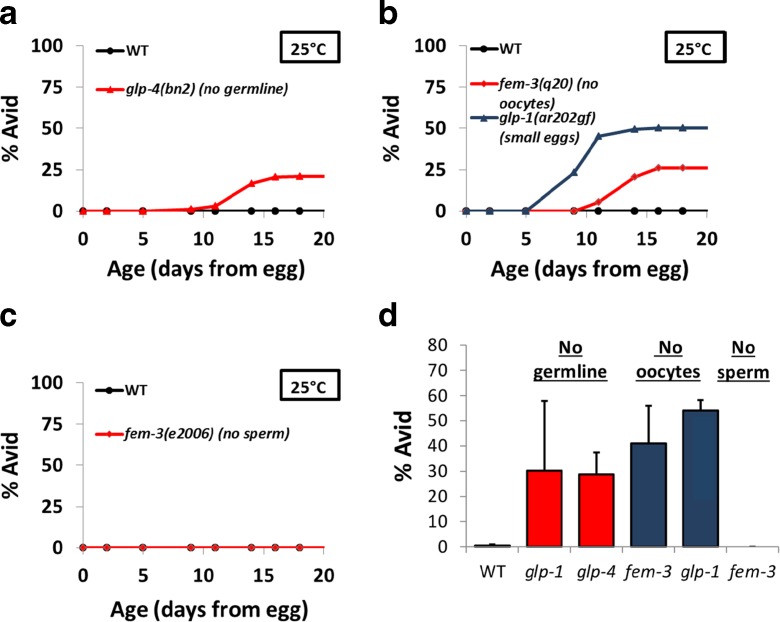
Since temperature interacts with Avid levels predominantly during reproductive life, we next asked whether the reproductive system (germline) itself was important for this phenotype. We utilized strains with mutations in genes reported to affect germline development (glp-1(e2141), glp-4(bn2)), oocyte development (fem-3(q20), glp-1(ar202gf)), and sperm development (fem-3(e2006)) to test what aspects of reproductive biology might affect Avid. Because these mutations are temperature-sensitive, we performed each experiment at 25 °C to observe the effects of the mutation. This is noteworthy because Avid is rarely observed in wild-type worms at 25 °C, as described above. Our results were striking and show that changes in the germline, including a mutation that prevents germline proliferation (glp-4(bn2)) (Beanan and Strome 1992), a gain of function in the germline that causes eggs to develop small and not absorb as much yolk (glp-1(ar202gf)) (Nadarajan et al. 2009), and a mutation that prevents development of oocytes (fem-3(q20)) (Barton et al. 1987), all cause a large increase in Avid (Fig. 4a–c), even at the warmer temperature where Avid is normally not observed. Interestingly, a mutation that only prevents the development of sperm and thus fertilization of eggs, fem-3(e2006) (Hodgkin 1986), did not cause Avid, suggesting that the presence of oocytes and eggs (fertilized or unfertilized) is crucial in the prevention of Avid in these strains. The results, summarized in Fig. 4d, show that the loss of germline proteins and specifically loss of oocyte development significantly increase the rate of Avid, even at 25 °C, and suggest that the germline plays a major role in Avid.
Fig. 4.
The germline affects the development of Avid. a Avid levels over the lifespan of wild-type (N2) worms and worms with a mutation that prevents development of the germline (glp-4(bn2)). b Avid levels over the lifespan of wild-type (N2) worms and worms with mutations that prevent development of oocytes (fem-3(q20) and glp-1(ar202gf)). c Avid levels over the lifespan of wild-type (N2) worms and worms with a mutation that prevents development of sperm, but not oocytes (fem-3(e2006)). d Summary of the total Avid in wild-type worms and in strains lacking germline, oocyte, or sperm development
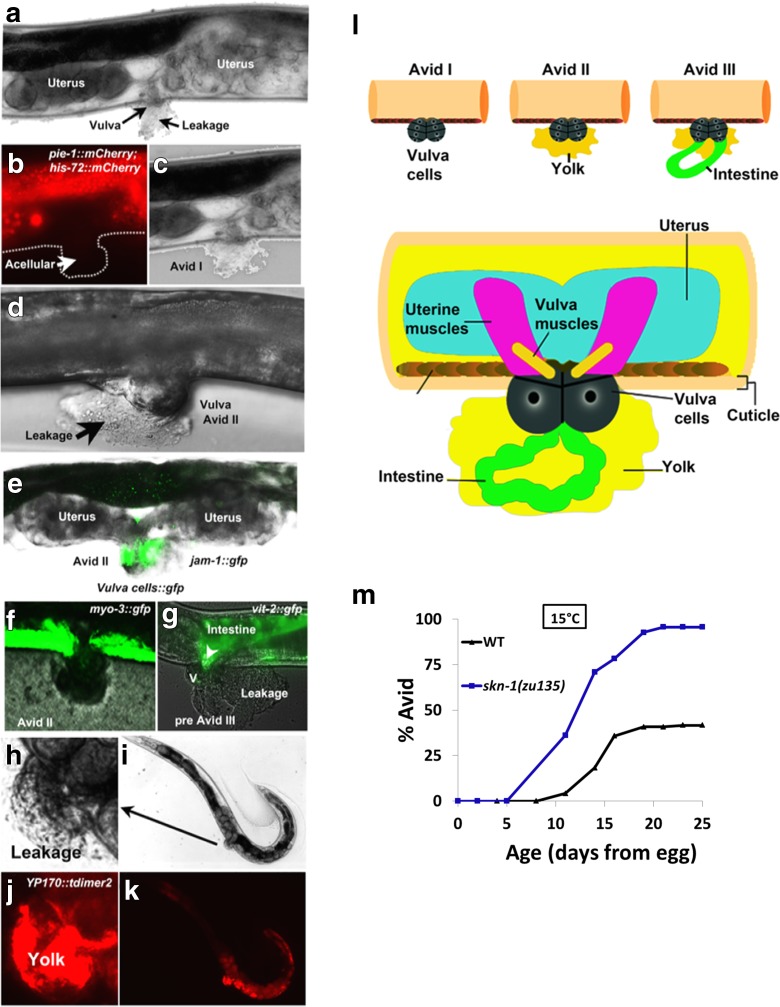
With the understanding that oocyte and egg production are important in preventing Avid, we next examined the reproductive cells, tissues, and proteins that are involved in Avid. We used several fluorescent markers, including markers for nuclei (pie-1::mcherry and his-72::mcherry), a marker for vulva cells (jam-1::gfp), a body wall muscle marker (myo-3::gfp), an intestinal marker (vit-2::gfp), and a marker for yolk (YP170::tdimer2). As noted earlier, the earliest phases of Avid are accompanied by leakage of an unidentified material from the vulva (Fig. 5a). This leakage does not appear to contain cells (Fig. 5b–d). As Avid progresses, the leakage is followed by protrusion of the vulva epithelial cells (Fig. 5e), but not body wall muscle cells (Fig. 5f, Fig. S1). We next observed that as Avid progresses toward stage III, intestine tissue fills the void inside the vulva, followed by leakage of yolk proteins and intestinal components from the weakened vulva (Fig. 5g, h). These results are illustrated in Fig. 5l and suggest that Avid observed in the absence of eggs (Fig. 4) may result from excess, unutilized yolk production. To test this hypothesis, we asked whether worms with mutations in the transcription factor skn-1, recently reported to be important for the digestion of yolk fat in the adult worm (Steinbaugh et al. 2015), had any effect on Avid rates. The results show that loss of skn-1 activity, with the accompanying loss of yolk digestion, leads to a dramatic increase in Avid rates (Fig. 5m), supporting a potential role for excess yolk in the formation of Avid.
Fig. 5.
Microscopic visualization of the vulval region during Avid. a Avid begins with leakage from the vulva at a time when the posterior uterus is paler than anterior uterus. b Picture of a nuclei labeled (pie-1::mcherry and his-72::mcherry) worm with Avid confirming that leakage does not contain cells. c Bright-field image of a and b. d DIC image of Avid II displaying leakage and vulval protrusion. e Image of labeled vulva epithelial cells (pjam-1::gfp) showing protrusion of these cells in Avid II. f Visualization of the body wall muscles during Avid with myo-3::gfp labeled worms. g Worms labeled with vit-2::gfp to show the intestinal location during late Avid II/pre Avid III. V = vulva. h–k Bright-field and fluorescent images of a worm with Avid II expressing the YP170::tdimer2 labeled yolk protein. l Illustrated schematic of the three stages of Avid and the resulting visible phenotypes. m Avid levels at death during lifespans of wild-type N2 worms and skn-1(zu135) mutant worms at 15 °C
Prevention of Avid
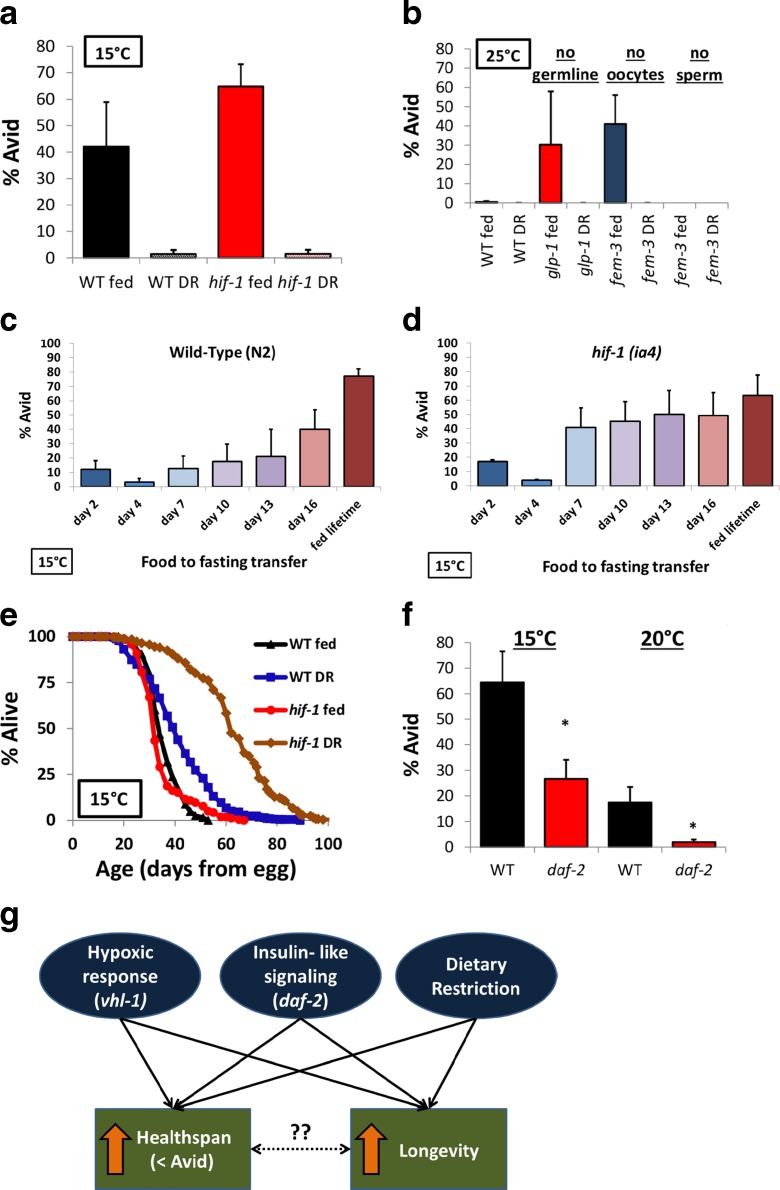
Having established a link between reproductive biology and Avid, we next asked whether we could prevent Avid through non-genetic means. Since yolk is made in the intestine from the bacterial food source, an easy way to decrease yolk production is to remove worms from food (Depuydt et al. 2013). Utilizing a dietary restriction (DR) protocol for lifespan extension published by our lab (Kaeberlein et al. 2006), we deprived worms of food (bacteria) in early adulthood (day 2 at 25 °C, day 4 at 15 °C) to test whether this might prevent Avid. DR almost completely prevents Avid in reproductive mutants (at 25 °C) and in worms lacking hif-1 (at 15 °C) (Fig. 6a, b, Table S2). We next asked how early in adulthood worms must be removed from food in order to prevent Avid and found that dietary restriction decreases Avid to a degree that correlates with the age at which they are subjected to DR in both wild-type and hif-1 worms (Fig. 6c, d). This suggests that the process that causes Avid is continual with respect to food and that the accumulation of food either in the gut or elsewhere may directly lead to the Avid phenotype. This adds further support to a model where gradual accumulation of excess yolk (depending on availability of dietary resources) leads to Avid.
Fig. 6.
Interventions that increase longevity prevent Avid. a Average Avid levels of control N2 worms and hif-1(ia4) worms in fed and dietary restriction (DR) conditions. b Avid levels of various germline mutants in fed and DR conditions. c–d Fraction of worms with Avid over their lifetime when removed from food at different times during adulthood. e Lifespans of wild-type (N2) worms and hif-1(ia4) worms in fed and bacterial deprivation conditions. f Average Avid levels of wild-type and daf-2(RNAi) worms at 15 °C and 20°. g Model describing how increased lifespan and reduced healthspan are commonly observed together. All bars represent the average of all experiments at the given temperature. All error bars represent standard error of the mean, asterisk represents p value <0.05 by paired t test
As mentioned above, many laboratories routinely censor worms with Avid from lifespan data. The censoring of Avid worms and the effect this can have on population lifespan in wild-type animals is illustrated in Fig. 2 and Table S3, where removing the Avid worms can improve longevity of strains that exhibit more Avid. We wondered whether the processes underlying Avid may play a more pronounced role in longevity under conditions where Avid is more frequent, such as lower temperature. To test this idea, we combined low temperature (15 °C) with a mutation that induces a high rate of Avid (hif-1) and subjected these animals to DR by bacterial deprivation, as described above. DR increased wild-type lifespan as expected (Kaeberlein et al. 2006), but also increased lifespan in the hif-1 mutant worm substantially more than in wild-type worms (Fig. 6e). In other words, the effect of hif-1 mutation on lifespan is dramatically different under conditions where Avid is high (normally fed, 15 °C) compared to conditions where Avid is prevented (DR, 15 °C). This difference in lifespan cannot be explained by simply censoring animals that demonstrate the overt Avid phenotype, consistent with the hypothesis that worms that do not lose vulval integrity may still have an underlying defect that limits lifespan and, perhaps, healthspan. This model suggests that censoring Avid worms only removes a subpopulation of the phenotype and may confound interpretation of lifespan results.
Since previous results showed that hypoxic signaling increases lifespan and decreases Avid (Leiser et al. 2011) and our results showed that dietary restriction, which also increases lifespan, prevents Avid, we next asked whether decreased Avid is common to a third longevity-extending pathway. We measured the Avid levels over lifespans of wild-type worms and worms depleted of the insulin-like receptor daf-2 (RNAi) and found that loss of daf-2 leads to a decrease in Avid at both 15 and 20 °C (Fig. 6f). Mutation or RNAi knockdown of daf-2 extends lifespan by reducing signaling through the insulin-like signaling pathway (Kenyon et al. 1993; Kimura et al. 1997). Thus, we find that at least three distinct longevity pathways, the hypoxic response (vhl-1), dietary restriction, and insulin-like signaling (daf-2), each decrease Avid levels and improve this measure of healthspan while also extending lifespan (Fig. 6g).
Discussion
The data reported here describe a healthspan marker, termed age-associated vulval integrity defects, or Avid, that is widely observed but rarely discussed in the fields of nematode biology and nematode aging. This term was first used several years ago by our laboratory (Leiser et al. 2011) to differentiate this phenotype from morphologically similar phenotypes (Pvl and Rup) which do not show an age dependence. We previously discovered a high rate of Avid in loss-of-function mutants for hif-1 and reciprocal low rate of Avid in long-lived vhl-1 worms with stabilized HIF-1 (Leiser et al. 2011). Here, we show that Avid is a strongly temperature- and life stage-dependent phenotype. While the overt Avid phenotype is detected primarily during post-reproductive adulthood, the effect of temperature on subsequent Avid is greatest during reproductive adulthood. In agreement with this pattern, we find that the germline is an important modulator of Avid, and that loss of oocyte production, but not sperm production, leads to a dramatic increase in Avid. We also find that the vulva cells do not show obvious defects in Avid worms apart from increased swelling, and that yolk proteins are well represented in the substance that is ejected during Avid. The data show that Avid is prevented by removing worms from food, suggesting that blocking yolk production and/or bacterial load in the gut is sufficient to robustly prevent Avid. Finally, we find that loss of the insulin-like signaling receptor daf-2 prevents Avid.
The loss of vulval integrity and accompanying ejection of fluids and/or bodily organ(s) during adulthood is an easily identifiable and robust marker of worm healthspan in worm populations. In contrast to a recent report suggesting that longevity can be dissociated from certain measures of healthspan under some conditions (Bansal et al. 2015), our results show that three genetically distinct longevity pathways (HIF-1, ILS, DR) also clearly modulate Avid with long-lived strains having reduced levels of Avid. Thus, increased healthspan can, in fact, be a byproduct of increased longevity in C. elegans. Interestingly, this correlation is reversed in the case of temperature, where Avid rates are elevated at low temperatures where lifespan is longest. This healthspan marker has, thus far, been largely overlooked, and our observations further suggest that establishing clear measures of invertebrate healthspan will be crucial for interpreting future experiments.
Unlike other worm-specific phenotypes like bagging, the Avid phenotype has no obvious selective value, as it occurs post-reproductively. It may be that Avid is selectively neutral because worms in their natural environment rarely live long enough to experience Avid; worms caught in the wild have almost invariably been found at larval stages, including dauer (Félix and Duveau 2012). Avid could also be an example of antagonistic pleiotropy, where reproduction causes the vulva to be a site of weakness that is exacerbated by excess yolk production. The hyperfunction theory of aging, which proposes that failure to shut down processes essential for growth and reproduction during adulthood contributes to age-related hypertrophy and hyperplasia (Blagosklonny 2012; Blagosklonny 2013; Gems and Partridge 2013), may provide an explanation for overproduction of yolk leading to Avid. Alternatively, overproduction of yolk may provide an advantage to larval progeny, if larval worms are able to take up the yolk and other proteins ejected from the parental animal (Rompay et al. 2015).
The data described here suggest that the Avid phenotype may also relate to accumulation of food in the gut or pseudocoelomic space that then leads to increased pressure and loss of vulval integrity. A variation of this phenomenon is also seen in humans in pelvic organ prolapse, where loss of integrity of the female reproductive organs causes prolapse of the pelvic floor. This is often accompanied by incontinence and is strongly associated with obesity (Machin and Mukhopadhyay 2011). In a sense, it can be argued that Avid is a phenotype analogous to obesity in higher organisms. When the worms have too much yolk (protein and fat), they literally burst. We find this hypothesis intriguing, especially when modified to describe the increased yolk as akin to visceral fat. This would draw a parallel between worms and mammals where increased subcutaneous fat (in worms, hypodermal fat, known to be increased in long-lived models including daf-2 mutant worms (Ogg et al. 1997)) is necessary and potentially beneficial, whereas visceral fat (analogous to yolk in worms) leads to a number of health problems (Seidell et al. 1990). This potential link between Avid and obesity is not yet proven but may connect what is typically assumed to be a worm-specific phenotype directly to mammalian health.
There are also a number of alternative hypotheses that could explain portions of our data. In Fig. 3, we show that the amount of time spent at higher or lower temperature has a powerful effect on the rate of Avid. We suggest that the temperature in which worms spend their reproductive life is most important for their rate of Avid, but other possibilities exist. It is possible, for example, that turning on heat shock proteins in the more stressful 25 degree temperatures prevents Avid, and our results simply show the loss of worm’s ability to activate heat shock machinery over time. We think that this is less likely since just a short time at 25 °C is known to be sufficient to extend worm lifespan (Zhang et al. 2015) but has little effect on Avid, but it is also possible that other mechanisms are at work. We note that these experiments did not include the intermediate and more common 20 °C condition because at that temperature, Avid rates are both lower than at 15 °C and variable to the point where obtaining robust data is challenging. In Fig. 6, we show the synergistic effects of combining a null mutation to hif-1 with dietary restriction. While we suggest that this synergy is likely due to a complete reversal of the Avid phenotype, both the observed and underlying phenotype, it is also possible that the loss of hif-1 interacts with DR in a novel way. Previous reports by us and others have shown both HIF-1 and DR to be important in neurons (Bishop and Guarente 2007; Leiser et al. 2015) and that hif-1 may require aspects of the DR pathway to affect longevity (Chen et al. 2009).
The finding that Avid levels increase in germline mutants, even at 25 °C, further suggests that the mechanisms of Avid can be separated from lifespan in certain instances. Glp-1(e2141) worms have a complete loss of the germline that leads to an increase in longevity dependent upon the presence of daf-16 (Berman and Kenyon 2006). Mutation in glp-4(bn2) also causes loss of germline proliferation but does not lead to increased longevity (TeKippe and Aballay 2010), but leads to a similar increase in Avid as the glp-1 mutation. This suggests that loss of the germline, and not changes in longevity, are more important to the Avid phenotype. This assertion is supported by two mutants that either lack oocytes (fem-3(q20)) or have small eggs (glp-2(ar202gf)), neither of which have strong lifespan phenotypes but both of which show increased Avid. Our results support a model in which the germline strongly affects Avid and can affect lifespan, but the effects are distinct.
In the simple, post-mitotic nematode C. elegans, metabolism is largely geared toward the production for 200–300 viable eggs per adult hermaphrodite. Therefore, any changes in the number of eggs or the ability of the eggs to take up nutrients will cause downstream effects that could lead to excess nutrients in the adult. This offers an explanation for the temperature dependence of Avid: a previous report shows that worms at 25 °C produce more oocytes than worms at 15 °C (Klass 1977). This change in oocyte number, similar to the drastic change in oocyte numbers in the germline mutants we describe, could explain why temperature is so important to the formation of Avid. Alternatively, changes in food quantity, food quality, and/or digestive capacity at different temperatures could plausibly lead to similar effects. Regardless, we submit that the Avid phenotype, while its mechanisms may be somewhat complex, is a robust measure of a specific C. elegans health outcome that may share conserved molecular mechanisms with human diseases of aging.
Avid in aging
It is clear from these studies that Avid is, at the very least, an important marker of nematode healthspan. We suggest that a more careful assessment of this phenotype in aging studies could benefit the entire field. Further, we submit that our data demonstrate that censoring Avid animals from survival studies based on the claim that Avid is not part of “normal aging” is not justified, as this is clearly an age-associated phenotype. It may be that the mechanisms underlying the Avid phenotype will closely relate to one or more fundamental mechanisms of aging, and that Avid is a phenotype that represents an important marker of longevity in addition to healthspan. Further studies on this phenotype may yield important insights into one or more of the various causes of death in nematodes and shed light on the mechanisms of aging in other organisms.
Experimental procedures
Growth conditions
Standard C. elegans procedures were used as previously described (Kaeberlein et al. 2006; Sutphin and Kaeberlein 2008). Worms were maintained on solid Nematode Growth Medium (NGM) using UV-killed E. coli OP50 throughout life. All experiments were conducted at the temperature noted (except in cases where worms were transferred between temperatures). All worms were kept at the noted temperature for at least two generations prior to measurement. All strains used in this study are listed in Table S4.
Lifespans/Avid measurements
Lifespans were carried out as previously described (Kaeberlein et al. 2006; Sutphin and Kaeberlein 2008; Sutphin and Kaeberlein 2009). Gravid adults were placed on NGM plates seeded with UV-killed OP50 lawns for a timed egg-lay. Subsequently, adults were removed from the plates, and eggs were allowed to develop. At late L4/early adult stage, worms were transferred to FUdR to prevent the development of progeny. The presence or absence of FUdR did not significantly alter Avid rates (Fig. S2). Approximately 75 worms were placed on each NGM + FUdR plate seeded with 20× UV-killed OP50. A minimum of two plates per strain per condition were used. Worms were transferred periodically to prevent starvation and avoid contamination. Worms were scored as dead when they did not move in response to prodding under a dissection microscope, and their Avid state was noted. Worms that crawled off the plate were not considered. On the rare occasions where a male was present on the plate, that male was removed promptly at the L4/YA stage.
Microscopy
The worms for each strain were synchronized by a timed egg lay and allowed to develop at different temperatures (15, 20, or 25 °C, according to the desired experiment). Microscope slides were prepared 1 h prior to microscopy with a 2 % agar mount. Worms of various Avid states were selected from each strain and washed in M9. The specimens were immobilized in 10 μl of 30 mM sodium azide placed on the agar pad for 8 min. Pictures were taken immediately after slide preparation using a Nikon Eclipse E600 microscope using a QImagingRetigia EX CCD Camera, and the fluorescence intensity was analyzed using ImageJ (Schneider et al. 2012).
Statistical analysis
Individual lifespan experimental data are provided in Table S3. A two-tailed Student’s t test was used to derive p values for comparisons such as Avid rate. All error bars shown in figures represent the standard error of the mean.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 40 kb)
(PDF 110 kb)
(PDF 59 kb)
(PDF 52 kb)
(PDF 305 kb)
(PDF 49 kb)
Acknowledgments
Strains were provided by the Caenorhabditis Genetics Center. This work was supported by NIH grant R01AG038518 to MK and NIH grant K99AG045200 to SFL. SFL was supported by NIH Training Grant T32AG000057. SFL was also supported by an AFAR post-doctoral fellowship. Additional support was provided by the UW Healthy Aging and Longevity Research Institute, the UW Nathan Shock Center of Excellence in the Basic Biology of Aging (NIH grant P30AG013280), and an award to MK from the M. J. Murdock Charitable Trust.
Compliance with Ethical Standards
Authors’ contributions
SFL, GJ, and MK designed the experiments. SFL, GJ, MP, GLS, JD, AL, and MF performed the experiments. SFL, GJ, and MK analyzed the experiments. SFL, GJ, and MK wrote the paper.
Footnotes
Scott F. Leiser and Gholamali Jafari contributed equally to this work.
Contributor Information
Scott F. Leiser, Phone: 206 221 4849, Email: sleiser@uw.edu
Matt Kaeberlein, Phone: 206 543 4849, Email: kaeber@uw.edu.
References
- Bansal A, Zhu LJ, Yen K, Tissenbaum HA. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc Natl Acad Sci U S A. 2015;112:E277–286. doi: 10.1073/pnas.1412192112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton MK, Schedl TB, Kimble J. Gain-of-function mutations of fem-3, a sex-determination gene in Caenorhabditis elegans. Genetics. 1987;115:107–119. doi: 10.1093/genetics/115.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beanan MJ, Strome S. Characterization of a germ-line proliferation mutation in C. elegans. Development. 1992;116:755–766. doi: 10.1242/dev.116.3.755. [DOI] [PubMed] [Google Scholar]
- Berman JR, Kenyon C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell. 2006;124:1055–1068. doi: 10.1016/j.cell.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Answering the ultimate question “what is the proximal cause of aging?”. Aging (Albany NY) 2012;4:861–877. doi: 10.18632/aging.100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging is not programmed: genetic pseudo-program is a shadow of developmental growth. Cell Cycle. 2013;12:3736–3742. doi: 10.4161/cc.27188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch JB, Augustine AD, Frieden LA, Hadley E, Howcroft TK, Johnson R, Khalsa PS, Kohanski RA, Li XL, Macchiarini F, Niederehe G, Oh YS, Pawlyk AC, Rodriguez H, Rowland JH, Shen GL, Sierra F, Wise BC. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S1–3. doi: 10.1093/gerona/glu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Thomas EL, Kapahi P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000486. doi: 10.1371/journal.pgen.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Caswell-Chen EP. Facultative Vivipary is a Life-History Trait in Caenorhabditis elegans. J Nematol. 2004;36:107–113. [PMC free article] [PubMed] [Google Scholar]
- Coburn C, Allman E, Mahanti P, Benedetto A, Cabreiro F, Pincus Z, Matthijssens F, Araiz C, Mandel A, Vlachos M, Edwards SA, Fischer G, Davidson A, Pryor RE, Stevens A, Slack FJ, Tavernarakis N, Braeckman BP, Schroeder FC, Nehrke K, Gems D. Anthranilate fluorescence marks a calcium-propagated necrotic wave that promotes organismal death in C. elegans. PLoS Biol. 2013;11:e1001613. doi: 10.1371/journal.pbio.1001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi AK, Wightman B, Chalfie M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics. 2015;200:387–407. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt G, Xie F, Petyuk VA, Shanmugam N, Smolders A, Dhondt I, Brewer HM, Camp DG, Smith RD, Braeckman BP. Reduced insulin/insulin-like growth factor-1 signaling and dietary restriction inhibit translation but preserve muscle mass in Caenorhabditis elegans. Mol Cell Proteomics. 2013;12:3624–3639. doi: 10.1074/mcp.M113.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix MA, Duveau F. Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae. BMC Biol. 2012;10:59. doi: 10.1186/1741-7007-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Partridge L. Genetics of longevity in model organisms: debates and paradigm shifts. Annu Rev Physiol. 2013;75:621–644. doi: 10.1146/annurev-physiol-030212-183712. [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton B, Dong Y, Shindo M, Liu W, Odell I, Ruvkun G, Lee SS. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 2005;19:1544–1555. doi: 10.1101/gad.1308205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. Sex determination in the nematode C. elegans: analysis of tra-3 suppressors and characterization of fem genes. Genetics. 1986;114:15–52. doi: 10.1093/genetics/114.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE, Cypser J, de Castro E, de Castro S, Henderson S, Murakami S, Rikke B, Tedesco P, Link C. Gerontogenes mediate health and longevity in nematodes through increasing resistance to environmental toxins and stressors. Exp Gerontol. 2000;35:687–694. doi: 10.1016/S0531-5565(00)00138-8. [DOI] [PubMed] [Google Scholar]
- Kaeberlein TL, Smith ED, Tsuchiya M, Welton KL, Thomas JH, Fields S, Kennedy BK, Kaeberlein M. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006;5:487–494. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Peterson C. Healthspan, translation, and new outcomes for animal studies of aging. J Gerontol A Biol Sci Med Sci. 2009;64:209–212. doi: 10.1093/gerona/gln063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass M. A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mech Ageing Dev. 1983;22:279. doi: 10.1016/0047-6374(83)90082-9. [DOI] [PubMed] [Google Scholar]
- Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Leiser SF, Begun A, Kaeberlein M. HIF-1 modulates longevity and healthspan in a temperature-dependent manner. Aging Cell. 2011;10:318–326. doi: 10.1111/j.1474-9726.2011.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Miller H, Rossner R, Fletcher M, Leonard A, Primitivo M, Rintala N, Ramos FJ, Miller DL, Kaeberlein M (2015). Cell nonautonomous activation of flavin-containing monooxygenase promotes longevity and health span. Science 350(6266):1375–1378 [DOI] [PMC free article] [PubMed]
- Machin SE, Mukhopadhyay S. Pelvic organ prolapse: review of the aetiology, presentation, diagnosis and management. Menopause Int. 2011;17:132–136. doi: 10.1258/mi.2011.011108. [DOI] [PubMed] [Google Scholar]
- Martin GM, Bergman A, Barzilai N. Genetic determinants of human health span and life span: progress and new opportunities. PLoS Genet. 2007;3:e125. doi: 10.1371/journal.pgen.0030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee MD, Weber D, Day N, Vitelli C, Crippen D, Herndon LA, Hall DH, Melov S. Loss of intestinal nuclei and intestinal integrity in aging C. elegans. Aging Cell. 2011;10:699–710. doi: 10.1111/j.1474-9726.2011.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R, Steinkraus KA, Sutphin GL, Ramos FJ, Shamieh LS, Huh A, Davis C, Chandler-Brown D, Kaeberlein M. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science. 2009;324:1196–1198. doi: 10.1126/science.1173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajan S, Govindan JA, McGovern M, Hubbard EJ, Greenstein D. MSP and GLP-1/Notch signaling coordinately regulate actomyosin-dependent cytoplasmic streaming and oocyte growth in C. elegans. Development. 2009;136:2223–2234. doi: 10.1242/dev.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Petrascheck M, Ye X, Buck LB. A high-throughput screen for chemicals that increase the lifespan of Caenorhabditis elegans. Ann N Y Acad Sci. 2009;1170:698–701. doi: 10.1111/j.1749-6632.2009.04377.x. [DOI] [PubMed] [Google Scholar]
- Rompay LV, Borghgraef C, Beets I, Caers J, Temmerman L. New genetic regulators question relevance of abundant yolk protein production in C. elegans. Sci Rep. 2015;5:16381. doi: 10.1038/srep16381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidell JC, Björntorp P, Sjöström L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39:897–901. doi: 10.1016/0026-0495(90)90297-P. [DOI] [PubMed] [Google Scholar]
- Steinbaugh MJ, Narasimhan SD, Robida-Stubbs S, Moronetti Mazzeo LE, Dreyfuss JM, Hourihan JM, Raghavan P, Operaña TN, Esmaillie R, Blackwell TK. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. Elife. 2015;4:e07836. doi: 10.7554/eLife.07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphin GL, Kaeberlein M. Dietary restriction by bacterial deprivation increases life span in wild-derived nematodes. Exp Gerontol. 2008;43:130–135. doi: 10.1016/j.exger.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphin GL, Kaeberlein M (2009) Measuring Caenorhabditis elegans life span on solid media. J Vis Exp 12(27):1152. doi:10.3791/1152 [DOI] [PMC free article] [PubMed]
- Sutphin GL, Bishop E, Yanos ME, Moller RM, Kaeberlein M. Caffeine extends life span, improves healthspan, and delays age-associated pathology in Caenorhabditis elegans. Longev Healthspan. 2012;1:9. doi: 10.1186/2046-2395-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TeKippe M, Aballay A. C. elegans germline-deficient mutants respond to pathogen infection using shared and distinct mechanisms. PLoS One. 2010;5:e11777. doi: 10.1371/journal.pone.0011777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shao Z, Zhai Z, Shen C, Powell-Coffman JA. The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans. PLoS One. 2009;4:e6348. doi: 10.1371/journal.pone.0006348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Xiao R, Ronan EA, He Y, Hsu AL, Liu J, Xu XZ. Environmental Temperature Differentially Modulates C. elegans Longevity through a Thermosensitive TRP Channel. Cell Rep. 2015;11:1414–1424. doi: 10.1016/j.celrep.2015.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 40 kb)
(PDF 110 kb)
(PDF 59 kb)
(PDF 52 kb)
(PDF 305 kb)
(PDF 49 kb)