Abstract
Older individuals who exercise regularly exhibit greater resistance to oxidative stress than their sedentary peers, suggesting that exercise can modify age-associated loss of resistance to oxidative stress. However, we recently demonstrated that a single bout of exercise confers protection against a subsequent oxidative challenge in young, but not older adults. We therefore hypothesized that repeated bouts of exercise would be needed to increase resistance to an oxidative challenge in sedentary older middle-aged adults. Sedentary older middle-aged men and women (50–63 years, n = 11) participated in an 8-week exercise intervention. Maximal oxygen consumption was measured before and after the intervention. The exercise intervention consisted of three sessions per week, for 45 min at an intensity corresponding to 70–85 % maximal heart rate (HRmax). Resistance to oxidative stress was measured by F2-isoprostane response to a forearm ischemia/reperfusion (I/R) trial. Each participant underwent the I/R trial before and after the exercise intervention. The intervention elicited a significant increase in maximal oxygen consumption (VO2max) (P < 0.0001). Baseline levels of F2-isoprostanes pre- and post-intervention did not differ, but the F2-isoprostane response to the I/R trial was significantly lower following the exercise intervention (time-by-trial interaction, P = 0.043). Individual improvements in aerobic fitness were associated with greater improvements in the F2-isoprostane response (r = −0.761, P = 0.011), further supporting the role of aerobic fitness in resistance to oxidative stress. These data demonstrate that regular exercise with improved fitness leads to increased resistance to oxidative stress in older middle-aged adults and that this measure is modifiable in previously sedentary individuals.
Keywords: Redox balance, F2-isoprostanes, Aging, Ischemia-reperfusion
Introduction
Aging is associated with significant impairment in tolerance to acute oxidative stressors, and this impairment appears to contribute to the increased rate of morbidity and mortality of older adults (Finkel and Holbrook 2000). Therefore, increasing the capacity to handle these stressors may play an important role in the prevention of age-related disease leading to more successful aging. It is projected that health care spending will increase by 25 % due to the shift in demographics (Jacobzone and Oxley 2002). In addition, less than 16 % of adults currently aged 55–64 meet the minimum recommended guidelines for physical activity, which may further exacerbate health problems in the future (Statistics 2015). While it is well accepted that exercise is beneficial, it is less clear whether the effects of sedentary lifestyle can be reversed when started later in life, in terms of redox balance and disease risk.
Previously, we have shown in a cross-sectional study in older adults that physical fitness is associated with lower oxidative stress and greater capacity to resist an oxidative challenge (Traustadottir et al. 2012), and that a single bout of exercise is protective against a subsequent non-exercise oxidative challenge in young, but not older adults (Nordin et al. 2014). These data suggest not only that older adults have a reduced capacity to constrain an oxidative insult, but that there may be a loss of signal transduction in the endogenous antioxidant response in older adults.
Studies in traditional models of oxidative stress have shown that repeated exposure to transient oxidizing environments, such as repeated doses of hydrogen peroxide, lead to upregulation of defense enzymes resulting in increased protection against later oxidative exposures of higher concentration or duration (Pickering et al. 2013). In a similar fashion, regular exercise repeatedly flexes the redox environment, which could in turn strengthen the endogenous antioxidant defense network. Thus, regular exercise may be an effective and inexpensive means for improving redox balance.
Exercise intervention studies have traditionally measured the effect of the intervention on basal levels of circulating biomarkers of oxidative stress pre- and post- treatment or have used an exercise challenge as the method of increasing oxidative stress for measurement (Campbell et al. 2010). However, neither of these methods for measuring oxidative stress necessarily translates into how well an individual can tolerate an oxidative insult. The endogenous antioxidant defense system is dynamically upregulated in response to shifts in redox balance, thus measuring the dynamic response to such a shift provides a clearer picture of the individual’s ability to constrain an oxidative challenge when compared to measuring basal levels. Additionally, in order to answer the question of whether adaptations to exercise translate to resistance towards non-exercise oxidative stressors, it is important to choose a stressor that is different from that of exercise. Forearm ischemia/reperfusion (I/R) is a well-established means for measuring the dynamic response to a non-exercise oxidative challenge (Davies et al. 2009; Traustadottir et al. 2012). I/R causes a robust increase in the production of reactive oxygen species, and models common acute events experienced in older adults such as cardiovascular disease and trauma (Kharbanda et al. 2002). By measuring the F2-isoprostane response to forearm I/R, individual responses can be quantified.
The purpose of this study was to investigate whether a regular exercise intervention could increase resistance to oxidative stress as measured by the F2-isoprostane response to forearm I/R. Perturbations caused by acute exercise are not limited to active skeletal muscle (Tanaka et al. 2006); therefore, it is reasonable to assume that adaptations to regular exercise should confer systemic resistance as measured by the forearm I/R trial, similar to remote preconditioning (Kharbanda et al. 2002; Seeger et al. 2015). We hypothesized that successive bouts of acute aerobic exercise during an 8-week exercise intervention would improve this resistance by stimulating the signaling pathways for production of endogenous antioxidants, in turn providing increased protection against a non-exercise oxidative challenge.
Methods
Participants
Eleven inactive men (n = 4) and women (n = 7) aged 50–63 years were recruited from the community. The study participants were generally healthy (no overt disease), non-smokers, not taking antioxidant supplementation in excess of a daily multi-vitamin, not regularly taking non-steroidal anti-inflammatory drugs, and not overly obese (BMI ≤ 33.0 kg/m2). Subjects were eligible to participate if they had not been actively exercising for 6 months prior to the study, as per self-report. Individuals were excluded if they had experienced a myocardial infarction within the last 6 months or if there were any clinically significant electrocardiogram (EKG) abnormalities at rest or during the maximal oxygen consumption (VO2max) test. Any condition that would contraindicate maximal exercise testing, including elevated blood pressure at rest or musculoskeletal problems, excluded subjects from participating in the study. Women were post-menopausal and were not taking any hormone replacement therapy as we have previously shown that estrogen has a significant effect on the F2-isoprostane response to the I/R trial (Davies et al. 2009). All the participants signed a written informed consent approved by the Northern Arizona University Institutional Review Board.
Study design
The study was a longitudinal study employing an 8-week supervised aerobic exercise intervention. The participants were inactive for at least 6 months prior to enrolling in the study, and were screened for eligibility as described below. Each participant completed a maximal graded exercise test on a cycle ergometer to determine aerobic fitness (VO2max) and maximal heart rate to inform the training intensity used during the intervention. The study participants completed a forearm ischemia/reperfusion (I/R trial) prior to beginning the exercise intervention, and following completion of the intervention in order to determine their ability to constrain an oxidative insult. These visits were separated from the VO2max test or any other acute exercise by a minimum of 48 h to control for any confounding effects of acute exercise. Due to funding limitations, a control group for the I/R trial was not included; however, we have previously shown that there is no preconditioning effect to the I/R trial in time periods even as short as 2 and 4 weeks apart (Traustadóttir et al. 2009).
Screening visit
Prior to any exercise testing, the participants completed a health history questionnaire. Height, weight, waist circumference, body composition (skinfolds), and resting blood pressure were measured, and a 12-lead supine resting electrocardiogram was obtained for screening any abnormalities that would exclude the participant. Waist circumference was measured at the level of the umbilicus using a tape measure. Skinfolds were taken at five sites: triceps, subscapular, thigh, abdominal, and suprailiac. All skinfold measures were taken by the same technician using Lange skinfold calipers. Enrolled subjects underwent these same measures following the exercise intervention.
Maximal oxygen consumption test
VO2max was measured with a graded exercise test performed on a cycle ergometer as previously described (Traustadottir et al. 2012; Traustadóttir et al. 2008). The starting workload (typically 15 or 20 W) was selected based on the predicted maximal workload for each individual, and was increased every minute until volitional exhaustion. The participants were instructed to maintain a pedaling rate of 60–70 rpm throughout the test. Oxygen consumption was measured by indirect calorimetry using a metabolic measurement cart (Vmax29, CareFusion, Yorba Linda, CA, USA). Heart function was monitored with continuous 12-lead electrocardiogram. VO2max was considered achieved if two of the following three criteria were met: (1) a plateau in VO2 with an increase in workload, (2) a respiratory exchange ratio ≥1.10, and (3) heart rate within ten beats of age-predicted maximal heart rate (Kohrt et al. 1991). Standard contraindications to exercise testing and termination criteria outlined by American College of Sports Medicine were followed at all times.
Exercise intervention
The study participants reported to the Northern Arizona University Recreation Center for supervised aerobic training three times per week utilizing treadmill, elliptical trainer, and stationary cycling modalities for 45 min per session. The participants wore heart rate monitors (Polar RS4000, Polar Electro Inc., Lake Success, NY, USA) throughout exercise sessions to monitor heart rate. Heart rate was chosen as the measure of exercise intensity in order to avoid the need for additional fitness testing during the intervention, as maximal heart rate is unlikely to change significantly in response to a training program. Target heart rate was set at 70–85 % maximal heart rate as measured by maximal exercise testing. The participants were introduced to the exercise protocol over the course of 2 weeks, starting at 30 min for the first two sessions, with an increase of 5-min total exercise time every two sessions until the final target exercise time of 45 min was achieved. After the completion of each exercise session, the participants performed an active cool-down.
Forearm ischemia/reperfusion trial
The study participants reported to the laboratory pre- and post-exercise intervention to complete the forearm ischemia/reperfusion trial as previously described (Davies et al. 2009; Traustadóttir et al. 2009; Traustadottir et al. 2012). Briefly, an intravenous catheter was inserted into the arm, and a baseline blood sample was collected (pre). The catheter was kept in situ with a slow saline drip throughout the trial. A blood pressure cuff was placed on the same arm, inflated to 200 mmHg, and kept inflated for 10 min then released for 2 min. This inflation procedure was repeated twice more (total time: 34 min). After the three ischemia/reperfusion periods, additional blood samples were obtained at 15, 30, 60, 120, 180, and 240 min after the final cuff deflation.
F2-isoprostanes analyses
Samples for F2-isoprostane analyses were collected into serum-separating vacutainer tubes (SST) and kept at room temperature for 30 min to clot, then placed in a refrigerator (4 °C) until being centrifuged at 3000 rpm for 15 min. Plasma was aliquoted into micro-centrifuge tubes for storage at −80 °C until analysis. All samples were analyzed at the Vanderbilt Eicosanoid Core. Free F2-isoprostanes in plasma were quantified, after purification and derivatization, using gas chromatography/negative ion chemical ionization–mass spectrometry with [2H4]15-F2t-isoprostane as an internal standard (Morrow and Roberts 1999). Compounds were analyzed as pentafluorobenzyl ester, trimethylsilyl ether derivatives by monitoring mass-to-charge ratios of 569 and 573 for endogenous F2-isoprostanes and the [2H4]15-F2t-isoprostane internal standard, respectively.
Statistical analyses
Participants’ anthropometric and exercise testing data were compared pre- and post-intervention by paired t test to determine the effect of the exercise intervention on improving fitness. The plasma F2-isoprostane response across time comparing IR-pre and IR-post was analyzed by 2 × 7 repeated-measures ANOVA (trial × time point). The effectiveness of the I/R challenge is reported by the quadratic effect of time as the expected response of the trial is an increase followed by a decrease in F2-isoprostanes. The integrated F2-isoprostane responses were calculated for each individual as area under the curve (AUC) and area under the response curve (AURC) by the method of the trapezoidal rule. Mean AURC and AUC responses were analyzed by paired t test, comparing I/R-pre and I/R-post. Pearson correlation and linear regression were used to establish the relationship between various outcome variables. All comparisons were considered significant at P < 0.05. All data are reported as means ± SEM. Analyses were conducted using the statistical package “R” and IBM SPSS Statistics 22 Software (IBM Corp., Armonk, NY, USA).
Results
Thirty-two subjects were screened for participation in the study. Of those, 21 were excluded due to their physical activity levels, fitness level, scheduling conflicts, or other exclusion criteria. The final cohort included 11 participants comprised of four men and seven women. Participant characteristics are provided in Table 1.
Table 1.
Subject characteristics pre- and post-exercise intervention
| Variable | Pre | Post |
|---|---|---|
| Age (years) | 57 ± 1 | – |
| Height (cm) | 170.7 ± 3.1 | – |
| Weight (kg) | 81.2 ± 6.9 | 80.2 ± 6.9 |
| BMI (kg/m2) | 27.4 ± 1.7 | 27.1 ± 1.7 |
| Body fat (sum of skinfolds) | 123.6 ± 14.3 | 111.5 ± 13.5 |
| WC (cm) | 95.5 ± 5.0 | 94.4 ± 5.5 |
| SBP (mmHg) | 122 ± 3 | 119 ± 4 |
| DBP (mmHg) | 80 ± 3 | 78 ± 2 |
| VO2max (ml kg−1 min−1) | 26.9 ± 1.7 | 30.4 ± 1.9*** |
| WLmax (W) | 156 ± 14 | 184 ± 17*** |
| AUC (pg ml−1 min−1) | 9948 ± 1479 | 8892 ± 1010 |
| AURC (pg ml−1 min−1) | 1942 ± 267 | 1513 ± 313 |
| Baseline F2-isop (pg ml−1) | 27.7 ± 4.1 | 28.1 ± 2.9 |
Values are means ± SEM
BMI body mass index, WC waist circumference, SBP systolic blood pressure, DBP diastolic blood pressure, VO 2max maximal oxygen consumption,WL max maximal workload, AUC area under the curve, AURC area under the response curve
***P<0.001; difference from pre- to post-intervention
Effects of an 8-week exercise intervention on subject characteristics and fitness
The 8-week exercise intervention significantly improved aerobic fitness by 13.4 %, with a mean increase of 3.6 ± 0.5 ml kg−1 min−1 in relative VO2max (P < 0.0001). The participants attended an average of 23 of the total 24 required visits, for an adherence of 94 %. Post-exercise intervention VO2max data were missing for one subject due to health complications unrelated to the study during the time frame allotted for post-intervention VO2max testing, resulting in an n = 10 for all analyses requiring VO2max data. The maximal workload reached during the maximal exercise test significantly improved by 27 ± 6 W (P = 0.0008). There were no significant changes in any clinical measures (SBP, DBP, and maximal heart rate (HRmax)) or body composition measures (WC, BMI, weight, and sum of skinfolds) as shown in Table 1.
Response to forearm I/R trial
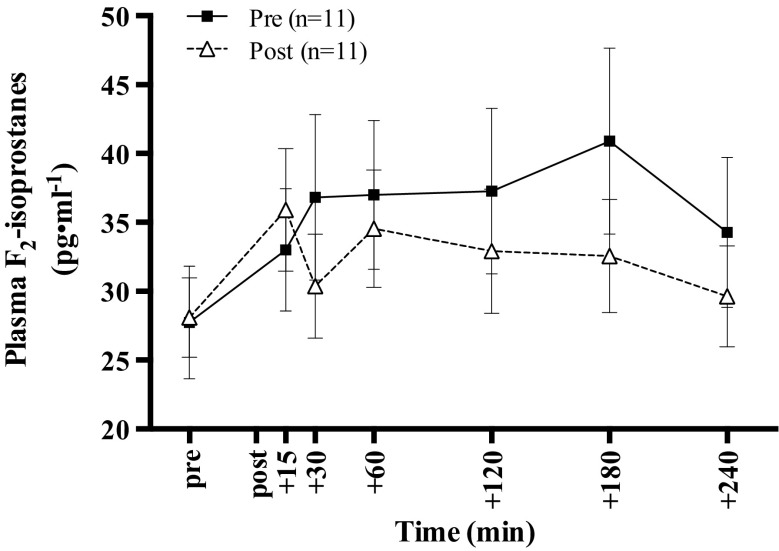
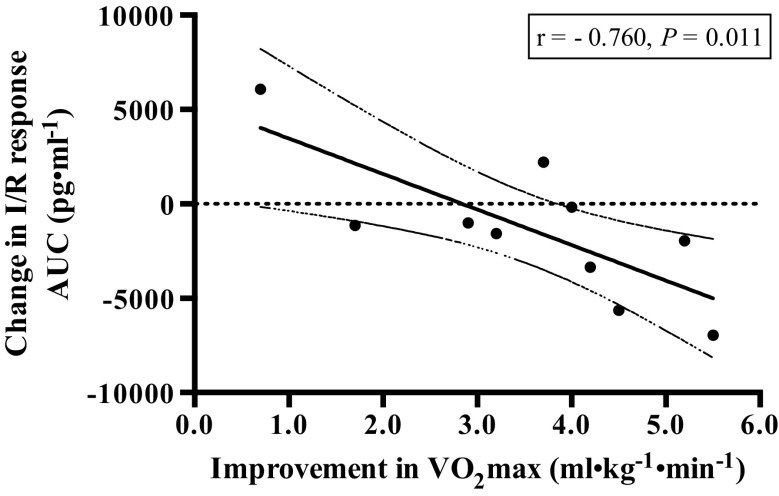
Baseline values for F2-isoprostanes did not differ pre- and post-exercise intervention. F2-isoprostane levels were significantly increased in response to the I/R trial in all visits (P = 0.001) as shown in Fig. 1. A significant interaction between time point and trial was present (P = 0.043), but main effect of trial was not significant. Time point indicates the F2-isoprostane values at each blood draw during the I/R trial, while trial indicates the I/R trials pre- and post-exercise intervention. The overall integrated F2-isoprostane responses (AURC and AUC) did not differ significantly following the exercise intervention; however, there was a significant negative correlation between changes in aerobic fitness and AUC (r = –0.760, P = 0.011) as shown in Fig. 2.
Figure 1.
Plasma F2-isoprostane response to the I/R trial pre- and post-exercise intervention. All values are mean ± SEM. Change across time before and after the 8-week exercise intervention. The time period between pre and post on the x-axis denotes the forearm ischemia/reperfusion trial. Baseline values did not differ between trials (P = 0.887). There was a significant interaction between time (I/R trial) and trial (P = 0.043). In addition, there was a significant main effect of time (P = 0.001) but not of trial (P = 0.421)
Figure 2.
Relationship between changes in aerobic capacity and changes in response to the I/R trial. There was a significant negative Pearson correlation between improvements in relative fitness (VO2max) and changes in the F2-isoprostane response to the I/R trial (AUC) following the exercise intervention (r = −0.760, P = 0.011). The solid line denotes the linear regression line of best fit. Dashed lines indicate the 95 % confidence intervals
Discussion
Cross-sectional studies have shown that older adults who are fit have a lower F2-isoprostane response to an ischemic challenge than their unfit peers, and individuals reporting high indices of lifetime physical activity have reduced systemic levels of oxidative damage (Traustadottir et al. 2012). Here, we report the results from a small longitudinal pilot study where we investigated whether an exercise intervention could increase resistance to an acute oxidative challenge in previously sedentary older adults. We tested the hypothesis that an 8-week aerobic exercise intervention would improve the capacity to resist an oxidative challenge in sedentary older middle-aged adults as measured by changes in F2-isoprostanes in response to a known oxidative challenge, forearm ischemia/reperfusion (I/R trial) (Davies et al. 2009; Traustadottir et al. 2012). Our hypothesis was supported by a significant time-by-trial interaction showing a lower F2-isoprostane response to the I/R trial following completion of the exercise intervention. While differences of pre- to post-exercise training in AUC and AURC did not reach statistical significance, the pattern of response is key to interpretation of these results. The response to the I/R trial pre-exercise intervention resulted in a sustained elevation of F2-isoprostanes, while post-intervention F2-isoprostanes peaked and then returned to baseline over the course of 4 h. The differences in the pattern of response are supported by the time-by-trial interaction and suggest that older middle-aged adults can in fact restore their resilience to acute oxidative challenges through regular exercise.
Acute exercise is a potent inducer of oxidative, metabolic, mechanical, and thermal stress. These responses are not limited to the actively exercising skeletal muscle (Peake et al. 2015). Data from multiple studies across species and exercise modalities have demonstrated that a single bout of exercise increases biomarkers of oxidative stress in nearly all tissues (Nikolaidis et al. 2012; Nikolaidis et al. 2011; Shing et al. 2007). This signaling, along with other biochemical messengers such as growth factors, cytokines, and eicosanoids, regulates the adaptive responses (Egan and Zierath 2013). However, the acute response of the endogenous redox system appears to be impaired with aging. In healthy young and active older adults, skeletal muscle contractions induce production of reactive oxygen species through increased mitochondrial and NADPH oxidase activity (Powers et al. 2011; Sakellariou et al. 2014; Sakellariou et al. 2013); yet, the increases in reactive oxygen species (ROS) in response to exercise are attenuated in sedentary older adults and animals. For example, Nyberg et al. (2014) demonstrated that a single session of leg exercise increased venous concentrations of the oxidized form of the antioxidant glutathione in young sedentary and older active adults in an intensity-dependent manner. However, no changes were observed in venous oxidized glutathione levels in the older sedentary group, despite undergoing the same exercise stimulus (Nyberg et al. 2014). In addition, resting levels of glutathione were lower in the older sedentary group, whereas the young sedentary and older active adults had significantly higher levels. Reactive oxygen species are known mediators of exercise adaptations; thus, in the absence of transient shifts in the redox balance, the benefits of a single session of exercise are likely lost in older adults.
Recent data from our laboratory also support this notion. We found that a single session of aerobic exercise in young adults offers protection against a subsequent non-exercise oxidative challenge (forearm I/R trial) as evidenced by a lower F2-isoprostane response. In contrast, older adults did not receive any protection from a single session of exercise performed on the day preceding the challenge (Nordin et al. 2014). Similarly, other studies have demonstrated that benefits of ischemic preconditioning are attenuated in older adults (Powers et al. 2004; van den Munckhof et al. 2013). The evidence suggests a loss in signal transduction, and that a single session of exercise is insufficient to elicit adaptations in older adults. However, there is little doubt that the most effective strategy for slowing age-related physiological declines and oxidative stress is regular exercise (Booth and Hargreaves 2011).
While a single exercise session may be insufficient to stimulate the redox system, repeated bouts of exercise, as occur in a regular exercise program, could flex the redox balance acting in an additive fashion as a primer against ensuing larger oxidative challenges. Studies in fruit flies and mammalian cells suggest that “priming the system” with repeated exposure to ROS—comparable to repeated bouts of acute exercise—confers greater resistance to an oxidative challenge, compared to a single exposure (Pickering et al. 2013). Similarly, it has been shown in rodent models of aging that regular exercise training restores the responsiveness of the antioxidant network, primarily through ROS-regulated transcription factors (Gounder et al. 2012; Zhao et al. 2013). We also know that lifelong physical activity is associated with reduced levels of oxidative stress, and an increased capacity to handle acute oxidative challenges in older adults (Cobley et al. 2014; Lessiani et al. 2015; Nyberg et al. 2014; Traustadottir et al. 2012).
The results from the present study are in disagreement with some previous studies that failed to find significant differences in markers of oxidative stress following an exercise intervention (Campbell et al. 2010). However, these studies often employed only baseline measures that may not capture the ability of the system to adapt to dynamic changes to the redox balance. For instance, our baseline measures of F2-isoprostanes were not different between pre- and post-testing suggesting that simply measuring basal (resting) markers without any type of challenge presents a missed opportunity to capture systemic adaptations that only become apparent in response to a challenge. The differences between studies could also be explained by their use of less sensitive or less stable markers of oxidative damage. A particular strength of our study is the measure of F2-isoprostanes, which are considered the gold standard for measuring oxidative stress in vivo (Yin et al. 2005).
Our targeted intervention of 8 weeks of supervised aerobic exercise significantly improved aerobic fitness as measured by VO2max and cycling leg power. An interesting finding in this study was the relationship between changes in fitness and improvements in the capacity to resist the oxidative challenge. We found a strong, significant correlation between improvements in VO2max and the response to the I/R trial. Individuals with the greatest improvements in fitness displayed the greatest resistance to the I/R trial as measured by the changes in F2-isoprostane AUC pre- to post-training. These data suggest resistance to oxidative stressors hinges on a component of fitness rather than simple activity. Although our study was under rigorous control and all the subjects enrolled participated in the same exercise training program, it is well known that individuals respond differently to the same exercise training. For instance, a large-scale 20-week exercise intervention including 742 participants demonstrated that even with full compliance, substantial variability exists in the percent improvement of VO2max with approximately 5 % of subjects having little or no improvement (<5 %) despite a mean improvement of 19 % (Skinner et al. 2000). In contrast, 5 % of the subjects had an improvement of 40 to >50 %. In line with this, one of our subjects showed an improvement of only 3.1 % in VO2max despite a mean group improvement of 13.4 %.
These data demonstrate that simply increasing activity may not be enough to improve resilience to acute oxidative challenges. Campbell et al. had similar findings in their 12-month exercise intervention, where levels of F2-isoprostanes decreased linearly with gains in aerobic fitness (Campbell et al. 2010). While there is no question that low-intensity exercise such as walking improves health outcomes, exercise of higher intensity may be necessary to restore redox balance. While our exercise intervention was relatively short, but successful nonetheless, it is certainly possible that a longer intervention would amplify the benefits we saw in our study. While we did not investigate the effects of detraining in our study, we feel confident in stating that the increased resistance to oxidative stress in response to the intervention would not be maintained without continued exercise regimen.
To our knowledge, this is the first study to test the effects of an exercise intervention in older middle-aged adults on their ability to handle a non-exercise oxidative challenge. We acknowledge the limitation of a small sample size and not having a corresponding control group. Additionally, the use of self-reported physical activity as inclusion criteria may be seen as a limitation. However, all the subjects who exhibited a higher than “fair” classification of aerobic fitness as measured by VO2max testing, despite reporting activity consistent with a sedentary lifestyle, were excluded. Finally, we did not monitor physical activity outside of the supervised exercise sessions, so we are unable to address whether subjects further modified their activity levels during the course of the study.
This pilot study demonstrates three important points for future studies: (1) resistance to oxidative stress can be increased through regular exercise in sedentary older middle-aged adults, (2) these changes occur in a relatively short time period, and (3) improvement in aerobic fitness may be more important for this effect than are increases in physical activity. While these findings cannot be generalized specifically to improved health outcomes and reduced risk for disease, it seems likely that any improvements that would be observed could be at least partially explained by increased resistance to oxidative stressors, resulting from improved redox signaling and enhanced endogenous antioxidant network. Future studies should incorporate measures of antioxidant enzymes and/or redox-regulated transcription factors for further understanding of the mechanisms of improved resistance to oxidative stressors with exercise training.
Acknowledgments
We thank our participants for their hard work and commitment to the exercise program. We would also like to thank Armando Peña, Rebecca Russell, and Iginio Stoppa for supervising the training program, Akaylah Jaeke for facilitating our access to the Northern Arizona University Campus Recreation Center, and Dave Lang, MD, for providing medical supervision. This study was funded by the Northern Arizona University Faculty Grant Program and Health Research Initiatives Mini-Grant Program (to T. Traustadóttir).
References
- Booth FW, Hargreaves M. Understanding multi-organ pathology from insufficient exercise. J Appl Physiol. 2011;111:1199–1200. doi: 10.1152/japplphysiol.01034.2011. [DOI] [PubMed] [Google Scholar]
- Campbell PT, Gross MD, Potter JD, Schmitz KH, Duggan C, McTiernan A, Ulrich CM. Effect of exercise on oxidative stress: a 12-month randomized, controlled trial. Med Sci Sports Exerc. 2010;42:1448–1453. doi: 10.1249/MSS.0b013e3181cfc908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley JN, et al. Lifelong training preserves some redox-regulated adaptive responses after an acute exercise stimulus in aged human skeletal muscle. Free Radic Biol Med. 2014;70:23–32. doi: 10.1016/j.freeradbiomed.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Davies SS, Traustadottir T, Stock AA, Ye F, Shyr Y, Harman SM, Roberts LJ., 2nd Ischemia/reperfusion unveils impaired capacity of older adults to restrain oxidative insult. Free Radic Biol Med. 2009;47:1014–1018. doi: 10.1016/j.freeradbiomed.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Gounder SS, et al. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobzone S, Oxley H. Ageing and health care costs. Int Polit Ges Online (Int Polit Soc) 2002;1:137–156. [Google Scholar]
- Kharbanda RK, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.CIR.0000043806.51912.9B. [DOI] [PubMed] [Google Scholar]
- Kohrt WM, et al. Effects of gender, age, and fitness level on response of VO2max to training in 60-71 yr olds. J Appl Physiol. 1991;71:2004–2011. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- Lessiani G, et al. Arterial stiffness and sedentary lifestyle: role of oxidative stress. Vasc Pharmacol. 2015 doi: 10.1016/j.vph.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/S0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- Nikolaidis MG, Kerksick CM, Lamprecht M, McAnulty SR. Redox biology of exercise. Oxidative Med Cell Longev. 2012;2012:407978. doi: 10.1155/2012/407978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidis MG, Kyparos A, Vrabas IS. F(2)-isoprostane formation, measurement and interpretation: the role of exercise. Prog Lipid Res. 2011;50:89–103. doi: 10.1016/j.plipres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Nordin TC, Done AJ, Traustadottir T. Acute exercise increases resistance to oxidative stress in young but not older adults. Age (Dordr) 2014;36:9727. doi: 10.1007/s11357-014-9727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Mortensen SP, Cabo H, Gomez-Cabrera MC, Vina J, Hellsten Y. Roles of sedentary aging and lifelong physical activity in exchange of glutathione across exercising human skeletal muscle. Free Radic Biol Med. 2014;73:166–173. doi: 10.1016/j.freeradbiomed.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Peake JM, Markworth JF, Nosaka K, Raastad T, Wadley GD, Coffey VG. Modulating exercise-induced hormesis: does less equal more? J Appl Physiol. 2015;119:172–189. doi: 10.1152/japplphysiol.01055.2014. [DOI] [PubMed] [Google Scholar]
- Pickering AM, Vojtovich L, Tower J, A Davies KJ (2013) Oxidative stress adaptation with acute, chronic, and repeated stress. Free Radic Biol Med 55:109–118. doi:10.1016/j.freeradbiomed.2012.11.001 [DOI] [PMC free article] [PubMed]
- Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med. 2011;51:942–950. doi: 10.1016/j.freeradbiomed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Powers SK, Quindry J, Hamilton K. Aging, exercise, and cardioprotection. Ann N Y Acad Sci. 2004;1019:462–470. doi: 10.1196/annals.1297.084. [DOI] [PubMed] [Google Scholar]
- Sakellariou GK, Jackson MJ, Vasilaki A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic Res. 2014;48:12–29. doi: 10.3109/10715762.2013.830718. [DOI] [PubMed] [Google Scholar]
- Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, Jackson MJ. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal. 2013;18:603–621. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger JP, Lenting CJ, Schreuder TH, Landman TR, Cable NT, Hopman MT, Thijssen DH. Interval exercise, but not endurance exercise, prevents endothelial ischemia-reperfusion injury in healthy subjects. Am J Phys Heart Circ Phys. 2015;308:H351–H357. doi: 10.1152/ajpheart.00647.2014. [DOI] [PubMed] [Google Scholar]
- Shing CM, Peake JM, Ahern SM, Strobel NA, Wilson G, Jenkins DG, Coombes JS. The effect of consecutive days of exercise on markers of oxidative stress. Appl Physiol Nutr Metab = Physiologie Appliquee Nutr Metab. 2007;32:677–685. doi: 10.1139/H07-051. [DOI] [PubMed] [Google Scholar]
- Skinner JS, et al. Adaptation to a standardized training program and changes in fitness in a large, heterogeneous population: the HERITAGE Family Study. Med Sci Sports Exerc. 2000;32:157–161. doi: 10.1097/00005768-200001000-00023. [DOI] [PubMed] [Google Scholar]
- Statistics NCfH (2015) Health, United States, 2014: with special feature on adults aged 55-64. Hyattsville, MD [PubMed]
- Tanaka H, Shimizu S, Ohmori F, Muraoka Y, Kumagai M, Yoshizawa M, Kagaya A. Increases in blood flow and shear stress to nonworking limbs during incremental exercise. Med Sci Sports Exerc. 2006;38:81–85. doi: 10.1249/01.mss.0000191166.81789.de. [DOI] [PubMed] [Google Scholar]
- Traustadóttir T, Davies SS, Stock AA, Su Y, Heward CB, Roberts LJ, 2nd, Harman SM. Tart cherry juice decreases oxidative stress in healthy older men and women. J Nutr. 2009;139:1896–1900. doi: 10.3945/jn.109.111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traustadottir T, Davies SS, Su Y, Choi L, Brown-Borg HM, Roberts LJ, 2nd, Harman SM. Oxidative stress in older adults: effects of physical fitness. Age (Dordr) 2012;34:969–982. doi: 10.1007/s11357-011-9277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traustadóttir T, Stock AA, Harman SM. High-dose statin use does not impair aerobic capacity or skeletal muscle function in older adults. Age (Dordr) 2008;30:283–291. doi: 10.1007/s11357-008-9070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Munckhof I, et al. Aging attenuates the protective effect of ischemic preconditioning against endothelial ischemia-reperfusion injury in humans. Am J Physiol Heart Circ Physiol. 2013;304:H1727–H1732. doi: 10.1152/ajpheart.00054.2013. [DOI] [PubMed] [Google Scholar]
- Yin H, Porter NA, Morrow JD. Separation and identification of F2-isoprostane regioisomers and diastereomers by novel liquid chromatographic/mass spectrometric methods. J Chromatogr B Anal Technol Biomed Life Sci. 2005;827:157–164. doi: 10.1016/j.jchromb.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Zhao X, et al. Effects of moderate exercise over different phases on age-related physiological dysfunction in testes of SAMP8 mice. Exp Gerontol. 2013;48:869–880. doi: 10.1016/j.exger.2013.05.063. [DOI] [PubMed] [Google Scholar]