Abstract
Mitochondrial respiration releases reactive oxygen species (ROS) as by-products that can damage the soma and may in turn accelerate ageing. Hence, according to “the oxidative stress theory of ageing”, longer-lived organisms may have evolved mechanisms that improve mitochondrial function, reduce ROS production and/or increase cell resistance to oxidative damage. Cardiolipin, an important mitochondrial inner-membrane phospholipid, has these properties by binding and stabilizing mitochondrial inner-membrane proteins. Here, we investigated whether ROS production, cardiolipin content and cell membrane resistance to oxidative attack in freshly collected red blood cells (RBCs) are associated with longevity (range 5–35 years) in 21 bird species belonging to seven Orders. After controlling for phylogeny, body size and oxygen consumption, variation in maximum longevity was significantly explained by mitochondrial ROS production and cardiolipin content, but not by membrane resistance to oxidative attack. RBCs of longer-lived species produced less ROS and contained more cardiolipin than RBCs of shorter-lived species did. These results support the oxidative stress theory of ageing and shed light on mitochondrial cardiolipin as an important factor linking ROS production to longevity.
Keywords: Birds, Comparative methods, Free radicals, Longevity, Phospholipids
Introduction
There is tremendous variation in lifespan among species within the same taxa, with for instance a 26-time difference in maximum longevity between the shortest- and the longest-lived bird species (3 years for the red-faced warbler, Cardellina rubrifrons versus 79 years for the Andean condor, Vultur gryphus; http://genomics.senescence.info/species/). Understanding the proximate factors that allow some species to be longer-lived is a central issue in gerontology. Because life is sustained by energy, how organisms acquire, convert and allocate energy is thought to be important determinants of longevity (Hulbert et al. 2007; Salin et al. 2015). At the cellular level, energy conversion takes place in the mitochondrion, and thus, one hypothesis is that variation in the functioning of this organelle accounts for variation in the rate of ageing. An initial argument for this hypothesis came from Denham Harman who postulated in 1956 that the production of damaging reactive oxygen species (ROS) as a by-product of mitochondrial respiration could explain ageing (Harman 1956). Indeed, when produced in excess to the antioxidant defences and repair mechanisms, ROS can damage biomolecules such as lipids, proteins and DNA, and those oxidative damages can accumulate over time and ultimately lead to cellular dysfunctions and ageing (Beckman and Ames 1998; Finkel and Holbrook 2000). This “oxidative stress theory of ageing” hypothesis posits that longer-lived species should be better equipped to resist oxidative stress and/or have mitochondria that produce less ROS than shorter-lived species.
Cell membrane composition in phospholipids can affect both cell resistance to oxidative stress and mitochondrial ROS production and thus is of prime interest when trying to explain variation in the rate of ageing (Hulbert 2003; Hulbert et al. 2007; Pamplona et al. 2002). Unsaturated phospholipids are important to ensure membrane fluidity and, in turn, normal functions of transmembrane proteins and cell homeostasis (Hulbert et al. 2007). Yet, unsaturated phospholipids are also more susceptible to ROS, and high levels of lipid peroxidation can lead to a loss of cellular integrity and eventually cell and organismal death. Accordingly, variation across species in membrane composition in unsaturated phospholipids has been reported to correlate with variation in longevity (Galván et al. 2015; Hulbert 2003; Hulbert et al. 2007; Naudí et al. 2013; Pamplona et al. 2002). Correlative studies where cells were exposed to oxidative attack also indicate that longer-lived organisms and individuals tend to have more resistant cell membranes (Bize et al. 2014; Csiszar et al. 2012; Losdat et al. 2012).
Phospholipid composition of the inner-mitochondria membrane is also known to play a role in mitochondrial functions and ROS production. Indeed, the key molecular machinery responsible for converting food into energy, but also for ROS production, is the electron transport chain (ETC) located in the mitochondrial inner membrane. Electrons lost by the ETC (mostly by complex I and III; Fry and Green 1981; Houtkooper and Vaz 2008) during mitochondrial respiration can react with dioxygen and lead to the production of superoxide, which is the main source of mitochondrial ROS production. In eukaryotes, cardiolipins are unsaturated phospholipids that are found exclusively in the mitochondrial inner membrane. Cardiolipins are known to bind to the different ETC complexes and to determine ETC complexes’ quaternary (functional) structure, position and orientation (Mileykovskaya and Dowhan 2009; Schlame and Ren 2009). Cardiolipins can also act as proton trap for ATP phosphorylation (Haines and Dencher 2002), induce ROS-derived release of cytochrome c and activate the mitochondrial transition pore inducing cell apoptosis (Petrosillo et al. 2003). Because cardiolipins can have important effects on mitochondrial bioenergetics and ROS production (Paradies et al. 2010; Schlame et al. 2000), variation in cardiolipin content may explain part of the variation in ROS production and, potentially, variation in lifespan. Interestingly, it has recently been shown that longer-lived tropical bird species have less cardiolipin content per cell than shorter-lived temperate bird species do (Calhoon et al. 2014).
In the present study, we report a comparative study on 21 bird species showing large variation in body size and longevity (Table 1). Birds are interesting models to perform such a study since, besides the wealth of information on their life histories, they also possess nucleated red blood cells (RBCs) with functional mitochondria in their cytoplasm (Stier et al. 2013). Thus, a blood sample can be used to measure both in vivo mitochondrial functions and cell membrane resistance to oxidative attack, and the value of RBCs in ageing studies has recently been highlighted in (Stier et al. 2015). Here, we investigated the covariation between maximum species lifespan and RBC mitochondrial ROS production (measured by superoxide production), RBC mitochondrial cardiolipin content, and RBC membrane resistance to oxidative attack. In our comparative analyses, we controlled for phylogenetic relationships and for allometry by considering body mass and total oxygen consumption. According to the oxidative stress theory of ageing, we expected longer-lived species to produce less superoxide and/or to have more resistant RBC membranes to oxidative attack. We also expected superoxide production to decrease with the increase in RBC cardiolipin content.
Table 1.
Sampled species classified by Order, Family, Latin name and vernacular name
| Orders | Families | Species | Number | Longevity | Mass | Ox. cons. | SO | Cardiolipins | Membrane resistance | |
|---|---|---|---|---|---|---|---|---|---|---|
| Latin name | Vernacular name | |||||||||
| Accipitriformes | Accipitridae | Accipiter gentilis | Northern goshawk | 1 | 22.0 | 1044.0 | 0.9490 | 4.87 | 7.43 | 62.80 |
| A. nisus | Eurasian sparrowhawk | 1 | 20.2 | 237.5 | 0.9516 | 4.29 | 10.46 | 47.55 | ||
| Buteo buteo | Common Buzzard | 2 | 28.8 | 1012.0 | 3.7580 | 5.33 ± 0.94 | 4.71 ± 1.58 | 50.88 ± 5.61 | ||
| Anseriformes | Anatidae | Anas platyrhynchos | Mallard | 5 | 29.1 | 1048.0 | 4.0680 | 6.74 ± 1.99 | 2.14 ± 1.07 | 119.49 ± 13.76 |
| Columbiformes | Columbidae | Columba livia | Rock dove | 3 | 35.0 | 358.7 | 1.7190 | 3.52 ± 0.40 | 20.77 ± 10.00 | 54.20 ± 2.21 |
| Falconiformes | Falconidae | Falco subbuteo | Eurasian hobby | 1 | 14.9 | 209.5 | 1.3014 | 6.10 | 9.56 | 57.71 |
| F. tinnunculus | Common kestrel | 3 | 23.8 | 184.0 | 0.8230 | 4.47 ± 2.39 | 3.75 | 50.29 ± 4.00 | ||
| Galliformes | Odontophoridae | Colinus virginianus | Virginia quail | 2 | 6.4 | 194.0 | 1.1140 | 8.31 ± 1.42 | 3.74 ± 2.05 | 49.41 ± 5.73 |
| Phasianidae | Alectoris rufa | Red-legged partridge | 2 | 6.2 | 528.0 | 1.9610 | 5.87 ± 0.96 | 7.08 ± 0.32 | 61.35 ± 12.18 | |
| Coturnix japonica | Japanese quail | 8 | 6.0 | 115.0 | 0.9780 | 3.95 ± 0.72 | 12.24 ± 4.19 | 86.16 ± 23.41 | ||
| Perdix perdix | Grey partridge | 2 | 5.2 | 492.0 | 2.7178 | 5.87 ± 1.84 | 5.12 ± 3.95 | 51.00 ± 15.85 | ||
| Passeriformes | Corvidae | Corvus corone | Carrion crow | 3 | 19.2 | 472.7 | 3.3531 | 7.52 ± 1.69 | 5.84 ± 2.46 | 35.41 ± 13.35 |
| Fringillidae | Fringilla coelebs | Common chaffinch | 2 | 29.0 | 20.7 | 0.3730 | 4.01 ± 0.36 | 19.72 ± 0.62 | 59.12 ± 12.79 | |
| Hirundinidae | Hirundo rustica | Barn swallow | 4 | 16.0 | 18.3 | 0.3158 | 4.50 ± 0.76 | 11.90 ± 5.19 | 51.58 ± 2.18 | |
| Paridae | Parus major | Great tit | 4 | 15.4 | 17.9 | 0.3240 | 3.58 ± 0.96 | 8.58 ± 2.27 | 58.24 ± 11.10 | |
| Passeridae | Passer domesticus | House sparrow | 6 | 23.0 | 25.3 | 0.3340 | 4.58 ± 0.58 | 17.05 ± 6.07 | 52.84 ± 10.85 | |
| P. montanus | Eurasian tree sparrow | 2 | 13.1 | 20.5 | 0.2007 | 4.34 ± 0.93 | 22.04 ± 4.86 | 49.05 ± 4.55 | ||
| Sittidae | Sitta europaea | Eurasian nuthatch | 1 | 12.9 | 20.4 | 0.2320 | 4.66 | 9.39 | 40.70 | |
| Sturnidae | Sturnus vulgaris | Common starling | 3 | 22.9 | 74.0 | 0.8770 | 4.09 ± 0.53 | 15.04 ± 3.95 | 63.29 ± 2.05 | |
| Turdidae | Turdus merula | Common blackbird | 1 | 21.8 | 103.2 | 1.1040 | 4.14 | 17.47 | 23.92 | |
| Strigiformes | Strigidae | Asio otus | Long-eared owl | 3 | 27.8 | 249.7 | 0.9540 | 4.40 ± 1.99 | 6.67 | 56.74 ± 3.53 |
N is sample size per species. Longevity corresponds to maximum lifespan record in years, mass to mean adult body mass (g) and oxygen consumption (Ox. cons.) to mean oxygen consumption (W) as reported in “the animal ageing and longevity database” website (http://genomics.senescence.info/species/). For each species, means ± standard errors are given for RBC mitochondrial superoxide (SO) production (arbitrary unit), RBC mitochondrial cardiolipin content (arbitrary unit) and RBC membrane resistance to oxidative attack (minutes)
Methods
Blood sampling
We sampled 59 adult individuals belonging to 21 bird species from 15 Families and 7 Orders (Table 1). Great tits (Parus major) were sampled in the field in Lausanne (Switzerland), and mallards (Anas platyrhynchos) came from a flock of adults captured in the field and kept in captivity at the Department of Ecology, Physiology and Ethology at the University of Strasbourg (France). Galliformes were sampled in a breeding farm (Verney, Switzerland). The other 15 species were sampled at the “La Vaux-Lierre” rehabilitation centre in Etoy (Switzerland) where we took care to include only individuals that had already recovered from their injury.
Samples of 20 μl of whole blood were collected from the brachial vein using EDTA microvette (Sarstedt, Germany) and immediately diluted in 730 μl of bird KRL buffer (Kirial international, Laboratoires Spiral S.A., Dijon, France), a physiological buffer adjusted to bird cell osmolarity ((150 mM Na+, 120 mM Cl), 6 mM K+, 24 mM HCO3), 2 mM Ca2+, 340 mosm, pH 7.4, as described in Alonso-Alvarez et al. (2004)). These blood-diluted samples were stored on ice in the dark for a maximum of 10 h prior to laboratory analysis. In the tawny owl (Strix aluco), we found no evidence that RBC membrane resistance and ROS production were explained by the time elapsed between sampling in the field and analyses in the laboratory (mean time elapsed in hours ± standard deviation = 10:37 ± 06:20, range = 02:00–22:15; n = 325 and 271 samples for RBC membrane resistance and ROS production; all P values >0.20; Emaresi et al. 2016).
Once in the laboratory, blood cells were washed by centrifuging blood-diluted samples for 5 min at 500 relative centrifugal force (rcf) and 4 °C, and cell pellets were resuspended in a volume of 1200 μl of KRL buffer. Glucose was added in excess (30 mM final concentration) as a mitochondrial respiration substrate to control for possible inter-individual variations in cell substrate availability and to match high natural glucose concentration in avian blood (Braun and Sweazea 2008). This stock sample solution was then used as starting solution for the flow cytometric measurements of superoxide production and cardiolipin content and for spectrometric measurements of RBC membrane resistance to oxidative attack.
RBC mitochondrial superoxide production
We quantified the production of superoxide by mitochondrial electron transport chains in live red blood cells using the specific FluoroProbe MitoSOX Red (Molecular Probes, Invitrogen) (Mukhopadhyay et al. 2007). Two hundred fifty microliters of stock sample solution of blood were incubated for 30 min at avian body temperature (i.e. 40 °C) with MitoSOX Red fluorescent dye (12 μM of final concentration once dye was added to the sample, diluted in DMSO). At the end of the incubation period, cells were washed by centrifuging samples for 3 min at 300 rcf and 4 °C. Cells were then resuspended in 250 μl of KRL buffer and stored on ice in the dark until flow cytometer measurement on a FACS Calibur (Becton Dickinson) using the FL2 channel and excitation at 582 nm (red fluorescence). For each sample, we acquired 50,000 events and computed median fluorescence value (i.e. arbitrary unit) using the CellQuest Pro software.
RBC mitochondrial cardiolipin content
We quantified the amount of mitochondrial cardiolipins using the specific FluoroProbe nonyl acridine orange (NAO) (Mileykovskaya and Dowhan 2009). After superoxide quantification, samples were washed by centrifuging them for 3 min at 300 rcf and 4 °C. Cells were then resuspended in 250 μl of KRL buffer and incubated for 7 min at 40 °C with NAO (10 μM final concentration; Molecular Probes, Invitrogen). At the end of the incubation period, cells were washed again by centrifuging the samples for 3 min at 300 rcf and 4 °C before being resuspended in 250 μl of KRL buffer. Samples were stored on ice in the dark until flow cytometer measurement on a FACS Calibur using the FL1 channel and excitation at 200 nm (green fluorescence). For each sample, we acquired 20,000 events and computed median fluorescence value using the CellQuest Pro software. A preliminary parameterization of the flow cytometer channels was performed to control for potential overlapping signals of MitoSOX and NAO probes. Moreover, non-sequential (i.e. independent) measurements of MitoSOX and NAO signals showed similar results (J.D., unpublished data), discarding a potential influence of the MitoSOX dye on the following NAO signal measurement.
RBC membrane resistance to oxidative attack
We investigated resistance of RBC membranes to oxidative attack as previously described in Bize et al. (2008). Briefly, 90 μl of the stock blood sample solution were incubated at 40 °C and exposed to a radical attack by adding to it a solution of 150 mmol of 2,2′-azobis-(amidinopropane) hydrochloride diluted in 153 μl of KRL buffer. We measured cell membrane resistance to an oxidative attack as the time needed to haemolyse half of the red cells. Cell haemolysis was quantified using a microplate reader by following the decrease of optical density at the wavelength of 540 nm and using the Kirial International processing analysis software.
Maximum lifespan, adult body mass and total oxygen consumption
Information on species maximum longevity record (in years), adult body mass (species average, in grams) and total oxygen consumption (species average, in watt) were obtained from “the animal ageing and longevity database” website (http://genomics.senescence.info/species/) (Tacutu et al. 2013) (Table 1). Measures of whole organism metabolic rates were used as proxy of oxygen consumption. We had no information on the exact age of individuals used in this study, and hence, we could not control for this factor in our analyses. We cannot exclude that individuals were sampled at older ages in long-lived species when compared to short-lived ones. However, mitochondrial ROS production is expected to increase with age within species (Criscuolo et al. 2010; Passos et al. 2007a, b), implying that our analyses are conservative because this potential bias would reduce the potential detection of a negative association between maximum lifespan and ROS production across species. Moreover, we performed analyses of variance (ANOVAs) with species for which we sampled more than one individual to test whether variability is higher between than within species. The results showed that individuals were more similar within than between species (RBC mitochondrial superoxide production: F (15, 38) = 3.89, P = 0.0004; RBC mitochondrial cardiolipin content: F (13, 34) = 5.48, P < 0.001; RBC membrane resistance to oxidative attack: F (15, 38) = 9.15, P < 0.001). Hence, our analyses are probably not confounded by inter-individual differences in RBC mitochondrial attributes.
Statistical analyses
As presented in Table 2, some of our variables were inter-correlated. As explained in Quinn and Keough (2006), this is an important issue in linear models that can be solved via the use of a principal components analysis (PCA). We thus summarised the information enclosed in our different explanatory factors (i.e. RBC mitochondrial superoxide production, RBC mitochondrial cardiolipin content, RBC membrane resistance to oxidative attack, adult body mass and total oxygen consumption) with a PCA using the software JMP (version 9, SAS Institute). We used varimax rotation to maximize the contrasts of the variable loadings between the components, restricting further analyses to components with eigenvalues higher than 1, and to create independent, orthogonal, explanatory variables for later use in phylogenetic analyses (Table 3). Bartlett’s sphericity tests showed that there was enough redundancy between our variables to compute PCA components (all P values <0.001).
Table 2.
Correlation matrix of maximal longevity, RBC mitochondrial superoxide production, RBC mitochondrial cardiolipin content, RBC membrane resistance to oxidative attack, body mass and oxygen consumption among the 21 bird species
| Longevity | Superoxide | Cardiolipin | Resistance | Body mass | Oxygen consumption | |
|---|---|---|---|---|---|---|
| Longevity | – | 0.265 | 0.426 | 0.695 | 0.382 | 0.525 |
| Superoxide | −0.32 | – | 0.015 | 0.695 | 0.141 | 0.035 |
| Cardiolipin | 0.22 | −0.63 | – | 0.382 | 0.038 | 0.039 |
| Resistance | 0.10 | 0.09 | −0.25 | – | 0.141 | 0.265 |
| Body mass | 0.25 | 0.41 | −0.55 | 0.41 | – | 0.002 |
| Oxygen consumption | 0.17 | 0.57 | −0.53 | 0.32 | 0.79 | – |
Pearson correlation coefficients are given below the diagonal and P values after Bonferroni correction above the diagonal with significant values in bold
Table 3.
Summary results of principal components analysis. Eigenvalue and percentage of the variance explained are given for the three first principal components (PCs) for which the eigenvalue is larger than 1
| PC 1 | PC 2 | PC 3 | |
|---|---|---|---|
| Eigenvalue | 2.20 | 1.38 | 1.00 |
| Percentage explained | 44.0 | 27.6 | 20.0 |
| Superoxide | 0.0006 | 0.9070 | −0.0900 |
| Cardiolipin | −0.1892 | −0.8542 | −0.1739 |
| Membrane resistance | 0.0190 | 0.0438 | 0.9922 |
| Body mass | 0.9877 | 0.0950 | 0.0110 |
| Oxygen consumption | 0.9875 | 0.0936 | 0.0280 |
Loadings are expressed as correlation coefficient of each variable with each PC. Significant values are written in bold. Eigenvalue and percentage of the variance explained are written in italic and given for the three first principal components (PCs) for which the eigenvalue is larger than 1
We investigated the influence of the different principal components (PCs) on maximum lifespan by computing phylogenetic generalized least squares models using the pgls function in the package caper in R (Orme 2013). This method allows us to take into account the covariance among traits due to the phylogenetic relationships between species. It further permits to account for the relative effect of the phylogenetic tree on the linear model by estimating the parameter λ, which takes a value of 1 if the phylogenetic tree fully explains the covariance observed between species measurements and a value of 0 if the variation among species is independent from their shared evolutionary history. We built the initial model including each PC with eigenvalues higher than 1 and performed a backward model selection based on model significance and on parsimony.
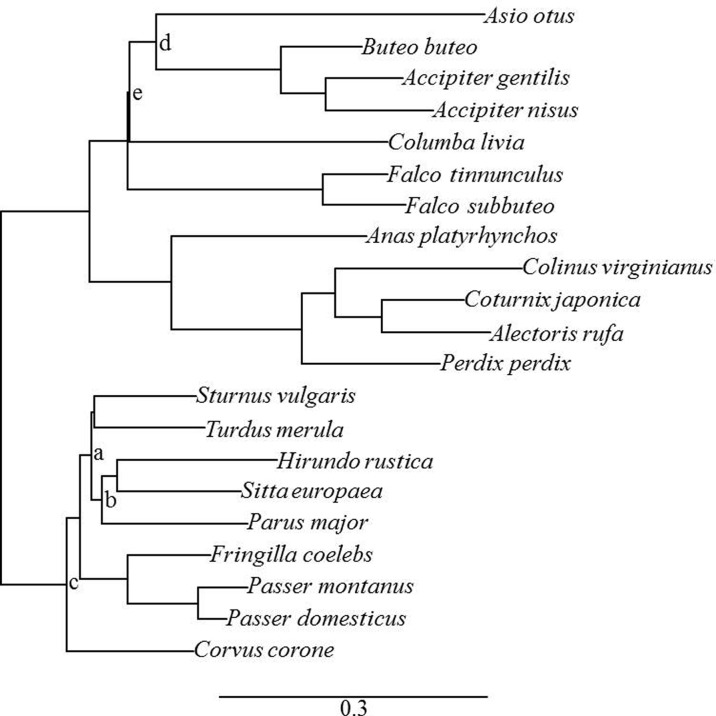
We built our own phylogeny in order to run the phylogenetic generalized least squares models including all the species sampled in our study. We used two DNA regions from the mitochondria (cytochrome b and the 12S ribosomal DNA) to build the phylogenetic tree among the 21 sampled species. Sequences were imported from GenBank (http://www.ncbi.nlm.nih.gov/genbank/). The two genes were concatenated and the alignment was performed with the software Muscle (Edgar 2004) as implemented in Seaview (version 4) (Gouy et al. 2010). The model of nucleotide substitution for each DNA region was estimated by AIC using the software MEGA (version 5) (Tamura et al. 2011). The model GTR + G + I was selected for both regions. We conducted a Bayesian analysis using MrBayes (version 3.2.2) (Ronquist et al. 2012). We ran the Markov chain Monte Carlo (MCMC) algorithm for 10,000,000 generations and sampled trees and model parameters every 1000 generations. We repeated the analyses twice, and we checked for the convergence of the MCMC chain by inspecting the trace of the likelihood and the estimated model parameters using the software Tracer (version 1.6). We considered that the first 25 % of the samples represented the burn-in of the MCMC chains and combined the two runs to estimate the posterior probabilities of the phylogenetic tree and model parameters. Fifteen out of the 20 nodes present in the phylogenetic tree were well supported with posterior probability superior to 0.907. The five remaining nodes had posterior probabilities ranging from 0.344 to 0.885 (Fig. 1). The obtained tree was congruent with published trees of birds (Hackett et al. 2008).
Fig. 1.
Phylogenetic tree of the 21 sampled bird species. Branch length and scale represent rate of substitution per site. Lowercase letters indicate nodes with posterior probability below 0.907 (a 0.885; b 0.857; c 0.835; d 0.681; e 0.344)
Results
There were correlations among the different explanatory factors (i.e. RBC mitochondrial superoxide production, RBC mitochondrial cardiolipin content, RBC membrane resistance to oxidative attack, adult body mass and total oxygen consumption; Table 2). Species consuming more oxygen were larger; their RBC produced a higher amount of mitochondrial superoxide and possessed lower content of mitochondrial cardiolipin (Table 2). Species possessing fewer cardiolipins per RBC were larger and produced more superoxides per RBC (Table 2). Therefore, there are clearly allometric relationships with the different variables. From the principal components analysis, the three first principal components, with eigenvalues greater than 1, explained together 91.6 % of the total variance (Table 3). PC1 was positively loaded with body mass and total oxygen consumption (Table 3), thus indicating that species with higher PC1 values were heavier and had a higher metabolism than species with lower PC1 values. PC2 was positively loaded with RBC mitochondrial superoxide production and negatively with RBC mitochondrial cardiolipin content, indicating that species with higher PC2 values had a higher superoxide production but a lower amount of cardiolipins per RBC than did species with lower PC2 values. PC3 was positively loaded with RBC resistance to oxidative attack (Table 3), indicating that species with higher PC3 values had RBC membranes that were more resistant to oxidative damage than species with lower PC3 values did. Hereafter, we refer to these components as descriptors of species size (PC1), RBC mitochondrial attributes (PC2) and cell resistance (PC3).
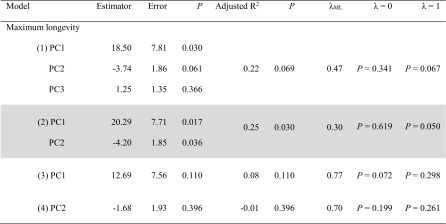
The most parsimonious model had an estimated phylogenetic signal of λ = 0.30, not significantly different from 0 (P = 0.619) but significantly different from 1 (P = 0.050) (model 2 in Table 4), indicating that only part of the covariance observed between species was explained by phylogenetic relationships between species. This model retained species size (PC1) and RBC mitochondrial attributes (PC2) as the best predictors of lifespan, but not cell resistance (PC3) (model 2 in Table 4). Long-lived species had higher adult body mass and higher oxygen consumption (significant positive effect of PC1: mean effect ± standard error = 20.29 ± 7.71; P = 0.017) than short-lived species, but also RBC of longer-lived species had higher cardiolipin content and produced less superoxides than RBC of shorter-lived species (PC2 −4.20 ± 1.85; P = 0.036) (Fig. 2).
Table 4.
Results of pgls models (package caper) on longevity variation between species (N = 21)
Lambda estimated by maximum likelihood (λ ML) represents phylogenetic dependence level varying from 0 (total phylogenetic independence) to 1 (total phylogenetic dependence). Results of comparison between models with estimated λ ML and models with λ = 0 and λ = 1 are given in the two last columns with P values (P). Based on model significance and parsimony, model 2 is retained as best model (highlighted in grey)
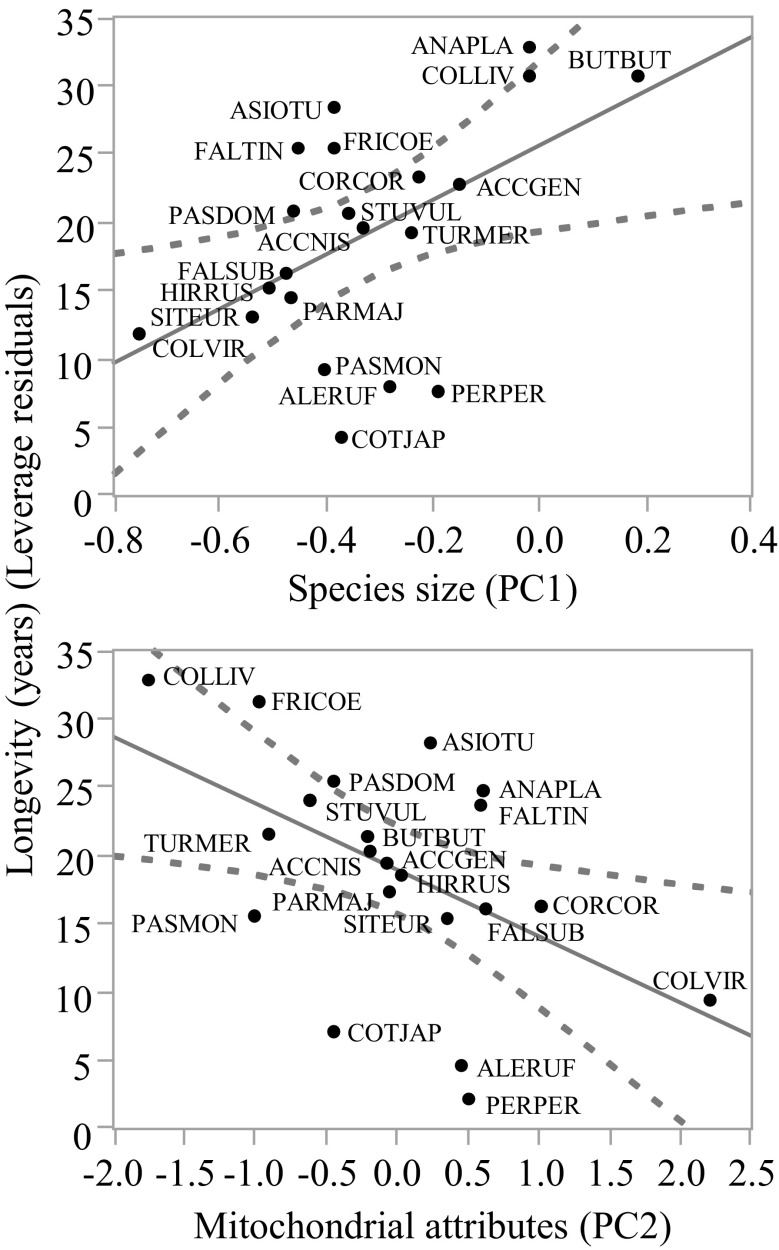
Fig. 2.
Maximal longevity in years in relation to principal components 1 and 2 (PC1: P = 0.009; PC2: P = 0.020). PC1 was positively loaded with species body mass and oxygen consumption and thus is referred to as “species size”. PC2 was positively loaded with RBC mitochondrial superoxide production and negatively with RBC mitochondrial cardiolipin content and thus is referred to as “RBC mitochondrial attributes”. Linear regression line and 95 % confidence interval are reported on each panel. Species codes reported in the panels correspond to ACCGEN: Accipiter gentilis, ACCNIS: Accipiter nisus, BUTBUT: Buteo buteo, ANAPLA: Anas platyrhynchos, COLLIV: Columba livia, FALSUB: Falco subbuteo, FALTIN: Falco tinnunculus, COLVIR: Colinus virginianus, ALERUF: Alectoris rufa, COTJAP: Coturnix japonica, PERPER: Perdix perdix, CORCOR: Corvus corone, FRICOE: Fringilla coelebs, HIRRUS: Hirundo rustica, PARMAJ: Parus major, PASDOM: Passer domesticus, PASMON: P. montanus, SITEUR: Sitta europaea, STUVUL: Sturnus vulgaris, TURMER: Turdus merula, ASIOTU: Asio otus
Discussion
We investigated the explanatory role of mitochondrial bioenergetics in variation in lifespan among 21 bird species with maximum lifespan ranging from 5 to 35 years. After controlling for phylogenetic relationships, variation in maximum longevity was significantly correlated with species size (i.e. body mass and oxygen consumption; P = 0.017) and RBC mitochondrial attributes (i.e. superoxide production and cardiolipin content; P = 0.036). As already described in the literature, species longevity was found to be positively associated with body mass (Holmes and Martin 2009; Speakman 2005; Stearns 1992). More interestingly, our correlative results also show that RBC produced less mitochondrial ROS and contained more cardiolipin in longer- than in shorter-lived species after controlling for body size. However, we did not detect any link between longevity and RBC membrane resistance to oxidative attack. Because we did not measure cell membrane composition per se, more work is required before rejecting the possibility that unsaturated phospholipids have an impact on longevity by shaping membrane resistance to oxidative attack. Furthermore, the cell membrane composition of a tissue can change through the removal/replacement of damaged acyl chains or after the turnover of the cells composing the tissue (discussed in Pamplona and Costantini 2011). Then, resistance of a given tissue may be linked with the turnover of its cells and investment in repair mechanisms may be more important in tissues with a low turnover. Based on this hypothesis, tissues with a high turnover, such as the blood, may be less subject to selection and therefore less likely to inform on the correlation between membrane resistance and longevity.
Although the negative correlation between longevity and ROS production at the cellular or mitochondrial levels has already been documented in comparative studies (Csiszar et al. 2012; Lambert et al. 2007; Robert et al. 2007; but see Montgomery et al. 2012), the importance of mitochondrial cardiolipin content in shaping ROS production and longevity had not yet been addressed. In eukaryotes, cardiolipins are found exclusively in the mitochondrial inner membrane. Laboratory measurements of cardiolipin content are often interpreted as a measure of mitochondrial (membrane) density per cell (Passos et al. 2007b). Indeed mitochondria are organized in dynamic networks: their membranes get constantly joined by the process of fusion and divided by the process of fission (Archer 2013). Among the variety of lipids and proteins constituting mitochondrial membranes, cardiolipins have a high affinity with a large number of proteins and thus play a central role in the structural organization of mitochondrial membranes (Schlame and Ren 2009). Then, an increase in the proportion of cardiolipins in the inner mitochondrial membrane is thought to help better regulating the proton gradient and to lessen ROS production (Hoch 1998; Paradies et al. 2010; Schlame and Ren 2009). Accordingly, our correlative analyses show that the production of superoxide per RBC is strongly and significantly decreasing with increasing cardiolipin content per RBC (r = −0.63; Table 2). The importance of the links between phospholipid composition of inner mitochondrial membranes, proton gradient and ROS production in shaping animal longevity is receiving growing attention (Galván et al. 2015; Hulbert 2010, 2008; Naudí et al. 2013; Valencak and Ruf 2013), and our results suggest a key role of mitochondrial content in cardiolipins. In the present study, we did not measure additional markers of mitochondrial membrane density or composition, and thus it cannot be excluded that the increase in cardiolipin content per RBC reported above is driven by a greater mitochondrial density (i.e. more mitochondria) rather than by a greater proportion of cardiolipins per mitochondrion. The finding that cardiolipin content per RBC is also declining with increasing species body mass (r = −0.55; Table 2) is nonetheless supporting the later hypothesis. It matches a previous finding in mammals that larger species have lower densities of mitochondria per gram of liver and are longer-lived (Passos et al. 2007b). Moreover, it has already been suggested that differences in mitochondria proton conductance between bird species is more likely to be explained by differences in inner-membrane properties than by variations in the amount of membranes (Brand et al. 2003). Altogether, our results suggest that longer lifespan in bird species may be associated both with lower densities of mitochondria per RBC and with greater amount of cardiolipin in their mitochondrial inner membrane, which are both expected to reduce mitochondrial ROS production.
The importance of cardiolipin in ageing research is new. Previous studies have shown a loss of cardiolipin content in old individuals that could account for the increased ROS production at older ages and may accelerate ageing (Paradies et al. 2002, 2000). Furthermore, it was shown that deficiency in complex I activity in mitochondria from old rats can be almost completely restored to the level of young rats by supplementing mitochondria with cardiolipin (Petrosillo et al. 2009). Finally, mutations associated with cardiolipin deficiency are also linked to shortened lifespan in Drosophila flies (Acehan et al. 2011; Zhou et al. 2006) and in humans (Schlame and Ren 2006). The costs of maintaining high amounts of cardiolipin in the mitochondrial inner membrane remain however unclear. More work is required to better understand inter- and intra-specific variability in cardiolipin content and its importance in shaping ROS production and animal life histories. To this end, RBCs of birds, fish, amphibians and reptiles may provide an interesting source of information since they are enclosing a nucleus and mitochondria (Stier et al. 2015).
Conclusion
Beside showing the ease with which RBC can be collected in various species to provide access to mitochondria, our study indicates that the variation observed in RBC mitochondrial attributes (i.e. superoxide production and cardiolipin content) do explain for part of the variation observed in longevity across species, as previously found in mitochondria isolated from metabolically very active tissues such as liver, heart or brain (Barja and Herrero 2000; Herrero and Barja 1998; Lambert et al. 2007; López-Torres et al. 2002; Navarro and Boveris 2004). Why and how variation in RBC mitochondria attributes can account for longevity deserves further studies (Stier et al. 2015).
Acknowledgments
This work was supported by the Swiss National Science Foundation (31003A-124988/1 to PB, 31003A-138187 and 31003A-159600/1 to PC). We are grateful to the staff of La Vaux-Lierre for giving us access to the bird care centre, to Sylvie Massemin and Jean-Patrice Robin for their help in data sampling and to Olivier Glaizot and two anonymous reviewers for their comments on the manuscript.
Footnotes
Pierre Bize and Philippe Christe contributed equally to this work.
References
- Acehan D, Malhotra A, Xu Y, Ren M, Stokes DL, Schlame M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys J. 2011;100:2184–2192. doi: 10.1016/j.bpj.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol Lett. 2004;7:363–368. doi: 10.1111/j.1461-0248.2004.00594.x. [DOI] [Google Scholar]
- Archer SL. Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369:2236–2251. doi: 10.1056/NEJMe1306684. [DOI] [PubMed] [Google Scholar]
- Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14:312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Bize P, Devevey G, Monaghan P, Doligez B, Christe P. Fecundity and survival in relation to resistance to oxidative stress in a free-living bird. Ecology. 2008;89:2584–2593. doi: 10.1890/07-1135.1. [DOI] [PubMed] [Google Scholar]
- Bize P, Cotting S, Devevey G, van Rooyen J, Lalubin F, Glaizot O, Christe P. Senescence in cell oxidative status in two bird species with contrasting life expectancy. Oecologia. 2014;174:1097–1105. doi: 10.1007/s00442-013-2840-3. [DOI] [PubMed] [Google Scholar]
- Brand MD, Turner N, Ocloo A, Else PL, Hulbert AJ. Proton conductance and fatty acyl composition of liver mitochondria correlates with body mass in birds. Biochem J. 2003;376:741–748. doi: 10.1042/bj20030984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun EJ, Sweazea KL. Glucose regulation in birds. Comp Biochem Physiol Part B Biochem Mol Biol. 2008;151:1–9. doi: 10.1016/j.cbpb.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Calhoon EA, Jimenez AG, Harper JM, Jurkowitz MS, Williams JB. Linkages between mitochondrial lipids and life history in temperate and tropical birds. Physiol Biochem Zool. 2014;87:265–275. doi: 10.1086/674696. [DOI] [PubMed] [Google Scholar]
- Criscuolo F, Font-Sala C, Bouillaud F, Poulin N, Trabalon M. Increased ROS production: a component of the longevity equation in the male mygalomorph, Brachypelma albopilosa. PLoS One. 2010;5:e13104. doi: 10.1371/journal.pone.0013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Podlutsky A, Podlutskaya N, Sonntag WE, Merlin SZ, Philipp EER, Doyle K, Davila A, Recchia FA, Ballabh P, Pinto JT, Ungvari Z. Testing the oxidative stress hypothesis of aging in primate fibroblasts: is there a correlation between species longevity and cellular ROS production? J Gerontol Ser A Biol Sci Med Sci. 2012;67:841–852. doi: 10.1093/gerona/glr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emaresi G, Henry I, Gonzalez E, Roulin A, Bize P (2016) Sex- and melanic-specific variations in the oxidative status of adult tawny owls in response to manipulated reproductive effort. J Exp Biol 219:73--79 [DOI] [PubMed]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fry M, Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981;256:1874–1880. [PubMed] [Google Scholar]
- Galván I, Naudí A, Erritzøe J, Møller AP, Barja G, Pamplona R. Long lifespans have evolved with long and monounsaturated fatty acids in birds. Evolution. 2015;69:2776–2784. doi: 10.1111/evo.12754. [DOI] [PubMed] [Google Scholar]
- Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Hackett SJ, Kimball RT, Reddy S, Bowie RCK, Braun EL, Braun MJ, Chojnowski JL, Cox WA, Han K-L, Harshman J, Huddleston CJ, Marks BD, Miglia KJ, Moore WS, Sheldon FH, Steadman DW, Witt CC, Yuri T. A phylogenomic study of birds reveals their evolutionary history. Science. 2008;320:1763–1768. doi: 10.1126/science.1157704. [DOI] [PubMed] [Google Scholar]
- Haines TH, Dencher NA. Cardiolipin: a proton trap for oxidative phosphorylation. FEBS Lett. 2002;528:35–39. doi: 10.1016/S0014-5793(02)03292-1. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Herrero A, Barja G. H2O2 production of heart mitochondria and aging rate are slower in canaries and parakeets than in mice: sites of free radical generation and mechanisms involved. Mech Ageing Dev. 1998;103:133–146. doi: 10.1016/S0047-6374(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Hoch FL. Mini review: cardiolipins and mitochondrial proton-selective leakage. J Bioenerg Biomembr. 1998;30:511–532. doi: 10.1023/A:1020576315771. [DOI] [PubMed] [Google Scholar]
- Holmes D, Martin K. A bird’s-eye view of aging: what’s in it for ornithologists? Auk. 2009;126:1–23. doi: 10.1525/auk.2009.1109. [DOI] [Google Scholar]
- Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert AJ. Life, death and membrane bilayers. J Exp Biol. 2003;206:2303–2311. doi: 10.1242/jeb.00399. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ. Explaining longevity of different animals: is membrane fatty acid composition the missing link? Age. 2008;30:89–97. doi: 10.1007/s11357-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert AJ. Metabolism and longevity: is there a role for membrane fatty acids? Integr Comp Biol. 2010;50:808–817. doi: 10.1093/icb/icq007. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R, Brand MD. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–618. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- López-Torres M, Gredilla R, Sanz A, Barja G. Influence of aging and long-term caloric restriction on oxygen radical generation and oxidative DNA damage in rat liver mitochondria. Free Radic Biol Med. 2002;32:882–889. doi: 10.1016/S0891-5849(02)00773-6. [DOI] [PubMed] [Google Scholar]
- Losdat S, Helfenstein F, Blount JD, Marri V, Maronde L, Richner H. Nestling erythrocyte resistance to oxidative stress predicts fledging success but not local recruitment in a wild bird. Biol Lett. 2012;9:20120888. doi: 10.1098/rsbl.2012.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskaya E, Dowhan W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta. 2009;1788:2084–2091. doi: 10.1016/j.bbamem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery MK, Hulbert AJ, Buttemer WA. Does the oxidative stress theory of aging explain longevity differences in birds? I. Mitochondrial ROS production. Exp Gerontol. 2012;47:203–210. doi: 10.1016/j.exger.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Yoshihiro K, Haskó G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun. 2007;358:203–208. doi: 10.1016/j.bbrc.2007.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudí A, Jové M, Ayala V, Portero-Otín M, Barja G, Pamplona R. Membrane lipid unsaturation as physiological adaptation to animal longevity. Front Physiol. 2013;4:1–13. doi: 10.3389/fphys.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1244–R1249. doi: 10.1152/ajpregu.00226.2004. [DOI] [PubMed] [Google Scholar]
- Orme D. The caper package: comparative analysis of phylogenetics and evolution in R. 2013. [Google Scholar]
- Pamplona R, Costantini D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am J Physiol Regul Integr Comp Physiol. 2011;301:R843–R863. doi: 10.1152/ajpregu.00034.2011. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Barja G, Portero-Otín M. Membrane fatty acid unsaturation, protection against oxidative stress and maximum life span. A homeoviscous-longevity adaptation? Ann N Y Acad Sci. 2002;959:475–490. doi: 10.1111/j.1749-6632.2002.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. The effect of reactive oxygen species generated from the mitochondrial electron transport chain on the cytochrome c oxidase activity and on the cardiolipin content in bovine heart submitochondrial particles. FEBS Lett. 2000;466:323–326. doi: 10.1016/S0014-5793(00)01082-6. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135–141. doi: 10.1016/S0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radic Biol Med. 2010;48:1286–1295. doi: 10.1016/j.freeradbiomed.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birket MJ, Harold G, Schaeuble K, Birch-Machin MA, Kirkwood TBL, von Zglinicki T (2007a) Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 5:e110. [DOI] [PMC free article] [PubMed]
- Passos JF, von Zglinicki T, Kirkwood TBL (2007b) Mitochondria and ageing: winning and losing in the numbers game. BioEssays. 29:908–917. [DOI] [PubMed]
- Petrosillo G, Ruggiero FM, Venosa NDI, Paradies G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J. 2003;17:714–716. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Matera M, Moro N, Ruggiero FM, Paradies G. Mitochondrial complex I dysfunction in rat heart with aging: critical role of reactive oxygen species and cardiolipin. Free Radic Biol Med. 2009;46:88–94. doi: 10.1016/j.freeradbiomed.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Keough MJ. Experimental design and data analysis for biologists. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Robert KA, Brunet-Rossinni A, Bronikowski AM. Testing the “free radical theory of aging” hypothesis: physiological differences in long-lived and short-lived colubrid snakes. Aging Cell. 2007;6:395–404. doi: 10.1111/j.1474-9726.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin K, Auer SK, Rey B, Selman C, Metcalfe NB. Variation in the link between oxygen consumption and ATP production, and its relevance for animal performance. Proc R Soc London B Biol Sci. 2015;282:20151028. doi: 10.1098/rspb.2015.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006;580:5450–5455. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Schlame M, Ren M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim Biophys Acta. 2009;1788:2080–2083. doi: 10.1016/j.bbamem.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/S0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208:1717–1730. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- Stearns SC. The evolution of life histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- Stier A, Bize P, Schull Q, Zoll J, Singh F, Geny B, Gros F, Royer C, Massemin S, Criscuolo F. Avian erythrocytes have functional mitochondria, opening novel perspectives for birds as animal models in the study of ageing. Front Zool. 2013;10:33. doi: 10.1186/1742-9994-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier A, Reichert S, Criscuolo F, Bize P. Red blood cells open promising avenues for longitudinal studies of ageing in laboratory, non-model and wild animals. Exp Gerontol. 2015;71:118–134. doi: 10.1016/j.exger.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Tacutu R, Craig T, Budovsky A, Wuttke D, Lehmann G, Taranukha D, Costa J, Fraifeld VE, de Magalhães JP. Human ageing genomic resources: integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res. 2013;41:D1027–D1033. doi: 10.1093/nar/gks1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencak TG, Ruf T. Phospholipid composition and longevity: lessons from Ames dwarf mice. Age (Omaha) 2013;35:2303–2313. doi: 10.1007/s11357-013-9533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhong Q, Greenberg ML. Decreased life span in cardiolipin mutants. FASEB. 2006;20:A1357. [Google Scholar]