Abstract
High consumption of fruits and vegetables has been associated with reduced risk of debilitating diseases and improved cognition in aged populations. These beneficial effects have been attributed to the phytochemicals found in fruits and vegetables, which have previously been shown to be anti-inflammatory and modulate autophagy. Tart cherries contain a variety of potentially beneficial phytochemicals; however, little research has been done to investigate the effects of tart cherry on the aging brain. Therefore, the purpose of this study was to determine if tart cherry supplementation can improve cognitive and motor function of aged rats via modulation of inflammation and autophagy in the brain. Thirty 19-month-old male Fischer 344 rats were weight-matched and assigned to receive either a control diet or a diet supplemented with 2 % Montmorency tart cherry. After 6 weeks on the diet, rats were given a battery of behavioral tests to assess for strength, stamina, balance, and coordination, as well as learning and working memory. Although no significant effects were observed on tests of motor performance, tart cherry improved working memory of aged rats. Following behavioral testing, the hippocampus was collected for western/densitometric analysis of inflammatory (GFAP, NOX-2, and COX-2) and autophagy (phosphorylated mTOR, Beclin 1, and p62/SQSTM) markers. Tart cherry supplementation significantly reduced inflammatory markers and improved autophagy function. Daily consumption of tart cherry reduced age-associated inflammation and promoted protein/cellular homeostasis in the hippocampus, along with improvements in working memory. Therefore, addition of tart cherry to the diet may promote healthy aging and/or delay the onset of neurodegenerative diseases.
Keywords: Aging, Cherry, Montmorency tart cherries, Memory, Inflammation, Autophagy
Introduction
Aging is associated with deficits in motor function, which include decreases in balance, muscle strength and coordination, and cognitive function, especially in tasks that require the use of spatial learning and memory. These decrements have been reported in numerous studies in both animals (Joseph et al. 1983; Shukitt-Hale et al. 1998) and humans (Brayne et al. 1995; Hofer et al. 2003). With the ongoing expansion of the aging population in many countries worldwide, various means have been explored to reduce or prevent age-related neurodegeneration, thus extending health-span and reducing the health care costs accrued by older adults.
Although the mechanisms underlying age-related deficits remain to be fully characterized, it is clear that inflammation, in conjunction with oxidative stress, plays a key role (Shukitt-Hale 1999; Hauss-Wegrzyniak et al. 2000). During aging, the brain’s endogenous anti-inflammatory defenses decline (Olanow 1993; Carney et al. 1994; Joseph et al. 1998), making the brain particularly susceptible to inflammatory insult. Because the brain has limited regenerative potential, any insult can lead to cumulative cellular damage that compromises normal neuronal function. Autophagy is one of the mechanisms responsible for sequestering and/or recycling intracellular damaged protein/lipid complexes, protein oligomers/aggregates, and cellular organelles. Autophagy is a well-regulated lysosomal degradation process whose disruption, which leads to neuronal dystrophy and death, is a potential mechanism of the aging process and the development of neurodegenerative diseases (Meijer and Codogno 2006; Rajawat et al. 2009). Neuroinflammation has been identified as a cause of autophagal dysregulation in aged rats (Poulose et al. 2011). Therefore, a potentially valuable approach to combat age-related deficits in behavior is to alter inflammation.
Epidemiologic studies have shown that high consumption of fruits and vegetables reduces the risk for common causes of death (Lock et al. 2005; He et al. 2006a, 2007) and improves cognitive performance in healthy elderly people (Polidori et al. 2009; Nurk et al. 2010). Previous research from our laboratory, and several others, suggests that the combinations of antioxidant/anti-inflammatory phytochemicals found in fruits and vegetables may effectively combat some of the effects of aging [see Miller and Shukitt-Hale (2012) for a review of these studies]. We found that supplementation of blueberry (BB), strawberry (SB), or spinach for 8 weeks significantly attenuated age-related motor and cognitive deficits in senescent rodents. When compared to the control group, all diet-supplemented groups showed improved working memory (short-term memory) performance in the Morris water maze (MWM) (Joseph et al. 1999). Short-term 2 % BB supplementation in aged rodents was also found to be effective in reversing cognitive declines in an object recognition task (Goyarzu et al. 2004; Malin et al. 2011). Subsequent experiments in our laboratory found similar protective effects against age-related behavioral declines with blackberries (Shukitt-Hale et al. 2009a, b), cranberries (Shukitt-Hale et al. 2005), black currants (Shukitt-Hale et al. 2005), Concord grape juice (Shukitt-Hale et al. 2006), plum juice (Shukitt-Hale et al. 2009a, b), and walnuts (Willis et al. 2009). In addition to their beneficial behavioral effects, the phytochemicals found in fruits and vegetables are believed to directly affect cell signaling in the brain by enhancing neuronal communication, increasing buffering capacity against intracellular calcium influx, promoting neurogenesis, upregulating heat-shock proteins, downregulating inflammatory gene expression, and protecting against excitotoxic stress [reviewed in Miller and Shukitt-Hale (2012)]. Furthermore, a recent study by Poulose and colleagues (2013) reported that aged rodents fed with a phytochemical-rich, walnut-supplemented diet for 15 weeks showed a marked increase in clearance of ubiquitinated protein aggregates in the hippocampus and activation of autophagy in the striatum and the hippocampus, areas of the brain that are crucial for learning and memory.
Tart cherry is a dietary source of fiber, potassium, and phytochemicals [reviewed in Ferretti et al. (2010) and McCune et al. (2011)]. Cyanidin is the major anthocyanin in tart cherries (Seeram et al. 2001a) followed by flavan-3-ols and flavonols (Bhagwat et al. 2014). Studies using cell lines, animal models, and humans demonstrated that the phytochemicals found in tart cherry confer health benefits by inducing cell-cycle arrest and apoptosis in precancerous and cancer cells (Kang et al. 2003; Bobe et al. 2006; Martin and Wooden 2012; Sehitoglu et al. 2014), decreasing triglyceride and total cholesterol levels (Seymour et al. 2008), reducing inflammation (Seeram et al. 2001b; Tall et al. 2004; Seymour et al. 2009; Ou et al. 2012), and decreasing oxidative stress (Kim et al. 2005; Traustadottir et al. 2009). Furthermore, a study by Kirakosyan showed that cherry anthocyanins accumulated in the brain of young rats after 3 weeks of feeding with either 1 or 10 % tart cherry-supplemented diets in a dose-dependent manner (Kirakosyan et al. 2015). Therefore, cherry anthocyanins may improve behavior by acting directly to improve brain cell function, signaling, and/or extraneuronal parameters of survival, such as inflammation, within the aging brain. The purpose of the present study was to determine whether dietary supplementation with 2 % tart cherry could improve the cognitive and motor function of aged rats via anti-inflammatory or neuronal housekeeping mechanisms.
Methods and materials
Chemicals and antibodies
The following chemicals and antibodies were used in this study: complete Mini EDTA-free protease inhibitor (Roche Diagnostic, Indianapolis, IN); PMSF (Sigma-Aldrich, St. Louis, MO); 30 % acrylamide/bis solution, 29:1, TEMED, 4× Laemmli sample buffer, 10× Tris/glycine/SDS buffer, 10× Tris/glycine buffer, polyvinyl difluoride (PVDF) membrane (Bio-Rad, Hercules, CA); Rapid Block (Amresco, Solon, OH); goat-anti-NADPH oxidase-2 (NOX-2), goat-anti-cyclooxygenase-2 (COX-2), goat-anti-β-actin (Santa Cruz Biotechnology, Dallas, TX); goat-anti-glial fibrillary acidic protein (GFAP) (EMD Millipore, Billerica, MA); rabbit-anti-phosphorylated mammalian target of rapamycin (pmTOR), rabbit-anti-Beclin1, and mouse-anti-p62/SQSTM1 (Cell Signaling Technology, Danvers, MA); ECL Plus reagents (GE Healthcare Life Sciences, Piscataway, NJ).
Animals
Nineteen-month-old male Fischer 344 rats (N = 30, 15 rats/group), acquired from the National Institute on Aging (NIA) rat colony (Taconic Farms), were maintained on a 12-h light/dark schedule in hanging wire cages with ad libitum food and water. After 2 weeks of acclimation, rats were weight-matched and assigned to diet groups. Body weights and food intakes were recorded every other week. All rats were observed daily for clinical signs of disease. At the conclusion of the study, there were 12 rats in each group, as some lost a significant amount of weight and were euthanized or died due to conditions related to old age during the course of the study; however, there were no pathological differences between the groups. Animals were utilized in compliance with all applicable laws and regulations as well as principles expressed in the National Institutes of Health, USPHS, Guide for the Care and Use of Laboratory Animals. This study was approved by the Animal Care and Use Committee of our Center.
Diet
Freeze-dried Montmorency tart cherry powder (Prunus cerasus L.; provided by Cherry Marketing Institute, Dewitt, MI) was incorporated into a pelleted NIH-31 diet (Harlan Teklad, Madison, WI). A 2 % (w/w) Montmorency tart cherry dose was chosen based on previous studies in humans (Kelley et al. 2006), as well as those conducted in our laboratory demonstrating the beneficial effects of phytochemical-rich berry fruits (i.e., blueberry, strawberry, cranberry, blackberry, black currant, and boysenberry) on cognition, motor function, and suppression of inflammation and oxidative stress in aged animals and accelerated aging models (Casadesus et al. 2004; Goyarzu et al. 2004; Shukitt-Hale et al. 2005; Galli et al. 2006; Shukitt-Hale et al. 2007, 2009a, b; Malin et al. 2011). The amount of ground corn was adjusted in the control diet to compensate for the added volume of the freeze-dried cherry powder, in accordance with established methodology (Shukitt-Hale et al. 1998; Youdim et al. 2000). Rats were maintained on these diets for the duration of the study.
Behavioral tests
The rats were tested on a battery of motor tests during week 6 of the diet and on the working memory version of Morris water maze (MWM) during week 7 of the diet (see below).
Psychomotor testing
A battery of age- and diet-sensitive tests of psychomotor behavior (Joseph et al. 1983, 1998; Shukitt-Hale et al. 1998; Joseph et al. 1999; Youdim et al. 2000; Shukitt-Hale et al. 2005) was administered in a randomized order to the animals at the end of the sixth week of dietary supplementation. Each test was performed once, separated by a break between tasks. Briefly, the tests included the following:
Rod walk: The ability of rats to balance on a stationary, horizontal rod measures psychomotor coordination and the integrity of the vestibular system. Animals were placed in the center of a rod (100 cm long, 26 mm in diameter, positioned 23 cm above the table surface), and their latency to fall off the rod onto a cushion below was recorded (max score = 60 s). If the rats fell immediately after being placed on the rod, they were given another opportunity.
Wire suspension: The prehensile reflex refers to an animal’s ability to grasp a horizontal wire (12-gauge) with its forepaws and to remain suspended; it is a measure of muscle strength. Rats were raised to a taut horizontal wire (55 cm above the tabletop), and the forepaws of each rat were placed on the wire. Each was given one trial, with the total hanging time in seconds recorded (60 s max).
Plank walk: Balance and coordination were measured by exposing the rats to one trial on each of three 100-cm-long horizontal planks (wide = 38 mm; medium = 25 mm; narrow = 13 mm), placed 34 cm above the tabletop, in counterbalanced order. Latency to fall (60 s max) was recorded and averaged for each trial.
Inclined screen: This test measures muscle tone, strength, and balance. Each rat was placed in one of six separate compartments of a wire mesh that was tilted 60° to the horizontal plane of the floor. Latency to fall from the screen was recorded (600 s max).
Accelerating rotarod: Fine motor coordination, balance, and resistance to fatigue were quantitated by measuring the amount of time that a rat could remain standing/walking on a rotating, accelerating rod (7-cm diameter; Ugo Basile, Italy). Each rat was placed on the rod at 2 rpm until it maintained its grip and orientation without assistance. The rod then accelerated steadily for 5 min (by 2 rpm every 30 s) until it reached 20 rpm. Latency to fall was recorded.
Working memory version of the Morris water maze (wMWM)
The wMWM (Morris 1984; Brandeis et al. 1989) is a learning paradigm that requires rats to explore a circular pool of water (134-cm diameter × 50-cm height, maintained at 23 ± 1 °C) and use extra-maze cues to locate a hidden platform (10-cm diameter) that is submerged 2 cm below the surface of the water. Accurate navigation to the platform is rewarded by escape from the water. The working memory version of the MWM (Morris 1984; Brandeis et al. 1989) was performed daily for four consecutive days, with a morning and an afternoon session, two trials per session, and a 10-min intertrial interval between the two trials. The platform was moved at the beginning of each session to one of four locations; these locations were chosen to frustrate a number of nonplace learning strategies that rats may adopt (Whishaw 1985). At the beginning of each trial, the rat was gently immersed in the water at one of four randomized start locations. Each rat was allowed 120 s to locate and escape onto the platform; if the rat failed to escape within this time, it was guided to the platform. Once the rat reached the platform, it remained there for 15 s (trial 1; reference memory or acquisition trial). The rat was returned to its home cage between trials (10 min). Trial 2 (the working memory or retrieval trial) used the same platform location and start position as trial 1. All trials were videotaped and analyzed with image tracking software (HVS Image, UK), which allows measurements of latency to find the platform (s), path length (cm), and swimming speed (cm/s). For a more detailed description of the maze and the paradigm used, see Shukitt-Hale and colleagues (1998).
Western blot analysis
After completion of behavioral tests, rats were euthanized by perfusion with phosphate-buffered saline (PBS). The hippocampus was then dissected out, snap-frozen, and stored at −80 °C until used. Samples were selected at random for western blot analysis (n = 7/group) to assess the total expression of inflammatory markers (glial fibrillary acidic protein (GFAP), NADPH oxidase-2 (NOX-2), and cyclooxygenase-2 (COX-2)) and autophagy markers (phosphorylated mammalian target of rapamycin (pmTOR), Beclin 1, and p62/sequestosome 1) as previously described in Poulose and colleagues (2012, 2013). Beta-actin was used as a loading control. The immunoreactive bands were captured, and optical densities were quantified with LabWorks Imaging Acquisition and Analysis software (UVP LLC, CA).
Statistical analyses
For each measure, between-subjects analysis of variance (ANOVA) models comparing the diet groups were performed using Systat (Cranes Software International Ltd., Chicago, IL) to test for statistical significance at an alpha level of p < 0.05. Days or trials, when appropriate, were included in the model as a within-subject variable. To determine differences between the diet groups, post hoc comparisons were performed using Fisher’s least significant difference (LSD) test.
Results
Effects of 2 % tart cherry supplementation on food intake and body weight
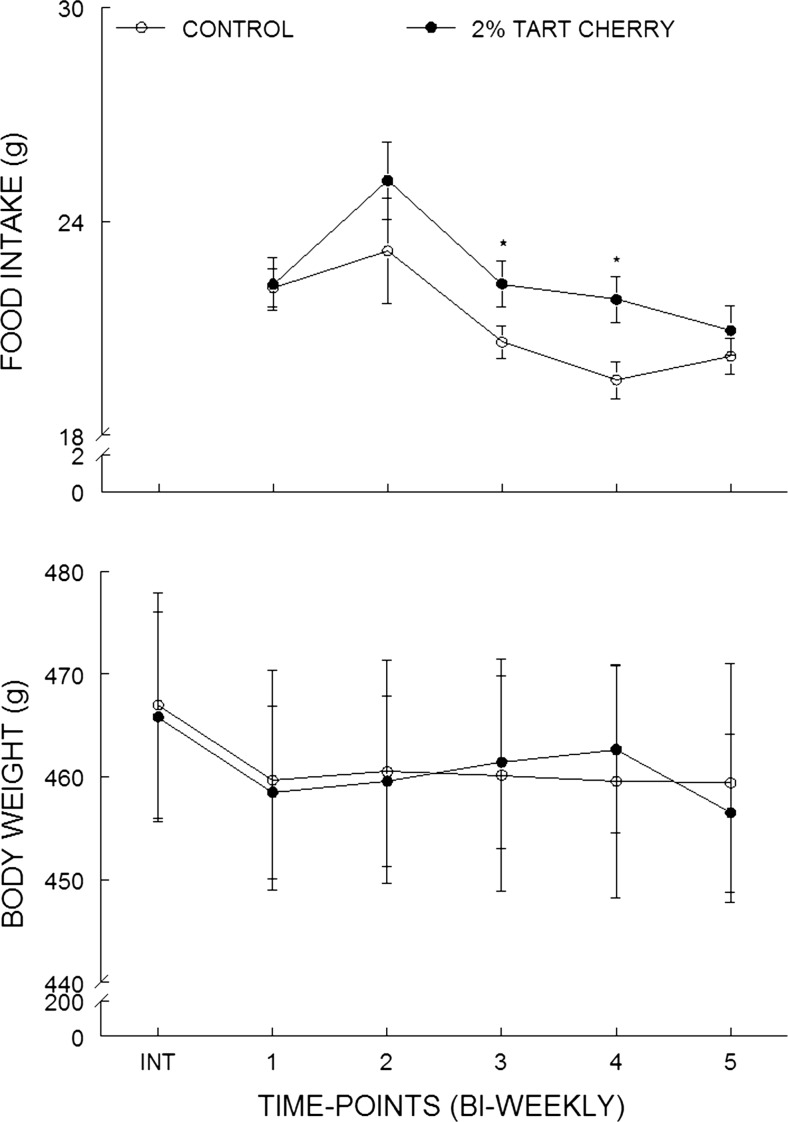
The tart cherry-supplemented group ate more than the control group initially, but food intake slowly declined to approximate that of controls by the end of the study (Fig. 1a). A one-way, repeated measures ANOVA showed a significant main effect of diet (p = 0.04) and a significant main effect of time (p < 0.001); however, no significant diet by time interaction was observed. Post hoc analysis indicated significant differences (p < 0.05) only at the second and third time point of food intake, during the fourth to sixth weeks after the initiation of the dietary supplementation.
Fig. 1.
Supplementation with tart cherry triggered transient increases in food consumption without affecting body weight. The results shown represent food intakes (a, grams/day; means ± SEM; n = 12/group) and body weight (b, grams; means ± SEM; n = 12/group) of rats over the course of the study for the control (open circles) and 2 % tart cherry-supplemented group (filled circles) measured bi-weekly. INT represents the body weight prior to initiation of diet. Asterisk indicates a statistically significant difference between the groups (p < 0.05)
Overall, the rats in both groups lost weight after their respective diets were implemented. Although there were significant differences in food intake between the two diet groups, there were no differences in body weight between the two groups at any time during the course of the study (Fig. 1b). Average body weights over the course of the study for the control and tart cherry groups were 460.8 ± 11.0 and 460.3 ± 8.2, respectively.
Effects of daily consumption of tart cherry on cognitive and motor function
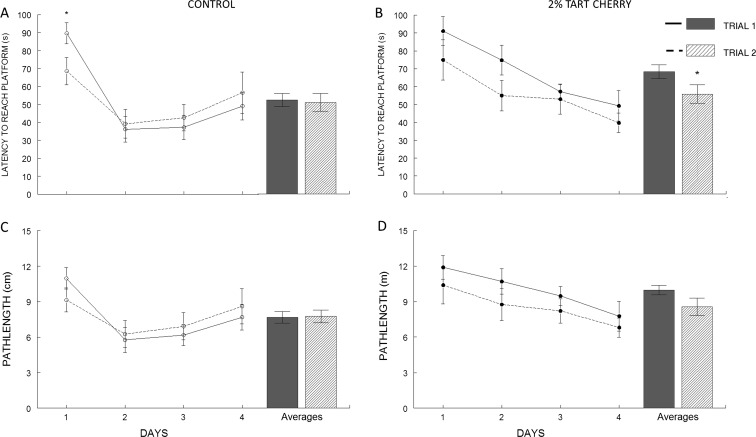
A wMWM was used to determine the effects of tart cherry supplementation on cognitive function. Rats fed the control diet reached maximum performance after 1 day of testing, as indicated by a plateau in the latency to reach the platform on days 2–4 (Fig. 2a), while rats in the tart cherry group continued to learn over the course of the test (Fig. 2b). For trial 1, a mixed model ANOVA revealed a significant main effect of diet (p = 0.009) and test day (p < 0.001), as well as significant interaction between diet and test day (p = 0.03). However, for trial 2, only a main effect of test day (p = 0.008) was observed.
Fig. 2.
Tart cherry supplementation improved spatial working memory. Performance of control (open circles) and 2 % tart cherry-supplemented group (filled circles) in a working memory version of the Morris water maze (wMWM), as assessed by latency (a, c) and path length (b, d) for trial 1 (solid line) and trial 2 (dashed line) during the four consecutive days of testing, and the averages between all test days (trial 1, open bar, and trial 2, hatched bar). Results represented as mean ± SEM; n = 12/group. Asterisk indicates statistically significant differences between trial 1 and trial 2 (p < 0.05)
The difference between trial 1 and trial 2 is a measure of working memory. Rats in the control group showed a significant reduction in their latency to reach the platform on trial 2 compared to trial 1 only on the first day of testing (Fig. 2a, p = 0.04), but took longer to reach the platform on trial 2 on days 3 and 4. In contrast, rats fed with tart cherry diet were able to reach the platform faster on trial 2 compared to trial 1 throughout the four test days. Separate t tests between the mean latencies for each group were performed to see if the tart cherry group significantly improved their performance from trial 1 to trial 2 (bar graphs in Fig. 2a, b) across testing days. Results showed that the cherry diet significantly (p < 0.005) improved working memory in the Morris water maze, but the control diet did not improve working memory. No group differences in swim speed were observed [average swim speed 0.150 ± 0.005 (control), 0.152 ± 0.004 (tart cherry), p > 0.05]; therefore, swim speed could not account for the group differences in swim latency.
Data for path length taken to reach the platform (Fig. 2c, d) were similar to the latency parameter. A mixed model ANOVA revealed a significant main effect of diet (p = 0.001) and test day (p = 0.002) for the first trial of each session (Trial 1); however, no significant main effects were seen for the second trial of each session (trial 2). No differences in path length were observed between trial 1 and trial 2 for the control group across testing days (Fig. 2c). However, a trend toward reduced path length from trial 1 to trial 2 among rats in the tart cherry group was observed (p = 0.08; Fig. d).
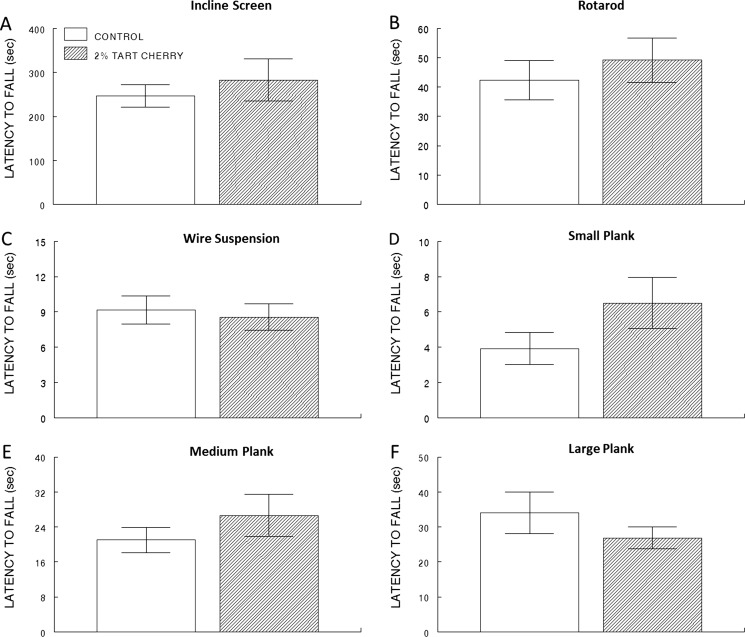
Rats completed a battery of motor tests after 6 weeks on either a control or tart cherry-supplemented diet. No significant differences were observed between the two diet groups on tests of balance, muscle strength, coordination, muscle tone, or stamina (Fig. 3a–f).
Fig. 3.
Motor performance was not affected by supplementation with tart cherry. Latency to fall (seconds, means ± SEM; n = 12/group) from inclined screen (a), rotarod (b), wire suspension (c), small plank (d), medium plank (e), and large plank (f) for the control (opened bar) and 2 % tart cherry-supplemented group (hatched bar)
Effect of tart cherry on inflammation and autophagy in the hippocampus
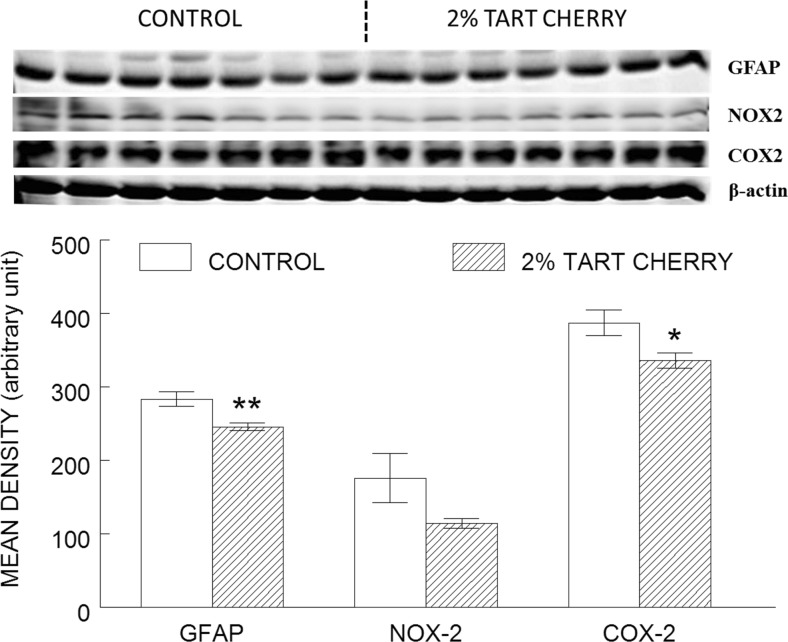
Inflammation in the hippocampus was assessed by western blot analysis of the inflammatory markers GFAP, NOX-2, and COX-2 (Fig. 4). Relative to controls, daily consumption of 2 % tart cherry led to reduction in GFAP, NOX-2, and COX-2, respectively. One-way ANOVA revealed a significant main effect of diet for GFAP (p = 0.006) and COX-2 (p = 0.03) and a trend toward an effect of NOX-2 (p = 0.09).
Fig. 4.
Tart cherry consumption reduced inflammation in the hippocampus. The results shown represent western blot (top) of inflammatory markers (glial fibrillary acidic protein [GFAP], NADPH oxidase-2 [NOX-2], and cyclooxygenase-2 [COX-2]) in the hippocampus of control (opened bar) and 2 % tart cherry-supplemented group (hatched bar), and densitometry analysis (bottom) of the immunoreactive bands normalized to β-actin, with results represented as mean ± SEM; n = 7/group. Asterisk indicates statistically significant difference between the groups (p < 0.05). Double asterisk indicates significant difference between the groups (p < 0.01)
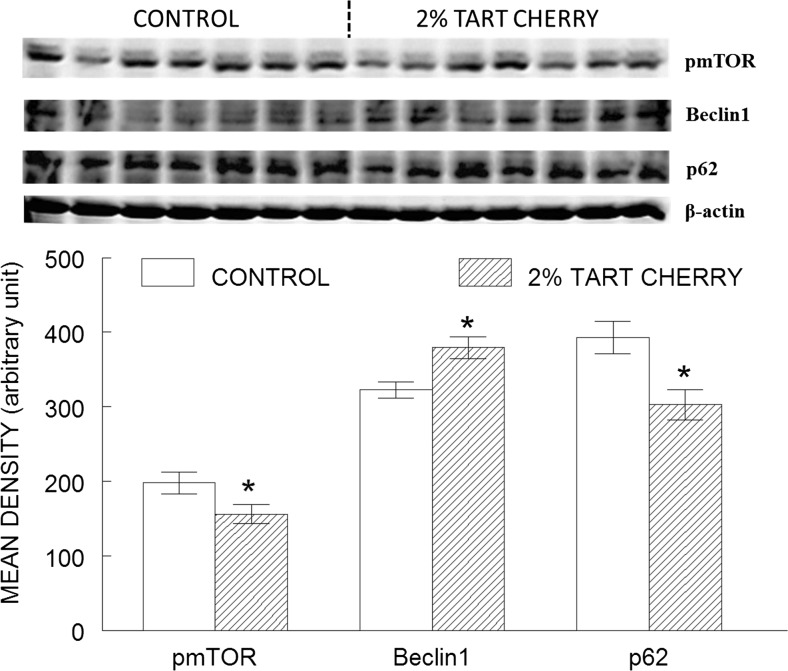
Additionally, autophagy markers were improved by the tart cherry diet (Fig. 5). One-way ANOVA revealed that the tart cherry-fed animals had decreased phosphorylation of mTOR (pmTOR) (p = 0.05) and p62/SQSTM 1 expression (p = 0.01) when compared to control rats, while Beclin 1 was upregulated (p = 0.01).
Fig. 5.
Tart cherry intake promoted autophagy in the hippocampus of aged rats. The results shown represent western blot (top) of autophagy markers (phosphorylated mTOR [pmTOR], Beclin 1, and p62/sequestosome 1 [p62]) in the hippocampus of control (opened bar) and 2 % tart cherry-supplemented group (hatched bar), and densitometry analysis (bottom) of the immunoreactive bands normalized to β-actin, with results represented as mean ± SEM; n = 7/group. Asterisk indicates statistically significant difference between the groups (p < 0.05)
Discussion
The present study investigated the effects of short-term 2 % tart cherry supplementation on motor and cognitive function, inflammation, and autophagy in aged rats. The major findings from this study were that supplementation with tart cherry: (1) transiently increased food intake without affecting body weight; (2) had no effects on motor function; (3) improved spatial learning and working memory; and (4) reduced inflammation and promoted autophagy in aged rats.
Previous studies from our laboratory and others found no differences in food intake when rats were fed diets supplemented with various berry fruits (e.g., blueberry, cranberry, and strawberry) (Joseph et al. 1999; Ahmet et al. 2009; Shaughnessy et al. 2009; Elks et al. 2011; Malin et al. 2011). Therefore, it was unexpected that tart cherry-fed rats would consume significantly more food than control-fed rats. Additionally, the increase in food intake did not result in altered body weight among tart cherry-fed rats. It is possible that the tart cherry-supplemented diet may increase the rate of metabolism. A study by Seymour and colleagues (2009) reported that 1 % tart cherry intake increased percentage of lean body mass, reduced body weight and decreased percentage fat mass in obesity-prone rats after 8 weeks of pair-feeding with high-fat controls. These findings suggest that consumption of tart cherry may shift body composition to favor a higher basal metabolic rate. However, more studies are needed to address this possibility.
When examining cognitive performance, tart cherry-fed rats exhibited improved spatial working memory that was not observed in the control rats. Specifically, trial 2 latencies were significantly less than trial 1, showing that rats in the tart cherry group demonstrated one-trial learning, even with the 10-min retention interval. This one-trial learning was not found in the control group. Studies using aged animals and aging models have reported that daily consumption of polyphenol-rich foods improves spatial learning and memory performance (Shukitt-Hale et al. 2007; Williams et al. 2008; Rendeiro et al. 2009; Shukitt-Hale et al. 2009a, b; Willis et al. 2009; Rendeiro et al. 2013). Because tart cherries contain a plethora of flavonoids and other phenolic compounds [(Kim et al. 2005), reviewed in (Ferretti et al. 2010; McCune et al. 2011)], and accumulate in the brain in a dose-dependent manner, it is not surprising that short-term intake of tart cherries led to improvement in spatial working memory.
The rapid drop in latency and path length between day 1 and day 2 observed in the control group appears to show that the control animals are learning faster than the tart cherry rats. However, examining the data in more detail shows that the controls actually exhibit higher latencies and path lengths for trial 2 after day 2, meaning that they show no working memory improvements. Therefore, it appears the control rats are not using spatial strategies to solve the maze, but rather may be using a different strategy, e.g., swimming until they bump into the platform. In using this strategy, performance gets better quickly but working memory (performing better on trial 2 based on information gleaned from trial 1) does not improve. As the tart cherry rats seem to be using spatial strategies, learning initially takes longer, but then working memory is improved. We see this working memory improvement in the tart cherry group, significantly for latency and as a trend for path length.
Daily intake of tart cherry had no effects on motor performance. It is possible that a 2 % tart cherry-supplemented diet may be insufficient to produce observable improvement in psychomotor performance. A study by Shukitt-Hale et al. (1999) showed that a 1 % strawberry-supplemented diet fed to aged rodents failed to produce beneficial effects on motor function, while a study by Joseph et al. (1999) reported that a 2 % strawberry-supplemented diet improved balance and coordination. Similarly, another study where aged rats were given either 10 or 50 % Concord grape juice for 8 weeks reported a significant improvement in motor function only in the 50 % Concord grape juice group when compared to the controls (Shukitt-Hale et al. 2006). In spite of evidence supporting beneficial effects of fruits and vegetables on motor function (Joseph et al. 1999; Shukitt-Hale et al. 2005; Galli et al. 2006; Neville et al. 2013; Shukitt-Hale et al. 2015), this null effect of tart cherry on motor function is supported by several studies where diets high in antioxidant capacity or fruits high in polyphenolic compounds fail to reverse age-related motor deficits in spite of other health benefits (Shukitt-Hale et al. 1999; Sumien et al. 2004; Shukitt-Hale et al. 2005). Because age-related motor deficits begin to manifest during early middle-age in rodents (Shukitt-Hale et al. 1998; de Fiebre et al. 2006) and deteriorate profoundly with age (Shukitt-Hale et al. 1999), it is possible that age-related damage may be too widespread by the time of late-life intervention, making it difficult to reverse (Bickford 1993). The age at which dietary supplementation is initiated, as well as the dose, may be the keys to forestalling age-related declines in motor function.
Many bioactive polyphenolic compounds with anti-inflammatory properties have been detected in both sweet and tart cherry varieties. Unsurprisingly, cherry-fed rats in this study showed less glial cell activation and lower expression of COX-2, an enzyme responsible for the formation of inflammatory mediators. Daily intake of cherry anthocyanins (40 mg/kg) for 28 days has been previously shown to significantly decrease TNF-α in the serum and prostaglandin E2 (PGE2) in the paws of rats using a model of arthritis (He et al. 2006b). Moreover, when 18 healthy male and female adults (age 45–61) consumed 280 g of Bing sweet cherries daily, a comparable amount of cherry (approximately 4 g/kg/day) as in this study, they showed a significant drop in serum C-reactive protein (hsCRP) levels compared to placebo controls (Kelley et al. 2006). These findings support the idea that cherry polyphenols have anti-inflammatory properties.
Recent studies have shown that consumption of a blueberry-, strawberry-, or walnut-supplemented diet can restore autophagy in aged rodents and rodent aging models (Poulose et al. 2013, 2014a, b). As with other berries (Poulose et al. 2014a, b) and walnuts (Poulose et al. 2013), dietary tart cherry improved markers of autophagy in the present study. The cherry-fed rats had decreased phosphorylation of mTOR, which negatively regulates the autophagal function, and decreased p62, a constituent of ubiquitinated protein aggregates, when compared to control rats. Consuming the tart cherry diet also led to overexpression of Beclin 1, which has been shown to regulate the onset of autophagy by interacting with a multi-protein complex (Liang et al. 1999; He and Levine 2010; Salminen et al. 2013). This cherry-induced stimulation of autophagy may contribute to the improvement in neuronal and cognition function.
Even though tart cherry had significant beneficial effects on cognition, inflammation, and autophagy, the sample size in this study is too small to generate a meaningful analysis linking biochemical changes to behavioral outcomes. Further studies are needed to determine the complete set of mechanisms involved and to examine the bioactive compound(s) in tart cherry that produce its beneficial effects. In summary, the findings from this study indicate that daily consumption of Montmorency tart cherry improved spatial learning and spatial working memory, reduced inflammation, and improved autophagy in the hippocampus of aged rats. Therefore, the regular addition of tart cherries to older adults’ diets may promote brain health and may reverse certain aspects of age-related cognitive decline.
Acknowledgments
This work was supported by USDA intramural funds and the Cherry Marketing Institute, Dewitt, MI. The authors would like to acknowledge the contributions of Francisco Ramirez for assistance with the western blots.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ahmet I, Spangler E, Shukitt-Hale B, Joseph JA, Ingram DK, Talan M. Survival and cardioprotective benefits of long-term blueberry enriched diet in dilated cardiomyopathy following myocardial infarction in rats. PLoS One. 2009;4:e7975. doi: 10.1371/journal.pone.0007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwat S, Haytowitz D, Holden J. USDA database for the flavonoid content of selected foods. Release. 2014;3:1. [Google Scholar]
- Bickford P. Motor learning deficits in aged rats are correlated with loss of cerebellar noradrenergic function. Brain Res. 1993;620:133–138. doi: 10.1016/0006-8993(93)90279-V. [DOI] [PubMed] [Google Scholar]
- Bobe G, Wang B, Seeram NP, Nair MG, Bourquin LD. Dietary anthocyanin-rich tart cherry extract inhibits intestinal tumorigenesis in APC(Min) mice fed suboptimal levels of sulindac. J Agric Food Chem. 2006;54:9322–9328. doi: 10.1021/jf0612169. [DOI] [PubMed] [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S. The use of the Morris water maze in the study of memory and learning. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Brayne C, Gill C, Paykel ES, Huppert F, O’Connor DW. Cognitive decline in an elderly population—a two wave study of change. Psychol Med. 1995;25:673–683. doi: 10.1017/S0033291700034930. [DOI] [PubMed] [Google Scholar]
- Carney JM, Smith CD, Carney AM, Butterfield DA. Aging- and oxygen-induced modifications in brain biochemistry and behavior. Ann N Y Acad Sci. 1994;738:44–53. doi: 10.1111/j.1749-6632.1994.tb21788.x. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Shukitt-Hale B, Stellwagen HM, Zhu X, Lee HG, Smith MA, Joseph JA. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci. 2004;7:309–316. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- de Fiebre NC, Sumien N, Forster MJ, de Fiebre CM. Spatial learning and psychomotor performance of C57BL/6 mice: age sensitivity and reliability of individual differences. Age. 2006;28:235–253. doi: 10.1007/s11357-006-9027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elks CM, Reed SD, Mariappan N, Shukitt-Hale B, Joseph JA, Ingram DK, Francis J. A blueberry-enriched diet attenuates nephropathy in a rat model of hypertension via reduction in oxidative stress. PLoS One. 2011;6:e24028. doi: 10.1371/journal.pone.0024028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti G, Bacchetti T, Belleggia A, Neri D. Cherry antioxidants: from farm to table. Molecules. 2010;15:6993–7005. doi: 10.3390/molecules15106993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli RL, Bielinski DF, Szprengiel A, Shukitt-Hale B, Joseph JA. Blueberry supplemented diet reverses age-related decline in hippocampal HSP70 neuroprotection. Neurobiol Aging. 2006;27:344–350. doi: 10.1016/j.neurobiolaging.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Goyarzu P, Malin DH, Lau FC, Taglialatela G, Moon WD, Jennings R, Moy E, Moy D, Lippold S, Shukitt-Hale B, et al. Blueberry supplemented diet: effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutr Neurosci. 2004;7:75–83. doi: 10.1080/10284150410001710410. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vannucchi MG, Wenk GL. Behavioral and ultrastructural changes induced by chronic neuroinflammation in young rats. Brain Res. 2000;859:157–166. doi: 10.1016/S0006-8993(00)01999-5. [DOI] [PubMed] [Google Scholar]
- He C, Levine B. The Beclin 1 interactome. Curr Opin Cell Biol. 2010;22:140–149. doi: 10.1016/j.ceb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006;367:320–326. doi: 10.1016/S0140-6736(06)68069-0. [DOI] [PubMed] [Google Scholar]
- He YH, Zhou J, Wang YS, Xiao C, Tong Y, Tang JC, Chan AS, Lu AP. Anti-inflammatory and anti-oxidative effects of cherries on Freund’s adjuvant-induced arthritis in rats. Scand J Rheumatol. 2006;35:356–358. doi: 10.1080/03009740600704155. [DOI] [PubMed] [Google Scholar]
- He FJ, Nowson CA, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J Hum Hypertens. 2007;21:717–728. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Berg S, Era P. Evaluating the interdependence of aging-related changes in visual and auditory acuity, balance, and cognitive functioning. Psychol Aging. 2003;18:285–305. doi: 10.1037/0882-7974.18.2.285. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Bartus RT, Clody D, Morgan D, Finch C, Beer B, Sesack S. Psychomotor performance in the senescent rodent: reduction of deficits via striatal dopamine receptor up-regulation. Neurobiol Aging. 1983;4:313–319. doi: 10.1016/0197-4580(83)90008-8. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Denisova N, Fisher D, Bickford P, Prior R, Cao G. Age-related neurodegeneration and oxidative stress: putative nutritional intervention. Neurol Clin. 1998;16:747–755. doi: 10.1016/S0733-8619(05)70092-X. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SY, Seeram NP, Nair MG, Bourquin LD. Tart cherry anthocyanins inhibit tumor development in Apc(Min) mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003;194:13–19. doi: 10.1016/S0304-3940(02)00583-9. [DOI] [PubMed] [Google Scholar]
- Kelley DS, Rasooly R, Jacob RA, Kader AA, Mackey BE. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J Nutr. 2006;136:981–986. doi: 10.1093/jn/136.4.981. [DOI] [PubMed] [Google Scholar]
- Kim DO, Heo HJ, Kim YJ, Yang HS, Lee CY. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J Agric Food Chem. 2005;53:9921–9927. doi: 10.1021/jf0518599. [DOI] [PubMed] [Google Scholar]
- Kirakosyan A, Seymour EM, Wolforth J, McNish R, Kaufman PB, Bolling SF. Tissue bioavailability of anthocyanins from whole tart cherry in healthy rats. Food Chem. 2015;171:26–31. doi: 10.1016/j.foodchem.2014.08.114. [DOI] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Lock K, Pomerleau J, Causer L, Altmann DR, McKee M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull World Health Organ. 2005;83:100–108. [PMC free article] [PubMed] [Google Scholar]
- Malin DH, Lee DR, Goyarzu P, Chang YH, Ennis LJ, Beckett E, Shukitt-Hale B, Joseph JA. Short-term blueberry-enriched diet prevents and reverses object recognition memory loss in aging rats. Nutrition. 2011;27:338–342. doi: 10.1016/j.nut.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Martin KR, Wooden A. Tart cherry juice induces differential dose-dependent effects on apoptosis, but not cellular proliferation, in MCF-7 human breast cancer cells. J Med Food. 2012;15:945–954. doi: 10.1089/jmf.2011.0336. [DOI] [PubMed] [Google Scholar]
- McCune LM, Kubota C, Stendell-Hollis NR, Thomson CA. Cherries and health: a review. Crit Rev Food Sci Nutr. 2011;51:1–12. doi: 10.1080/10408390903001719. [DOI] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P. Signalling and autophagy regulation in health, aging and disease. Mol Asp Med. 2006;27:411–425. doi: 10.1016/j.mam.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Miller MG, Shukitt-Hale B. Berry fruit enhances beneficial signaling in the brain. J Agric Food Chem. 2012;60:5709–5715. doi: 10.1021/jf2036033. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Neville CE, Young IS, Gilchrist SE, McKinley MC, Gibson A, Edgar JD, Woodside JV. Effect of increased fruit and vegetable consumption on physical function and muscle strength in older adults. Age (Dordr) 2013;35:2409–2422. doi: 10.1007/s11357-013-9530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurk E, Refsum H, Drevon CA, Tell GS, Nygaard HA, Engedal K, Smith AD. Cognitive performance among the elderly in relation to the intake of plant foods. The Hordaland Health Study. Br J Nutr. 2010;104:1190–1201. doi: 10.1017/S0007114510001807. [DOI] [PubMed] [Google Scholar]
- Olanow CW. A radical hypothesis for neurodegeneration. Trends Neurosci. 1993;16:439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- Ou B, Bosak KN, Brickner PR, Iezzoni DG, Seymour EM. Processed tart cherry products—comparative phytochemical content, in vitro antioxidant capacity and in vitro anti-inflammatory activity. J Food Sci. 2012;77:H105–H112. doi: 10.1111/j.1750-3841.2012.02681.x. [DOI] [PubMed] [Google Scholar]
- Polidori MC, Pratico D, Mangialasche F, Mariani E, Aust O, Anlasik T, Mang N, Pientka L, Stahl W, Sies H, et al. High fruit and vegetable intake is positively correlated with antioxidant status and cognitive performance in healthy subjects. J Alzheimers Dis. 2009;17:921–927. doi: 10.3233/JAD-2009-1114. [DOI] [PubMed] [Google Scholar]
- Poulose SM, Bielinski DF, Carrihill-Knoll K, Rabin BM, Shukitt-Hale B. Exposure to 16O-particle radiation causes aging-like decrements in rats through increased oxidative stress, inflammation and loss of autophagy. Radiat Res. 2011;176:761–769. doi: 10.1667/RR2605.1. [DOI] [PubMed] [Google Scholar]
- Poulose SM, Carey AN, Shukitt-Hale B. Improving brain signaling in aging: could berries be the answer? Expert Rev Neurother. 2012;12:887–889. doi: 10.1586/ern.12.86. [DOI] [PubMed] [Google Scholar]
- Poulose SM, Bielinski DF, Shukitt-Hale B. Walnut diet reduces accumulation of polyubiquitinated proteins and inflammation in the brain of aged rats. J Nutr Biochem. 2013;24:912–919. doi: 10.1016/j.jnutbio.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Poulose SM, Bielinski DF, Carrihill-Knoll KL, Rabin BM, Shukitt-Hale B. Protective effects of blueberry- and strawberry diets on neuronal stress following exposure to (56)Fe particles. Brain Res. 2014;1593:9–18. doi: 10.1016/j.brainres.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Poulose SM, Fisher DR, Bielinski DF, Gomes SM, Rimando AM, Schauss AG, Shukitt-Hale B. Restoration of stressor-induced calcium dysregulation and autophagy inhibition by polyphenol-rich acai (Euterpe spp.) fruit pulp extracts in rodent brain cells in vitro. Nutrition. 2014;30:853–862. doi: 10.1016/j.nut.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Rajawat YS, Hilioti Z, Bossis I. Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev. 2009;8:199–213. doi: 10.1016/j.arr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Rendeiro C, Spencer JP, Vauzour D, Butler LT, Ellis JA, Williams CM. The impact of flavonoids on spatial memory in rodents: from behaviour to underlying hippocampal mechanisms. Genes Nutr. 2009;4:251–270. doi: 10.1007/s12263-009-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendeiro C, Vauzour D, Rattray M, Waffo-Teguo P, Merillon JM, Butler LT, Williams CM, Spencer JP. Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. PLoS One. 2013;8:e63535. doi: 10.1371/journal.pone.0063535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Impaired autophagy and APP processing in Alzheimer’s disease: the potential role of Beclin 1 interactome. Prog Neurobiol. 2013;106-107:33–54. doi: 10.1016/j.pneurobio.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Bourquin LD, Nair MG. Degradation products of cyanidin glycosides from tart cherries and their bioactivities. J Agric Food Chem. 2001;49:4924–4929. doi: 10.1021/jf0107508. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Momin RA, Nair MG, Bourquin LD. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 2001;8:362–369. doi: 10.1078/0944-7113-00053. [DOI] [PubMed] [Google Scholar]
- Sehitoglu MH, Farooqi AA, Qureshi MZ, Butt G, Aras A. Anthocyanins: targeting of signaling networks in cancer cells. Asian Pac J Cancer Prev. 2014;15:2379–2381. doi: 10.7314/APJCP.2014.15.5.2379. [DOI] [PubMed] [Google Scholar]
- Seymour EM, Singer AA, Kirakosyan A, Urcuyo-Llanes DE, Kaufman PB, Bolling SF. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J Med Food. 2008;11:252–259. doi: 10.1089/jmf.2007.658. [DOI] [PubMed] [Google Scholar]
- Seymour EM, Lewis SK, Urcuyo-Llanes DE, Tanone II, Kirakosyan A, Kaufman PB, Bolling SF. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J Med Food. 2009;12:935–942. doi: 10.1089/jmf.2008.0270. [DOI] [PubMed] [Google Scholar]
- Shaughnessy KS, Boswall IA, Scanlan AP, Gottschall-Pass KT, Sweeney MI. Diets containing blueberry extract lower blood pressure in spontaneously hypertensive stroke-prone rats. Nutr Res. 2009;29:130–138. doi: 10.1016/j.nutres.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B. The effects of aging and oxidative stress on psychomotor and cognitive behavior. Age (Omaha) 1999;22:9–17. doi: 10.1007/s11357-999-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukitt-Hale B, Mouzakis G, Joseph JA. Psychomotor and spatial memory performance in aging male Fischer 344 rats. Exp Gerontol. 1998;33:615–624. doi: 10.1016/S0531-5565(98)00024-2. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Smith DE, Meydani M, Joseph JA. The effects of dietary antioxidants on psychomotor performance in aged mice. Exp Gerontol. 1999;34:797–808. doi: 10.1016/S0531-5565(99)00039-X. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Galli RL, Meterko V, Carey A, Bielinski DF, McGhie T, Joseph JA. Dietary supplementation with fruit polyphenolics ameliorates age-related deficits in behavior and neuronal markers of inflammation and oxidative stress. Age (Dordr) 2005;27:49–57. doi: 10.1007/s11357-005-4004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukitt-Hale B, Carey A, Simon L, Mark DA, Joseph JA. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition. 2006;22:295–302. doi: 10.1016/j.nut.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Carey AN, Jenkins D, Rabin BM, Joseph JA. Beneficial effects of fruit extracts on neuronal function and behavior in a rodent model of accelerated aging. Neurobiol Aging. 2007;28:1187–1194. doi: 10.1016/j.neurobiolaging.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Cheng V, Joseph JA. Effects of blackberries on motor and cognitive function in aged rats. Nutr Neurosci. 2009;12:135–140. doi: 10.1179/147683009X423292. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Kalt W, Carey AN, Vinqvist-Tymchuk M, McDonald J, Joseph JA. Plum juice, but not dried plum powder, is effective in mitigating cognitive deficits in aged rats. Nutrition. 2009;25:567–573. doi: 10.1016/j.nut.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Bielinski DF, Lau FC, Willis LM, Carey AN, Joseph JA (2015) The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br J Nutr:1–8 [DOI] [PubMed]
- Sumien N, Heinrich KR, Sohal RS, Forster MJ. Short-term vitamin E intake fails to improve cognitive or psychomotor performance of aged mice. Free Radic Biol Med. 2004;36:1424–1433. doi: 10.1016/j.freeradbiomed.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Tall JM, Seeram NP, Zhao C, Nair MG, Meyer RA, Raja SN. Tart cherry anthocyanins suppress inflammation-induced pain behavior in rat. Behav Brain Res. 2004;153:181–188. doi: 10.1016/j.bbr.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Traustadottir T, Davies SS, Stock AA, Su Y, Heward CB, Roberts LJ, 2nd, Harman SM. Tart cherry juice decreases oxidative stress in healthy older men and women. J Nutr. 2009;139:1896–1900. doi: 10.3945/jn.109.111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ (1985) Formation of a place learning-set by the rat: a new paradigm for neurobehavioral studies. Physiol Behav 35:139–143 [DOI] [PubMed]
- Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer JP. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Willis LM, Shukitt-Hale B, Cheng V, Joseph JA. Dose-dependent effects of walnuts on motor and cognitive function in aged rats. Br J Nutr. 2009;101:1140–1144. doi: 10.1017/S0007114508059369. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Shukitt-Hale B, Martin A, Wang H, Denisova N, Bickford PC, et al. Short-term dietary supplementation of blueberry polyphenolics: beneficial effects on aging brain performance and peripheral tissue function. Nutr Neurosci. 2000;3:383–397. doi: 10.1080/1028415X.2000.11747338. [DOI] [Google Scholar]