Summary
The magnitude of coagulation abnormalities, and the definition and treatment of coagulopathy in burn patients are inadequately understood and continue to be discussed in the literature. We aimed to analyse physicians’ views on monitoring and treating coagulation abnormalities in burn patients. A total of 350 questionnaires were distributed electronically to burn ICU physicians. Participation was voluntary and anonymous. Responses were analysed electronically and comparisons were made according to the region of the ICU or the specialty of the physician. Of the 350 questionnaires distributed, 55 (15.7%) were returned. The majority of burn specialists consider sepsis-induced coagulopathy to be the most frequent coagulopathy in burn patients, and 74.5% declare that they do not use any specific definition/scoring system in their department to detect coagulopathy. The majority of specialists (70.8%) use standard coagulation tests. The most frequent indications for plasma transfusion are massive bleeding (32.8%) and Disseminated Intravascular Coagulation syndrome treatment (20%). The main specific factors reported in our study are cryoprecipitate (23.2%) and fibrinogen concentrate (18.9%). 21.1% of respondents state that they do not use any specific coagulation factor substitution in burn patients. Specific coagulation factor substitution is not a routine practice. The low response rate precludes the generalization of our results.
Keywords: burn, blood coagulation, monitoring, treatment
Abstract
La définition, l’importance et le traitement des anomalies de la coagulation chez les patients brûlés sont mal connues et font régulièrement l’objet de controverse dans la littérature. Nous avons analysé le point de vue des praticien sur le monitorage et le traitement de ces anomalies. Trois cent cinquante questionnaires ont été envoyés par voie électronique à des médecins travaillant en USI pour brûlés. La participation était volontaire et anonyme. Les réponses ont été comparées en tenant compte de la géographie et de la spécialité du répondant. Cinquante cinq (15,7%) ont été remplis. La majorité des praticiens considèrent que le sepsis est la cause la plus fréquente de coagulopathie chez les brûlés. Les ¾ n’utilisent pas de définition ni de score spécifiques, 70,8% utilisant les tests standard. Les indication le plus fréquentes de transfusion plasmatique (32,8%) sont le saignement massif et la CIVD (20%). Les facteurs spécifiques le plus souvent utilisés sont les cryoprécipités (23,2%) et le fibrinogène (18,9%), et 21,1% des sondés n’utilisent jamais de tels dérivés du sang. L’utilisation en routine de facteurs de coagulation est donc rare chez les brûlologues. Le faible taux de réponse ne permet pas d’inférer ces résultats à la population brûlologique générale.
Introduction
Burn injury is traditionally referred to as a common triggering cause of acute coagulopathy, ranging from subclinical activation of coagulation to fulminant overt Disseminated Intravascular Coagulation (DIC). Coagulopathy associated with burn injury was well recognized as early as the 1970s.1,2 Coagulopathy in burn patients is considered to be driven by an endothelial injury, release of tissue factor and inflammatory cytokines.3 Blood loss, hypovolaemia or excessive volume expansion, hypothermia and acidosis further aggravate the situation. 4,6 Coagulation system activation is characterized initially by thrombin generation, hypercoagulability and hyperfibrinosysis.7 Activation of both thrombosis and fibrinolysis and increased consumption of coagulation factors leads thereafter to the development of consumption coagulopathy.3,6-8 The coagulation system abnormalities may be further enhanced by surgery; wound excision may be associated with extensive blood loss, dilution and consumption of coagulation factors which may have an additional negative impact on the coagulation system. Additionally, the activation of inflammatory and coagulation cascade in septic burn patients can lead to microvascular injury and subsequent multiple organ dysfunction or failure.3,6
The literature on coagulopathy in burn patients is relatively heterogeneous, so the incidence of coagulopathy in burns is still undefined and depends on diagnostic criteria and the definitions used in each study. In terms of diagnosis, assessment of the levels of specific coagulation markers has been reported to be helpful.7,8 The use of new diagnostic methods such as thromboelastometry and thromboelastography may also improve our diagnostic abilities in coagulopathy. Although a few studies have recently been published on the use of thromboelastography in burn patients,9,10 there are still insufficient data on the use of the tool in this specific area. Controversies persist over the treatment of coagulopathy in burn patients. Modern treatment strategies suggest using specific coagulation factors instead of plasma, in an effort to minimize patients’ exposure to blood products. The use of specific coagulation factors in burn patients seems to be effective and reduces allogeneic blood product requirements perioperatively.10,11
Although there is extensive literature exploring the attitudes of physicians on diagnosis and management of traumainduced coagulopathy treatment over the last decade,12,18 still little is known about practices in specialised burn units. The questionnaire used in our study was created to evaluate aspects of monitoring and treatment of coagulation abnormalities in burn patients.
Materials and methods
The questionnaire was designed by the authors and was assessed by two intensive care consultants who work in the specialised burn ICU of the first author. The reviewing consultants were not involved in conducting the survey, and their comments resulted in minor modifications to improve the clarity of the questionnaire.
A total of 350 questionnaires were distributed electronically to burn ICU physicians. Participation in the survey was voluntary and anonymous. Collection time (time the survey remained open) was 2 months.
The questionnaire consisted of three parts: the first part collected physician and institutional demographics, the second part explored the opinions and attitudes of the burn specialists regarding diagnostic approach to coagulopathy, and the third explored their opinions and attitudes regarding therapeutic approach to coagulopathy in burn patients.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Appropriate descriptive statistics were used to explore basic response rates in every question, while the chi-square test was utilized to reveal differences between the responses of burn specialists in different regions or from different backgrounds. Test results were considered to be statistically significant if the p value was less than 0.05.
Results
Of the 350 questionnaires distributed, 55 (15.7%) were returned. Regarding the geographic distribution of respondents, 54.5% were from centres in Europe, 20% in North America, and 25.5% in other regions. The majority of respondents were from burn centres with more than 60 admissions per year (80% of centres in Europe, 100% in North America, and 71% in other regions). Most of the physicians (89.1%) were senior members of staff (heads of department and consultants); 56.4% of respondents were surgeons and 43.6% were anaesthesiologists or intensivists.
Physician and institutional demographics are shown in Table I. Diagnostic approaches to coagulopathy are displayed in Table II. The majority of participants (74.5%) declare that they do not use any specific definition and scoring system in their department to detect coagulopathy. One third of physicians (30.9%) claim that there is not enough data to support the use of viscoelastic tests in burn patients, and 25.5% of respondents state that they are not aware of these techniques.
Table I. Characteristics of respondents and burn centres.
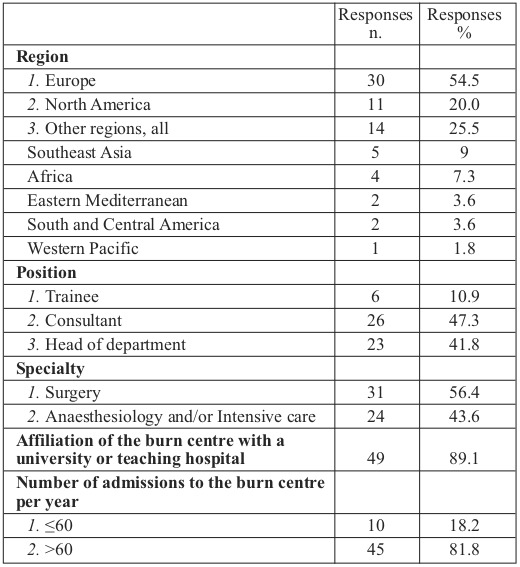
Table II. Diagnostic approaches to coagulopathy.
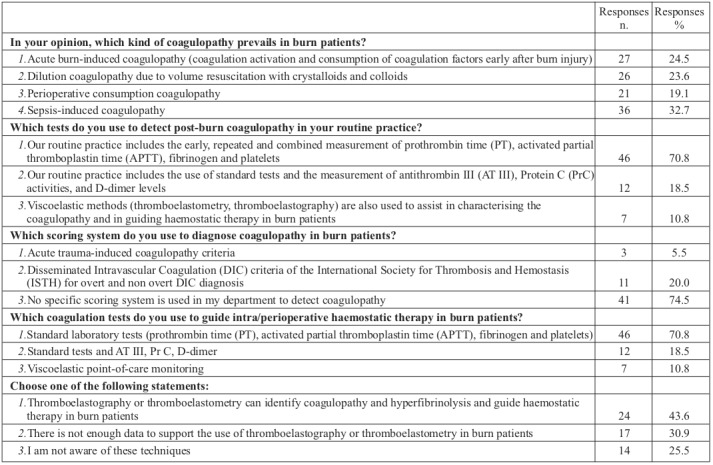
Treatment approaches to coagulopathy are shown in Table III. The minority of burn specialists (16.8%) use a transfusion protocol with a fixed blood product ratio; 43.6% of respondents suggest an optimal red blood cell:plasma ratio of at least 1:3 and 36.4% of respondents suggest at least 1:1. The most frequent indications for plasma transfusion are massive bleeding (32.8%) and Disseminated Intravascular Coagulation syndrome treatment (20%). A platelet count <50 x 109 /L is a common trigger for perioperative platelet transfusion for 31.9% of burn specialists, however 33.3% of respondents use individualized platelet substitution in each patient. The most used specific factors reported by participants in our study are cryoprecipitate (23.2%) and fibrinogen concentrate (18.9%), while 21.1% of respondents state that they do not use any specific coagulation factor substitution in burn patients. We observed statistically significant differences in replies to particular questions about origins and kind of treatments for coagulopathy between regions and respondents’ specialties (Table IV).
Table III. Therapeutic approaches to coagulopathy.
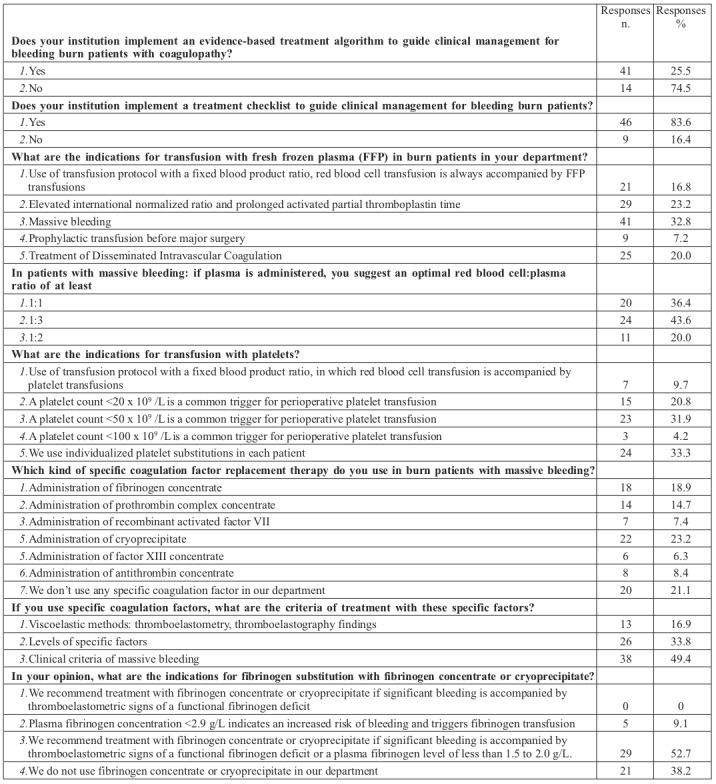
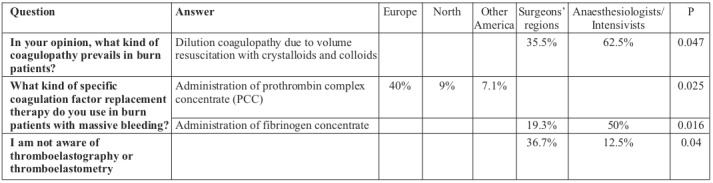
Table IV. Differences between regions and specialties.
Discussion
The magnitude of coagulation abnormalities, and the definition and treatment of coagulopathy in burn patients are inadequately understood and continue to be discussed in the literature.4-8,19,20 Furthermore, the majority of studies on coagulopathy in burns were conducted more than a decade ago, before the dramatic increase in the use of specific coagulation factors and before the implementation of new monitoring tools such as the measurement of specific coagulation factors and the use of viscoelastic tests. Our questionnaire aimed to explore the views and practices of burn ICU physicians regarding this challenging matter.
Combined mechanisms contribute to coagulopathy in burn patients, such as excessive consumption of coagulation factors and platelets, dilution coagulopathy due to administration of large volumes of fluids and sepsis-induced coagulopathy.4,6 More than thirty percent of the study participants (32.7%) consider sepsis-induced coagulopathy to be the most frequent coagulopathy in burn patients, followed by early post-burn coagulopathy (24.5%) and dilution coagulopathy (23.6%) due to volume resuscitation. Interestingly, differences were observed between surgeons and anaesthesiologists/intensivists; the prevailing coagulation disorder reported by 62.5% of anaesthesiologists/intensivists was dilution coagulopathy, whilst only 35.5% of surgeons considered dilution coagulopathy to be the principal cause of coagulation disorders in burns.
The majority of the respondents (70.8%) report that their routine practice includes the use of standard coagulation tests (measurement of prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen and platelets). More recent evidence, however, suggests that classical tests for coagulation, such as international normalized ratio, PT and PTT are not reliable, and fail to accurately describe the complex processes occurring in acute trauma patients.12,13,15,18
Major differences in the definitions and criteria used and, consequently, the incidence of coagulation abnormalities can be noted in recent literature. Coagulopathy is defined as acute traumatic coagulopathy, acute burn-induced coagulopathy or overt and non-overt disseminated intravascular coagulation. 4,7,21,22 Surprisingly, 74.5% of participants declare that they do not use any specific definition and scoring system in their department to detect coagulopathy, and only a few of them (5.5%) use the acute trauma-induced coagulopathy criteria. Conventional plasma-based coagulation tests (prothrombin time, international normalized ratio and activated partial thromboplastin time) are used by most respondents (70.8%) to assess haemostatic status of bleeding patients, and to guide intra/perioperative haemostatic therapy. This is of particular interest, as recent publications found no evidence that standard coagulation tests are predictive of bleeding or have the potential to guide coagulation therapy.13,23
Viscoelastic tests such as TEG (thrombography) or ROTEM (rotational thromboelastometry), which use whole blood and provide measurements of the entire clotting mechanism, have become more popular in the management of trauma patients. 13,18 The use of Point of Care coagulation monitoring (POC), which is based on viscoelastic tests, may improve our understanding and diagnostic abilities in coagulopathy, and additionally, seems to lower transfusion-related costs in cardiac surgery, trauma and liver transplantation.13 The use of a specific coagulopathy treatment algorithm, based on viscoelastic techniques, has reduced allogeneic blood product requirements in burn patients perioperatively.10 Our study reveals that only the minority of respondents (10.8%) use viscoelastic point-of-care monitoring to guide haemostatic therapy in their routine practice, with 25.5% of respondents claiming that they are not aware of these techniques. Interestingly, differences were observed on this point between surgeons and anaesthesiologists/intensivists; a much greater percentage of surgeons (36.7%) were unaware of the use of viscoelastic tests compared to anaesthesiologists and intensivists (12.5%).
With growing concern about the need for optimizing transfusion practices and improving treatment of bleeding patients, algorithms for the clinical evaluation and control of bleeding in perioperative clinical settings have been created. 10,13,24 The majority of respondents (74.5%) report that they do not implement an evidence-based treatment algorithm to guide clinical management of a bleeding burn patient with coagulopathy. These findings highlight the importance of a structured approach for clinicians in order to achieve earlier and more effective bleeding control.
Many treatment protocols for perioperative bleeding use fixed ratios of allogeneic blood products. Studies from both military and civilian trauma centres have shown that early transfusions with a high ratio of fresh frozen plasma to red cells outcomes in trauma patients with severe hemorrhage.18,25-27 However, transfusion of allogeneic blood products increases morbidity and mortality, and fixed ratios might not improve outcomes.13 Our study reveals that only a low percentage of burn specialists (16.8%) use a transfusion protocol with a fixed blood product ratio, where red blood cell transfusion is always accompanied by FFP transfusion, and only 9.7% of respondents use a transfusion protocol with a fixed blood product ratio, in which red blood cell transfusion is accompanied by platelet transfusions. Interestingly, 20% of respondents consider the use the plasma transfusion for Disseminated Intravascular Coagulation syndrome treatment, despite the fact that the use of plasma for this purpose is no longer recommended.3,13
Modern treatment strategies suggest using specific coagulation factors instead of plasma, thus minimizing patients’ exposure to blood products. The principal advantages of specific coagulation factors include their immediate availability, defined and high concentrations of the coagulation factors which can be administered without volume expansion.13,16,18 We searched for evidence on the use of fibrinogen concentrate, cryoprecipitate, factor XIII concentrate, recombinant activated factor VII (rFVIIa), prothrombin complex concentrate and antithrombin concentrate in severe perioperative bleeding. Our results show that in 49.4% of centres, treatment with specific coagulation factors is mainly based on pragmatic clinical aspects (clinical signs of massive bleeding) rather than guided by specific tests. The most frequently used specific factors reported by participants in our study are cryoprecipitate (23.2%) and fibrinogen concentrate (18.9%), while 21.1% of respondents state that they do not use any specific coagulation factor substitution in burn patients. The potential concerns about the treatment-associated cost of these factors might partially explain the last finding, although current guidelines report that goal-directed therapy with specific coagulation factor concentrates may reduce transfusion- associated costs.13 However, specific administrative rules and cost control initiatives of hospitals could be an additional actor that affected the burn specialists’ decision about the replacement of specific factors in our study.
We observed statistically significant differences regarding the use of specific coagulation factor replacement therapy when comparing different regions and specialties. The use of prothrombin complex concentrate is more frequent in Europe (40%), whereas in N. America and other regions it accounts for 9% and 7.1% of specific factors, respectively. Anaesthesiologists and/or intensivists administer fibrinogen concentrate more frequently in comparison to surgeons (50% vs. 19.5%). Lack of sufficient evidence that using specific coagulation factors improves outcome in burn patients, and the high cost of treatment with specific factors, may partially explain the observed reluctance of burn physicians to use this treatment option. Low availability of coagulation factor concentrates and point-of-care testing might also lead to insufficient use of targeted therapy for haemostatic defects. More clinical trials should aim to extend our knowledge of the effects of specific coagulation factors and should therefore address important biologically- based coagulopathy treatment uncertainties in burn patients. Clear indications, efficacy, and the economic feasibility of the use of specific coagulation factors in burn patients should be targeted in these future trials. Our study was the first international study evaluating the opinions and attitudes of burn specialists regarding diagnostic and therapeutic approaches to coagulopathy in burn patients. However, there were several limitations: the use of the questionnaire was not formally validated and the response rate was unfortunately low. This might be attributed partly to the absence of support by an official scientific association, which would make completion of the questionnaire more appealing. The resulting small number of responses did not allow us to analyse differences between geographic regions and countries in depth, although our sample was not country specific. Additionally, the small number of respondents makes the results of this study susceptible to randomization errors. Another limitation is that the study was limited to burn specialists and did not address a multidisciplinary group of specialists involved in burn care, which could be an interesting target group for analogous future investigations.
In conclusion
The majority of burn specialists consider sepsis-induced coagulopathy to be the most frequent coagulopathy in burn patients
The majority of burn specialists do not use any specific scoring system to detect coagulopathy.
Standard coagulation tests are the most commonly used coagulation tests in burn units.
Very few burn physicians use viscoelastic point-of-care monitoring to guide haemostatic therapy in their routine practice.
Specific coagulation factor replacement therapy includes the use of cryoprecipitate, fibrinogen concentrate and prothrombin complex concentrate; however, quite a few physicians do not use any specific coagulation factor substitution in burn patients.
Additional clinical trials should aim to extend our knowledge about diagnostic and therapeutic approach to coagulopathy in burn patients.
Acknowledgments
Conflict of interest.None
References
- 1.Curreri PW, Rayfield DL, Vaught M, Baxter CR. Extravascular fibrinogen degradation in experimental burn wounds: a source of fibrin split products. Surgery. 1975;77(1):86–91. [PubMed] [Google Scholar]
- 2.Curreri PW, Wilterdink ME, Baxter CR. Coagulation dynamics following thermal injury: effect of heparin and protamine sulfate. Ann Surg. 1975;181(2):161–163. doi: 10.1097/00000658-197502000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippi G, Ippolito L, Cervellin G. Disseminated intravascular coagulation in burn injury. Semin Thromb Hemost. 2010;36(4):429–436. doi: 10.1055/s-0030-1254051. [DOI] [PubMed] [Google Scholar]
- 4.Sherren PB, Hussey J, Martin R, Kundishora T. Lethal triad in severe burns. Burns. 2014;40(8):1492–1496. doi: 10.1016/j.burns.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Mitra B, Wasiak J, Cameron PA, O’Reilly G. Early coagulopathy of major burns. Injury. 2013;44(1):40–43. doi: 10.1016/j.injury.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 6.García-Avello A, Lorente JA, Cesar-Perez J, García-Frade LJ. Degree of hypercoagulability and hyperfibrinolysis is related to organ failure and prognosis afterburn trauma. Thromb Res. 1998;15:59–64. doi: 10.1016/s0049-3848(97)00291-0. [DOI] [PubMed] [Google Scholar]
- 7.Lavrentieva A, Kontakiotis T, Bitzani M, Papaioannou-Gaki G. Early coagulation disorders after severe burn injury: impact on mortality. Intensive Care Med. 2008;34(4):700–706. doi: 10.1007/s00134-007-0976-5. [DOI] [PubMed] [Google Scholar]
- 8.Kowal-Vern A, Gamelli RL, Walenga JM, Hoppensteadt D. The effect of burn wound size on hemostasis: a correlation of the hemostatic changes to the clinical state. J Trauma. 1992;33(1):50–56. doi: 10.1097/00005373-199207000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Park MS, Martini WZ, Dubick MA, Salinas J. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67(2):266–275. doi: 10.1097/TA.0b013e3181ae6f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaden E, Kimberger O, Kraincuk P, Baron DM. Perioperative treatment algorithm for bleeding burn patients reduces allogeneic blood product requirements. Br J Anaesth. 2012;109(3):376–381. doi: 10.1093/bja/aes186. [DOI] [PubMed] [Google Scholar]
- 11.Johansson PI, Eriksen K, Nielsen SL, Rojkjaer R, Alsbjørn B. Recombinant FVIIa decreases perioperative blood transfusion requirement in burn patients undergoing excision and skin grafting - results of a single centre pilot study. Burns. 2007;33(4):435–440. doi: 10.1016/j.burns.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Davenport R, Manson J, De’Ath H, Platton S. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. 2011;39(12):2652–2658. doi: 10.1097/CCM.0b013e3182281af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozek-Langenecker SA, Afshari A, Albaladejo P, Santullano CA. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30(6):270–382. doi: 10.1097/EJA.0b013e32835f4d5b. [DOI] [PubMed] [Google Scholar]
- 14.Pham HP, Shaz BH. Update on massive transfusion. Br J Anaesth. 2013;111 Suppl 1::i71–i82. doi: 10.1093/bja/aet376. [DOI] [PubMed] [Google Scholar]
- 15.Simmons JW, Pittet JF, Pierce B. Trauma-Induced Coagulopathy. 2014;4(3):189–199. doi: 10.1007/s40140-014-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Innerhofer P, Westermann I, Tauber H, Breitkopf R. The exclusive use of coagulation factor concentrates enables reversal of coagulopathy and decreases transfusion rates in patients with major blunt trauma. Injury. 2013;44(2):209–216. doi: 10.1016/j.injury.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 17.Joseph B, Pandit V, Khalil M, Kulvatunyou N. Use of prothrombin complex concentrate as an adjunct to fresh frozen plasma shortens time to craniotomy in traumatic brain injury patients. Neurosurgery. 2015;76(5):601–607. doi: 10.1227/NEU.0000000000000685. [DOI] [PubMed] [Google Scholar]
- 18.Schöchl H, Voelckel W, Schlimp CJ. Management of traumatic haemorrhage - the European perspective. Anaesthesia. 2015;70 Suppl 1:102–107. doi: 10.1111/anae.12901. [DOI] [PubMed] [Google Scholar]
- 19.Sherren PB, Hussey J, Martin R, Kundishora T. Acute burn induced coagulopathy. Burns. 2013;39(6):1157–1161. doi: 10.1016/j.burns.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Lu RP, Ni A, Lin FC, Ortiz-Pujols SM. Major burn injury is not associated with acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013;74(6):1474–1479. doi: 10.1097/TA.0b013e3182923193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davenport R, Manson J, De’Ath H, Platton S. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. 2011;39(12):2652–2658. doi: 10.1097/CCM.0b013e3182281af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 23.Haas T, Fries D, Tanaka KA, Asmis L. Usefulness of standard plasmatic coagulation tests in perioperative haemostasis management – is there any evidence? Br J Anaesth. 2015;114(2):217–224. doi: 10.1093/bja/aeu303. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho M, Rodrigues A, Gomes M, Carrilho A. Interventional Algorithms for the Control of Coagulopathic Bleeding in Surgical, Trauma, and Postpartum Settings: Recommendations From the Share Network Group. Clin Appl Thromb Hemost. 2016;22(2):121–137. doi: 10.1177/1076029614559773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 26.Brown LM, Aro SO, Cohen MJ. A high fresh frozen plasma: packed red blood cell transfusion ratio decreases mortality in all massively transfused trauma patients regardless of admission international normalized ratio. J Trauma. 2011;71 (2 Suppl 3):S358–S363. doi: 10.1097/TA.0b013e318227f152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peiniger S, Nienaber U, Lefering R, Braun M. Balanced massive transfusion ratios in multiple injury patients with traumatic brain injury. Crit Care. 2011;15(1):R68. doi: 10.1186/cc10048. [DOI] [PMC free article] [PubMed] [Google Scholar]


