Summary
After a burn lesion, Acute Respiratory Distress Syndrome (ARDS) may occur via direct lung injury due to inhaled smoke and fumes or mediated by the inflammatory response associated with the burn or its infectious complications. The aim of the present study is to assess the epidemiologic profile of ARDS in adult burn patients admitted to intensive care in a burn unit at a university hospital. A prospective cohort study was performed from January to December 2012. Demographic and diagnostic data, prognostic scores, etiology and data on the extent and depth of burns were collected. Data related to risk factors for ARDS and death were also recorded. A total of 85 patients were included in the study. Patients were aged 41.7 (SD = 15.7) years old; 71.8% were male and the mean total body surface area burned was 28.3% (SD = 19.1%); 35.3% presented inhalation injuries. Invasive ventilatory support was required in 44 ICU inpatients (51.8%). ARDS was diagnosed in 38.6% of patients under invasive mechanical ventilation. In multivariate analysis, the presence of inhalation injuries was a risk factor for ARDS (OR = 9.75; CI 95% 2.79 – 33.95; P < 0.001). ARDS is a common complication in burn patients admitted to specialized intensive care units. Inhalation injuries were an independent risk factor for ARDS. Mortality rate observed in the study patients was high and associated with ARDS diagnosis.
Keywords: burn unit, acute respiratory distress syndrome, risk factors, mortality
Abstract
Après une brûlure, un SDRA peut survenir soit en raison d’une atteinte pulmonaire directe (inhalation de fumées) soit en raison de la réaction inflammatoire due à la brûlure ou à une complication infectieuse. Le but de ce travail est d’évaluer l’épidémiologie des SDRA survenus chez des adultes brûlés hospitalisés dans l’unité de réanimation dédiée d’un CHU durant l’année 2012. Les données démographiques, celles concernant la brûlure (cause, étendue, profondeur, scores pronostics) et les facteurs de risque de SDRA ont été relevés. Quatre vingt cinq patients ont été inclus. Les patients étaient âgés de 41,7+/-15,7 ans, 71,8% d’entre eux étaient des hommes, ils étaient brûlés sur 28,3+/-19,1% de la SCT, 35,3% d’entre eux avaient des lésions d’inhalation. Quarante quatre patients (51,8%) ont eu besoin de ventilation mécanique. Un SDRA a été diagnostiqué chez 38,6% des patients ventilés. En analyse multivariée, les lésions d’inhalation sont un facteur de risque de SDRA (OR 9,75 ; IC95 2,79-33,95 ; p<0,001). Le SDRA est une complication fréquente chez les brûlés admis en unité de réanimation spécialisée. Les lésions d’inhalation sont un facteur de risque indépendant de SDRA. La mortalité de la cohorte était élevée, et associée au diagnostic de SDRA
Introduction
Acute Respiratory Distress Syndrome (ARDS) is a heterogenic clinical condition with complex pathological mechanisms. It affects critically-ill patients and is associated with high mortality rates and treatment costs.1,2 ARDS is characterized by severe acute hypoxemia following alveolar injuries in the lungs. Injuries are inflammatory in nature, leading to protein- rich pulmonary edema and acute respiratory failure.3
Since ARDS was first described by Ashbaugh et al.,4 there have been great advances in the knowledge of its pathophysiology. However, sound epidemiological data on ARDS were only possible after its definition was standardized in the American European Consensus Conference (AECC).5 This definition was used by researchers and clinicians all over the world, and lead to advances in knowledge about the syndrome.
More recently, the definition criteria for ARDS were reviewed and updated, and a new definition was proposed.6 The Berlin definition describes ARDS as an acute condition that takes place within a week of a known insult or that presents worsening of respiratory symptoms within this period of time, followed by bilateral radiographic opacities that are not fully explained by effusions, pulmonary collapse or nodules. An objective assessment of cardiac function is required in cases where risk factors for ARDS are not identified. ARDS is classified according to hypoxemia levels: mild (PaO2/FiO2 ratio between 201 and 300 mmHg), moderate (PaO2/FiO2 ratio between 101 and 200 mmHg) and severe (PaO2/FiO2 ratio < 100 mmHg).
Rubenfeld et al.2 described pulmonary sepsis (46%), sepsis elsewhere (33%), bronchoaspiration (11%) and polytrauma (7%) as the main risk factors for ARDS. In a different study carried out in Australia, sepsis, pneumonia and bronchoaspiration were again shown to be the most frequent risk factors. The other risk factors involved were pulmonary contusion (10.7%), transfusions (3%), trauma (2.4%), pancreatitis (1.8%), overdose (0.6%) and other indiscriminate factors (3.6%).7
After a burn lesion, ARDS can occur via direct lung injury due to inhaled smoke and fumes or mediated by the inflammatory response associated with the burn or its infectious complications. 8 The increase in capillary permeability in patients with extensive burns is not only observed in the lesion site, but also in organs elsewhere. The increase in vascular permeability leads to the leakage of fluid to the interstitial space;9 under those circumstances, pulmonary edema is aggravated by thermal injuries due to inhaled smoke.10
Epidemiologic aspects of ARDS seem to have peculiar characteristics in burn patients. In a retrospective cohort of 469 burn patients, multivariate analysis showed that age was the only independent risk factor (p = 0.03).11 Few studies provide information on epidemiologic aspects of ARDS in burn patients;11-14 these were performed before the new ARDS definitions, or even before protective mechanical ventilation strategies were recommended.15 More recent studies describe a higher incidence of ARDS in burn patients applying the Berlin definition, and association with increased risk of death according to escalating severity of ARDS.16,17
Knowledge of epidemiologic data on clinical characteristics, risk factors and prognosis of burn patients who develop ARDS is crucial to the development of preventive and therapeutic interventions to improve clinical outcomes.
The purpose of this study was to assess incidence and risk factors for Acute Respiratory Distress Syndrome associated with thermal injury in adult burn patients admitted to intensive care in a burn unit at a university hospital.
Materials and methods
This study was approved by the local Human Research Ethics Committee (study number: 00826912.4.0000.5231); informed consent forms were not required. A prospective cohort study was conducted with adult patients admitted to the Intensive Care Unit (ICU) specialized in the treatment of burn patients at a university hospital. A consecutive sampling of all patients admitted to the ICU was performed from 1st January 2012 to 31st December 2012. Exclusion criteria were patients under 18 years of age and those staying in the ICU for less than 24 hours. Data were collected from patients’ records. Identification data, demographic characteristics, diagnosis, date of the thermal injury, date of ICU and hospital admission and release were collected. In the first 24 hours after ICU admission, clinical and laboratory data required to calculate Acute Physiology and Chronic Health Evaluation (APACHE II),18 Sequential Organ Failure Assessment (SOFA)19 and Abbreviated Burn Severity Index (ABSI)20 were collected.
Percentage of total body surface area burned (TBSA) was calculated with the Lund and Browder Chart21 and the presence of third-degree burns was noted. Inhalation injury was suspected in patients with the following symptoms: history of a closed-space fire and facial burns with singed nasal hair, carbonaceous sputum, hoarseness, stridor or laboured breathing. Bronchoscopy was performed in patients suspected of having inhalation injury to confirm diagnosis. If bronchoscopy was not available, patients underwent a direct examination of the oropharynx followed by laryngoscopy. If edema or blistering was seen during laryngoscopy, patients were intubated. Intubation and mechanical ventilation were indicated if inhalation injury with respiratory distress was present or anticipated. Chronic disease was defined according to the Charlson Comorbidity Index.22
The date of ARDS diagnosis was noted, as well as the start of mechanical ventilation and its duration. The diagnosis of ARDS was made according to Berlin criteria6 within one week of thermal injury, considering it to be the insult associated with ARDS. Time to ARDS diagnosis was expressed in days, considering the date of thermal injury and ARDS-grading was evaluated at the initial time of ARDS diagnosis. Mechanical ventilation was defined as time between the start of ventilator support until the withdrawal of mechanical ventilation for 24 hours or more, or death. Mechanical ventilation parameters were routinely set to employ pressure limitation modes to maintain airway pressure levels under 30 cmH2O. Outcome of the patients in the study was assessed when leaving the ICU.
Statistical analysis
Results of continuous variables were described by mean, standard deviation (SD) or median and interquartile range (ITQ), depending on data distribution. The Student’s t-test was used to compare the means of continuous variables with normal distribution and variance homogeneity, and the non-parametric test (Mann-Whitney U test) was applied for data with non-normal distribution and/or variance heterogeneity. Categorical factors are given as frequency and shown in charts and tables. Categorical variables were assessed with the chisquared test. The Kaplan-Meier curve was used to compare survival between patients with or without ARDS. Univariate analysis was used to compare the relevant groups (patients with and without ARDS diagnosis). Multivariate logistic regression with variable selection was performed using the forward stepwise method with the variables that were considered to be risk factors and that showed p <0.20 in the univariate analysis for the study outcome (ARDS diagnosis). Results were given as odds ratio (OR) and 95% confidence interval (CI 95%). Significance level was 5% and analyses were performed using MedCalc for Windows, version 15.2.2 (MedCalc Software bvba, Ostend, Belgium).
Results
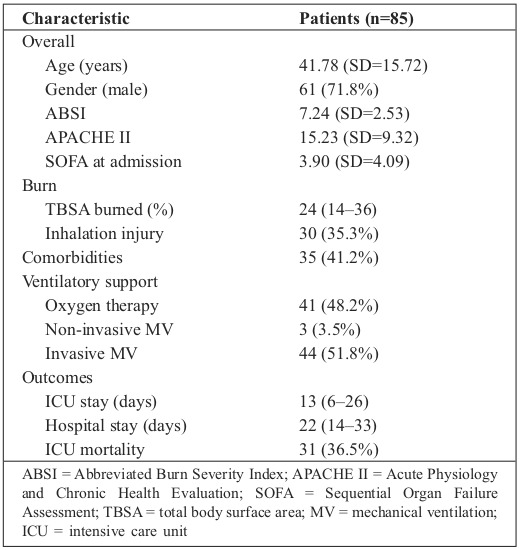
The study included 85 patients that met the eligibility criteria (Fig. 1). Table I describes their main clinical and demographic characteristics. Study patients were admitted to the ICU 2 days (1 – 4) after the thermal injury. Mean ABSI score was 7.24 (SD = 2.53) at ICU admission.
Fig. 1. Flowchart of study patients.
Table I. General characteristics of study patients.
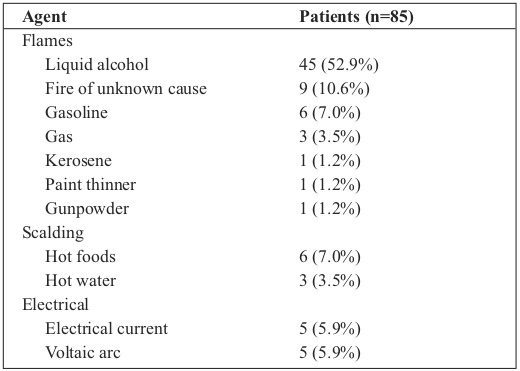
In terms of depth, third-degree burns were observed in 70 (82.4%) patients. The most common etiology among the study patients was flames due to accidents involving alcohol in open spaces (Table II). Fifty (58.8%) patients were deemed healthy before the thermal injury. Comorbidities were diagnosed in 35 patients (41.2%): 8 cases of alcoholism (9.4%); 5 diabetes mellitus (5.9%); 4 arterial hypertension (4.7%); 4 substance abuse (4.7%); 3 cases of seizures (3.5%); 2 coronary artery disease, acquired immunodeficiency, depression, and chronic obstructive pulmonary disease (2.4% each), and 3 cases of other comorbidities (3.5%).
Table II. Etiologic agents of burns in study patients.
Invasive ventilatory support was required in 44 ICU patients (51.8%). Duration of invasive mechanical ventilation was 18.5 (8.5 – 33.5) days. Median TBSA was higher among patients requiring invasive mechanical ventilation: 34% (21.5% – 50%) compared to 18% of other burn patients (9% – 28%; P < 0.001). Inhalation injuries were frequent (65.9%) among patients who required invasive mechanical ventilation.
Non-invasive ventilatory support was used in one patient in the post-extubation period as part of the invasive mechanical ventilation withdrawal plan and in two patients as part of the initial ventilatory strategy.
ARDS was diagnosed in 17 (20.0%) patients, which corresponds to 38.6% of patients who needed invasive mechanical ventilation. Five patients were classified as mild, nine as moderate, and three as severe according to the Berlin definition. Time to onset of ARDS was 3 (2 – 5) days after the thermal injury, and invasive mechanical ventilation was the initial ventilatory support method for all these patients.
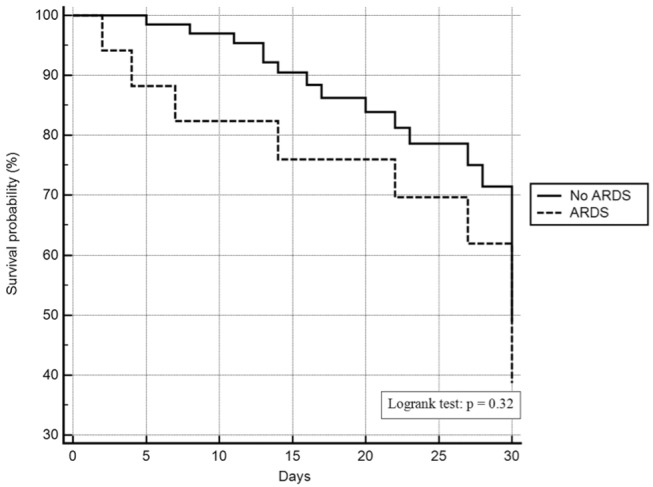
Longer stay in ICU was observed in patients with ARDS: 26 (14 – 44) days compared to 11 (6 – 23) days for patients without the condition (P = 0.01). No difference in length of hospital stay was observed between patients with or without ARDS: 27 (14 – 46) days and 20 (14 – 30.5) days respectively (P = 0.31). Fig. 2 shows the comparison of survival between patients with and without ARDS diagnosis
Fig. 2. Comparison of survival between patients with and without ARDS diagnosis.
The comparison of prognostic scores between the groups of patients with and without ARDS showed that mean ABSI was higher for patients with ARDS (8.8, SD = 2.5) compared to patients without ARDS (6.8, SD = 2.3; P = 0.002). Mean APACHE II was higher in patients with ARDS (23.1, SD = 10.4) compared to patients without ARDS (13.2, SD = 7.9; P < 0.001); mean SOFA was also higher in patients with ARDS (7.5, SD = 3.9) compared to patients without ARDS (3.0, SD = 3.6; P < 0.001).
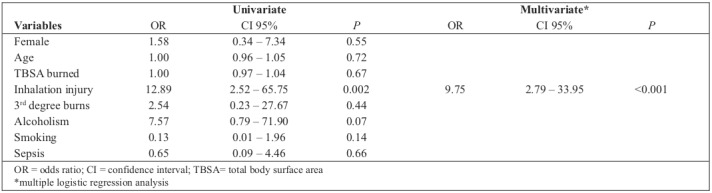
Median TBSA was higher in patients with ARDS: 30 (21 – 54) compared to 23 in those without ARDS (12.5 – 33.5; P = 0.04). In multivariate analysis, the presence of inhalation injuries was the only independent risk factor identified for ARDS (OR = 9.75; CI 95% 2.79 – 33.95; P < 0.001) (Table III). Hospital mortality was higher for patients with a diagnosis of ARDS (52.9%) compared to patients without ARDS (27.9%, P = 0.04).
Table III. Univariate and multivariate analysis of risk factors for the diagnosis of Acute Respiratory Distress Syndrome in burn patients admitted to an Intensive Care Unit.
Discussion
A high incidence of ARDS was observed in burn patients admitted to a specialized intensive care unit, particularly in those under invasive mechanical ventilation. Diagnosis of ARDS was associated with higher mortality rates in these patients, and inhalation injuries were associated with increased risk for developing ARDS.
Incidence of ARDS has been reported to be elevated in burn patients, and the Berlin definition seems to be more accurate in determining outcome when compared with the AECC definition.16 The Berlin definition was proposed to help early diagnosis and risk stratification, and these have been confirmed by the results of the present study and others.16,17
Inhalation lesions were an independent risk factor associated with ARDS in the present study. There are conflicting data in the literature about this association. Some studies report no correlation between inhalation lesions and incidence of ARDS,11,12 while others describe it as a strong risk factor.17,23
Masanès et al.24 were able to demonstrate this association when using bronchoscopy and biopsy to diagnose inhalation injury. It is difficult to identify such a correlation because of the complex diagnosis of inhalation injuries in burn patients. Literature does not have a clear, standardized definition for this diagnosis, and not all specialized centres use bronchoscopy to diagnose and classify lesions.
Inhaled smoke releases materials that occlude the airway lumen. Such material is composed of fibrin, neutrophils, mucus and epithelial cell debris.25 Occluded alveoli are hypoventilated, causing an increase in pulmonary shunt fraction that leads to changes in the ventilation:perfusion ratio. Open alveoli are over-distended during mechanical ventilation; the distention of the alveolar wall releases inflammatory cytokines and leads to damage induced by mechanical ventilation. These changes reduce gas exchange and cause hypoxemia.9
Evidence shows that inhalation lesions caused by smoke reduce gas exchange in animal models due to the increase in capillary permeability and fluid transport by the alveolar epithelial-endothelial barrier.10,26 Alpard et al. show a correlation between the incidence of ARDS and growing amounts of smoke inhaled by sheep.27 Inhalation injuries may be considered a predisposing factor for ARDS as they lead to an increase in local inflammatory responses and are associated with a greater need for mechanical ventilation. In these circumstances it would be appropriate to recommend the use of protective ventilation for burn patients with inhalation injuries to prevent ARDS.
Our results confirm that ARDS is associated with increased mortality in burn patients. Previous clinical study failed to find this association,11 while one study reported odds of death increased more than fivefold in moderate ARDS, and ninefold in severe ARDS compared to burn patients without this complication.17
Mortality rate of our study patients with ARDS may be considered high compared to the results of recent studies.16,17 However, it has been described that burned military patients on mechanical ventilation may present lower mortality despite larger burns and more severe injury when compared to civilian burn patients, probably due to their young age.28
High rates of mortality found in the present study might be explained by a number of factors, such as delayed admission after the accident, comorbidities, and association of ARDS with other complications, such as infections and kidney failure requiring dialysis.29 The observed mean delay of 2 days for admission can be justified by the small number of specialized burn care hospital beds available in the geographical area concerned.
Length of ICU stay was longer for burn patients who developed ARDS, but not hospital length of stay. Prolonged mechanical ventilation and possible complications after ARDS may have prolonged ICU stay, but since these patients have a long hospital stay due to multiple surgical interventions and rehabilitation, it is possible that this small sample could not capture the difference in hospital stay. The main limitation of the present study is that it was a single centre study with a small number of patients. The small sample size might have underestimated the effect of risk factors relevant for the studied outcomes. Besides, data on the ventilatory and fluid resuscitation strategies used were not analysed: these variables may be associated with the high incidence of ARDS observed and might have influenced its prognosis. Additional studies are required to assess the role of protective mechanical ventilation and restricted fluid therapy to prevent ARDS and reduce mortality rates in these patients
Conclusion
In conclusion, ARDS was common among burn patients admitted to the specialized intensive care unit, particularly among those requiring mechanical ventilation. The pathological mechanisms involved in the occurrence of ARDS in these patients are not fully understood, but are likely to be multifactorial. Inhalation lesions were associated with an increased risk of developing ARDS. ARDS was associated with increased mortality rates in burn patients.
References
- 1.Ware LB, Matthay MM. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1134–1149. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J. Incidence and Outcomes of Acute Lung Injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Pelosi P, D’Onofrio D, Chiumello D, Paolo S. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003;42:48s–56s. doi: 10.1183/09031936.03.00420803. [DOI] [PubMed] [Google Scholar]
- 4.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. The Lancet, Saturday 12 August 1967. Crit Care Resusc. 2005;7(1):60–61. [PubMed] [Google Scholar]
- 5.Bernard GR, Artigas A, Brigham KL, Carlet J. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 6.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND. ARDS definition task force: Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 7.Bersten AD, Edibam C, Hunt T, Moran J. Australian and New Zealand Intensive Care Society Clinical Trials Group: Incidence and Mortality of Acute Lung Injury and the Acute Respiratory Distress Syndrome in Three Australian States. Am J Respir Crit Care Med. 2002;165(4):443–448. doi: 10.1164/ajrccm.165.4.2101124. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202(2):145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 9.Enkhbaatar P, Traber DL. Pathophysiology of acute lung injury in combined burn and smoke inhalation injury. Clin Sci (Lond) 2004;107(2):137–143. doi: 10.1042/CS20040135. [DOI] [PubMed] [Google Scholar]
- 10.Soejima K, Schmalstieg FC, Sakurai H, Traber LD, Traber DL. Pathophysiological analysis of combined burn and smoke inhalation injuries in sheep. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1233–L1241. doi: 10.1152/ajplung.2001.280.6.L1233. [DOI] [PubMed] [Google Scholar]
- 11.Dancey DR, Hayes J, Gomez M, Schouten D. ARDS in patients with thermal injury. Intensive Care Med. 1999;25:1231–1236. doi: 10.1007/pl00003763. [DOI] [PubMed] [Google Scholar]
- 12.Liffner G, Bak Z, Reske A, Sjöberg F. Inhalation injury assessed by score does not contribute to the development of acute respiratory distress syndrome in burn victims. Burns. 2005;31(3):263–268. doi: 10.1016/j.burns.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Steinvall I, Bak Z, Sjoberg F. Acute respiratory distress syndrome is as important as inhalation injury for the development of respiratory dysfunction in major burns. Burns. 2008;34(4):441–451. doi: 10.1016/j.burns.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Higgins S, Fowler R, Callum J, Cartotto R. Transfusion-related acute lung injury in patients with burns. J Burn Care Res. 2007;28(1):56–64. doi: 10.1097/BCR.0b013E31802C88EC. [DOI] [PubMed] [Google Scholar]
- 15.ARDSNet: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 234;234:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 16.Bordes J, Lacroix G, Esnault P, Goutorbe P. Comparison of the Berlin definition with the American European consensus definition for acute respiratory distress syndrome in burn patients. Burns. 2014;40(4):562–567. doi: 10.1016/j.burns.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Belenkiy SM, Buel AR, Cannon JW, Sine CR. Acute respiratory distress syndrome in wartime military burns: application of the Berlin criteria. J Trauma Acute Care Surg. 2014;76(3):821–827. doi: 10.1097/TA.0b013e3182aa2d21. [DOI] [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 19.Vincent J, Moreno R, Takala J, Willatts S. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 20.Tobiasen J, Hiebert JM, Edlich RF. The abbreviated burn severity index. Ann Emerg Med. 1982;11:260–262. doi: 10.1016/s0196-0644(82)80096-6. [DOI] [PubMed] [Google Scholar]
- 21.Lund CC, Browder NC. The estimation of areas of burns. Surg Gynecol & Obst. 1944;79:352–358. [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Gajic O, Dabbagh O, Park PK, Adesanya A. U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS): Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masanès MJ, Legendre C, Lioret N, Saizy R, Lebeau B. Using bronchoscopy and biopsy to diagnose early inhalation injury. Macroscopic and histologic findings. Chest. 1995;107(5):1365–1369. doi: 10.1378/chest.107.5.1365. [DOI] [PubMed] [Google Scholar]
- 25.Cox RA, Burke AS, Soejima K, Murakami K. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol. 2003;29(3 Pt 1):295–302. doi: 10.1165/rcmb.4860. [DOI] [PubMed] [Google Scholar]
- 26.Herndon DN, Traber DL, Niehaus GD, Linares HA, Traber LD. The pathophysiology of smoke inhalation injury in a sheep model. J Trauma. 1984;24(12):1044–1051. doi: 10.1097/00005373-198412000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Alpard SK, Zwischenberger JB, Tao W, Deyo DJ. New clinically relevant sheep model of severe respiratory failure secondary to combined smoke inhalation/cutaneous flame burn injury. Crit Care Med. 2000;28(5):1469–1476. doi: 10.1097/00003246-200005000-00036. [DOI] [PubMed] [Google Scholar]
- 28.Waters JA, Lundy JB, Aden JK, Sine CR. A comparison of acute respiratory distress syndrome outcomes between military and civilian burn patients. Mil Med. 2015;180(3 Suppl):56–59. doi: 10.7205/MILMED-D-14-00390. [DOI] [PubMed] [Google Scholar]
- 29.Fagan SP, Bilodeau ML, Goverman J. Burn Intensive Care. Surg Clin North Am. 2014;94(4):765–779. doi: 10.1016/j.suc.2014.05.004. [DOI] [PubMed] [Google Scholar]


