Summary
Acute kidney injury (AKI) is an important complication in burn patients. Recently, it has been recommended that hydroxyethyl starch (HES) be avoided in burn patients because it increases the incidence of AKI. Our purpose was to study incidence of AKI in critically ill burn patients resuscitated with Ringer’s solution and supplements of HES. We conducted an observational study of 165 patients admitted to the critical care burn unit (with 30 ± 15% TBSA burned). The main outcome measures were incidence of AKI, contributions of colloids and crystalloids, various severity scores, comorbidities, complications and mortality. According to the RIFLE criteria, 10 (6.1%) patients presented with Risk, 11 (6.7%) presented with Injury and 11 (6.7%) presented with Failure. According to the AKIN criteria, 9.7% presented stage I, 3% stage II and 10.3% stage III. Replacement therapy (RRT) was performed in 15 patients (9.1%), but in 6 of them RRT was employed in the final stages of multi-organ failure. The incidence of AKI in severe burn patients is high according to the RIFLE or AKIN criteria and these patients experience more complications and higher mortality. Our study suggests that the use of HES in low doses in the burn resuscitation phase does not cause more AKI than resuscitation without HES, but further evaluation is required. Further studies should be conducted.
Keywords: burns, renal, resuscitation
Abstract
La souffrance rénale aiguë (SRA) est une complication sévère des patients brûlés. Il a récemment été recommandé d’éviter les HydroxyEthylAmidons (HEA) chez les patients brûlés en raison de l’augmentation de l’incidence des SRA. Le but de ce travail est d’évaluer l’incidence de la SRA chez des patients réanimés avec du Ringer Lactate et des HEA. Il s’agit d’une étude observationnelle conduite auprès de 165 patients admis en réanimation pour brûlés (surface 30 +/-15%). Les principaux paramètre recueillis étaient la SRA, les cristalloïdes et colloïdes utilisés, les scores de gravité, les comorbidités, les complications et la mortalité. Selon la classification de Rifle, 10 (6,1%) patients étaient dans le groupe à risque, 11 (6,7%) avaient une souffrance rénale et 11 (6,7%) une insuffisance rénale. Selon les critères AKIN, 9,7% des patients étaient au stade 1, 3% au stade 2 et 10,3% au stade 3. Une épuration extra-rénale a été nécessaire à 15 (9,1%) patients, 6 d’entre eux étant à un stade avancé de défaillance multiviscérale. Basée sur les scores Rifle comme AKIN, l’incidence de souffrance rénale est élevée chez les brûlés et ceux qui en souffrent ont une morbidité et une mortalité plus élevées. Toutefois, notre étude laisse à penser que les patients ayant reçu des HEA n’ont pas plus de souffrance rénale que ceux n’en ayant pas reçu, des études plus poussées restant nécessaires.
Introduction
Acute kidney injury (AKI) is one of the most important complications in burned patients. The Risk, Injury, Failure, Loss and End-stage kidney (RIFLE) and Acute Kidney Injury Network (AKIN) classification systems were developed to categorize the severity of renal dysfunction and have enabled investigators to examine the impact of acute kidney dysfunction in burn patients.1,3
To prevent renal dysfunction, it is essential to achieve adequate blood volume status. This is much more important in burn patients, because their plasma losses are higher than those of trauma or septic patients. Most forms of resuscitation are based on the Parkland formula using crystalloids in the first 24 h, sometimes followed by colloids.4 In recent years, factors such as haemodynamic monitoring, increased use of sedation and others have led to increased fluid intake, which is known as ‘fluid creep’ and leads to increased interstitial oedema associated with increased morbidity and mortality.5 Resuscitation centres exclusively using crystalloid reported more cases of compartment syndrome.6 To avoid excessive intake of crystalloid, albumin and synthetic colloids are used as replacements.7,8 Lower-molecular-weight hydroxyethyl starch (HES) 130/0.4/6% is a synthetic colloid that appears to exhibit reduced side effects compared with older HES.9 Vlachou et al. found that the use of HES was effective in avoiding excessive intake of crystalloid.10 However, the VISEP study,11 the 6S study,12 the CHEST study13 and others14,15 all noted an increased incidence of AKI or an increase in the number of days on which RRT was required in patients who received HES. In contrast, the CRYSTMAS study showed that significantly less volume was required to achieve haemodynamic stabilization for HES versus NaCl in the initial phase of fluid resuscitation in severe sepsis patients without any difference in adverse events in both groups.16 Moreover, the FIRST trial noted that in penetrating trauma, HES provided significantly better lactate clearance and less renal injury than saline.17 Recently, it has been recommended that HES be avoided in burn patients, although there are no serious studies showing increased AKI in burn patients treated with HES.
Therefore, we investigated the incidence of AKI in critically burned patients resuscitated with a protocol that included Ringer’s solution and HES 130/0.4/6% as a supplement. In addition, we studied whether there were any differences in comorbidity, severity scores, fluid therapy, morbidity and mortality among patients who developed AKI and those who did not.
Materials and methods
This study was approved for the observation of the effects of a resuscitation protocol in critically burned patients and was performed between October 2008 and December 2011. The study was approved by the Clinical Research Ethics Committee of the ‘La Paz’ University Hospital (Madrid, Spain) (HULP PI- 576. 2007) and all necessary written informed consent was obtained from the patients involved in the study. Data were collected from 165 patients admitted to our burn unit with critical burns covering >15% of the total body surface area (TBSA). Exclusion criteria were age <16 years or >80 years, burns over 85% of the TBSA, Baux score > 150, pregnancy, delayed admission over 6 h and chronic renal dysfunction (previous serum creatinine >2.0 mg/dL).
Resuscitation protocols
Resuscitation was initiated with Ringer’s lactate according to the Parkland formula. Subsequently, the fluid rate was increased when the hourly urine output was <0.5 mL/kg or MAP <65 mm Hg or decreased when the hourly urine output was >1 mL/kg. Re-evaluation according to our protocol (Fig. 1) was performed every 8 h and more frequently whenever urine output was below the target for consecutive 2 h or MAP was <55 mm Hg. In the first 12 h, a pulse-dose of 250 ml hydroxyethyl starch solution was only added whenever a patient with signs of hypovolemia developed acute hypotension (for bleeding, sedoanalgesia or other interventions). The dose was eventually repeated. At 12-24 h, continuous infusion of 6% hydroxyethyl starch 130/0.4 (Voluven® Fresenius Kabi, Bad Homburg, Germany) was added, the amount of Ringer’s lactate reduced, oral tolerance tested in patients not receiving mechanical ventilation and enteral tolerance tests performed in intubated patients. The doses of HES ranged from 0.3 to 0.4 mL/kg×% TBSA burned with a maximum of 20 mL/kg×day. On day 2 of admission, each patient received Ringer’s lactate plus HES and enteral feeding was started where possible. After the third day, the infusion of HES was removed, except in 17 patients who needed moderate doses of vasopressor. Analgosedation was performed with benzodiazepines, propofol, ketamine, opioids and other minor analgesics. Near-total burn wound excisions were made in the first 72 h (most of the time between 12 and 48 h) unless circumstances prevented it.
Fig. 1. Resuscitation decision tree.
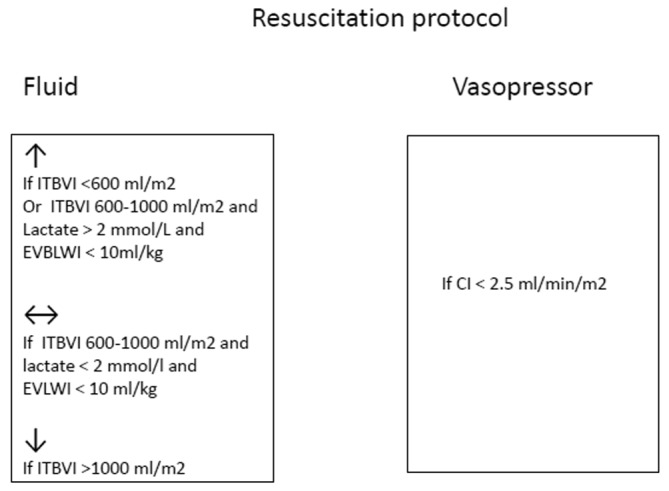
In addition to acute kidney injury (AKI) staging using the RIFLE and AKIN criteria, we studied aetiology, comorbidities, various severity scores such as Baux, Abbreviated Burned Severity Index (ABSI), Sequential Organ Failure Assessment (SOFA), other complications and mortality. The effects of crystalloid and colloid administration were evaluated every 8 h for the first 3 days, along with the daily relationship to the extravascular lung water index (EVLWI), intra-abdominal pressure (IAP) and arterial pressure of O2/fraction of inspired oxygen (PO2/FiO2).
Statistical analysis
The binary data are presented as proportions, and continuous variables are expressed as means ± standard deviation. To analyse the association between qualitative variables, a chi-square test (likelihood ratio test) was performed and the Kruskal-Wallis test was used to determine the association between quantitative and qualitative variables when normality was not evidenced in the distributions. The Spearman test was used to search for correlations between renal dysfunction and IAP, arterial PO2/FiO2, EVLWI, total volume and colloid volume, complications and mortality. The overall effect of the average period volume of colloids or crystalloids, time and renal dysfunction (RIFLE or AKIN stages) were studied using type III analysis associated with generalized estimation equations. Multiple regression analysis was used to study comorbidity, aetiology and inhalation syndrome. Two-sided tests were used and a P value <0.05 wasindicative of statistical significance. We used SAS 9.3 (SASInstitute Inc., Cary, NC, USA).
Results
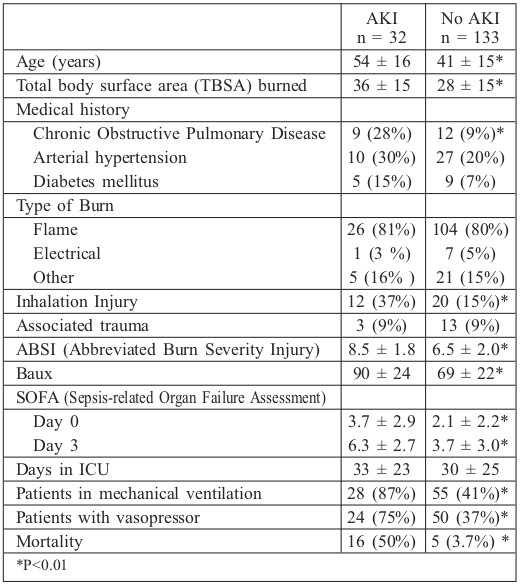
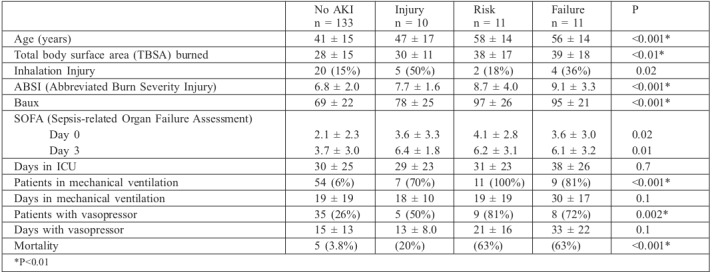
The study included 182 consecutive patients admitted to the critical care burn unit, of whom 17 had exclusion criteria and 165 were included: 112 males (68%) and 53 females (32%). Mean age was 43 ± 16 years and mean TBSA was 30 ± 15% (15%-20%: 48 patients, 21%-40%: 89 patients, 41%- 60%: 16 patients and >61%: 12 patients). More than half of the patients exhibited significant past medical histories. Patient characteristics are shown in Table I.
Table I. Characteristics of patients in AKI and no AKI groups (using RIFLE criteria).
The total volume provided in the first 24 h was 10.9 ± 5.5 L. Boluses of HES were only administered in the first 12 h in the presence of hypovolemia plus acute hypotension. On day 2, a smaller volume was provided (8.2 ± 2.1 L), in which <1 L of the medium was composed of HES. In 17 patients (10.3%), the administration of HES continued after the first 72 h. The mean fluids administered indexed to burn size in the first 24 h were 4.60 ± 2.3 mL/kg×TBSA burned (median 4.01) and between 24 and 48 h they were 3.60 ± 2.1 mL/kg×TBSA burned. The fluids administered are shown in Table II.
Table II. Volume administered and related parameters.
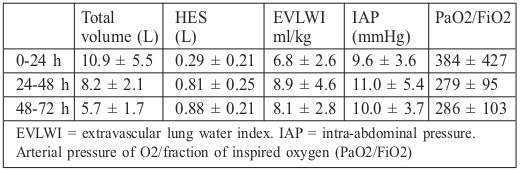
The average EVLWI did not exceed 10 mL/kg but was elevated on day 2, along with IAP, while arterial PO2/FiO2 decreased. There was no significant correlation with EVLWI or IAP.
Basal serum creatinine was 0.87 ± 0.28 mg/dL (median 0.80). At the end of resuscitation (third day), it was 0.85 ± 0.32 mg/dL (median 0.80) and rose to 0.90 ± 0.49 mg/dL (median 0.80) on day 7. The maximum creatinine was 1.25 ± 0.74 (median 1.00), and at discharge, serum creatinine was 0.94 ± 0.37 mg/dL (median 0.80).
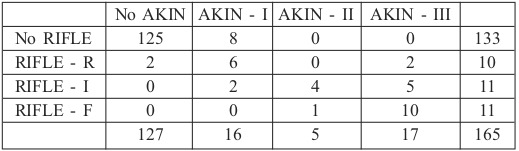
Incidences of AKI were as follows: 32 patients met the criteria for AKI using RIFLE criteria (19.3%): 10 met the criteria for risk (6.1%), 11 for injury (6.7%) and 11 for failure. Using the AKIN criteria, 38 patients met the criteria for AKI (23%): 16 for stage I (9.7%), 5 for stage II (3%) and 17 for stage III (10.3%) (Table III).
Table III. AKI using RIFLE or AKIN criteria.
Renal replacement therapy (RRT) was performed in 15 patients (9.1%). In 6 of these patients, RRT was employed in the final stages of multi-organ failure. There were significant differences between AKI and no-AKI groups with respect to age, TBSA burned, inhalation injury, severity scores, need for mechanical ventilation, need for vasopressors or mortality. However, there were no differences in burn aetiology or comorbidities (there was only an association with a history of COPD). The different stages of RIFLE were associated with age, TBSA burned, severity scores, need for mechanical ventilation or vasopressor and mortality. However, length of ICU stay, mechanical ventilation and need for vasopressors were not associated with RIFLE stage (Table IV).
Table IV. RIFLE criteria and patient characteristics.
Patients who received HES in the first 12 h showed no more AKI than those who did not. Moreover, there was an inverse relationship between use of HES in the first 12 h and AKI, though it was not statistically significant (−0.6236, p = 0.09).
We cannot rule out a relationship between degree of RIFLE or stage of AKIN and the amount of fluid provided in the first 24 h (p = 0.7 and p = 0.8, respectively) or in the first 72 h (p = 0.7 and p = 0.5, respectively). In addition, we studied whether the pattern of providing volume over time could influence renal dysfunction and showed that it was no different among patients who developed any stage of AKI.
For comparison with other studies, a subgroup of 117 patients with >20% TBSA burned was generated and we observed that in this group, median TBSA burned was 35 ± 15%, 72.6% were male, median age was 45 ± 16 years, 69.2% were burned by fire and 23% had inhalation syndrome. According to the RIFLE criteria, AKI occurred in 25.6% and 12.8% needed RRT. If we consider only the patients with >30% TBSA burned, mean TBSA burned was 45 ± 14%, 32% had AKI according to the RIFLE criteria and 16.3% needed RRT.
Global mortality rate was 12.5% and, using RIFLE criteria, mortality was 3.7% in no-AKI patients and 50% in AKI patients (Table IV). Using the AKIN criteria, mortality was 3.9% in no-AKI patients and 42% in AKI patients (16% in patients in stage I, 20% in stage II and 82% in stage III). All patients in whom RRT was used in the end stage of multipleorgan failure died, and in those without multiple-organ failure, mortality was 88%.
Discussion
AKI in a substantial number of patients suffering from burns. Using RIFLE/AKIN, we identified AKI as a common complication that is associated with other complications and worse outcome.
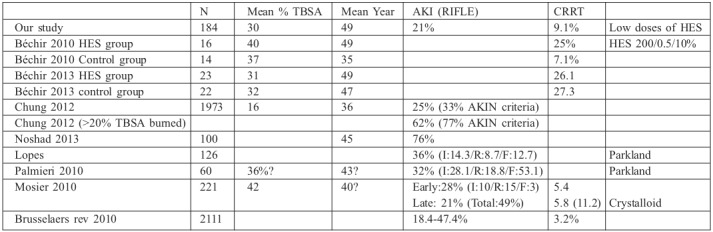
In our study, AKI incidence was 19.4% using the RIFLE criteria and 23% using the AKIN criteria. In previous studies on burn patients, the prevalence of AKI varied considerably. Brusselaers et al. reported in a review that AKI occurred in 25% of patients and that RRT was performed in almost 30% of AKI patients when the RIFLE classification was used.18 Among the most recent studies similar to ours, the following are noteworthy. Lopes et al. identified AKI using the RIFLE criteria in 36% of patients with severe burns.19 Chung KK et al. reported AKI in 33% of patients using the AKIN criteria and in 24% of patients using the RIFLE criteria; among those with >20% TBSA burned, the prevalence of AKI rose to 77% and 62%, respectively.20 Palmieri et al. applied the RIFLE criteria in burn patients and reported AKI in 53%.21 Mosier et al. performed another study using the Parkland formula for resuscitation and reported a 49% incidence of AKI using the RIFLE criteria for patients with a mean TBSA of 42% and a similar total 24-h resuscitation volume and colloid volume in patients who developed early AKI and those who did not.22 Finally, in a study by Noshad et al., AKI was observed in approximately 76% of patients.23 Therefore, the incidence of AKI varies greatly and depends on many factors, not only on the percentage of TBSA burned or the type of resuscitation fluid used. However, in any case, incidence appears to be between 26% and 49%, being as high as 76% in some studies (Table V).
Table V. AKI in previous studies on burns patients.
The incidence of AKI was higher when the AKIN criteria were used, although this was due in part to stage III, which includes all patients with RRT, but some of these patients received RRT by volume overload, respiratory dysfunction, final stage of multiple organ failure or other initially nonrenal causes.
We did not find more AKI in patients with diabetes mellitus, hypertension, heart failure or in any burn aetiology. As we expected, AKI was associated with age, TBSA burned, severity scores (Baux, ABSI), need for mechanical ventilation, need for vasopressors and mortality. However, different stages of RIFLE were not associated with length of ICU stay, mechanical ventilation or need for vasopressors. The distribution of the contribution of volume over time during resuscitation was no different among patients who developed any stage of AKI.
HES are a relatively inexpensive alternative to human albumin for correcting hypovolemia. There is a lack of evidence on the effectiveness of HES in avoiding excessive administration of fluids in the resuscitation phase of severe burn patients. Nonetheless, Vlachou et al. observed that the use of HES in the first 24 h after burns decreased fluid requirements (crystalloid group, 307 ml/% TBSA vs. colloid group, 263 ml/% TBSA).10 Renal dysfunction has been associated with HES use in septic patients. Few studies have investigated the incidence of AKI in burns patients resuscitated with HES supplementation. Bèchir et al. compared resuscitation according to the Baxter formula with another group of patients in which they administered 0.5 ml/kg×% TBSA of HES 200/0.5/10%.24 Although this study was a post hoc analysis of a small number of patients, it is one of the few that provide data on this subject; therefore, it might be of some interest to compare the results with those of our study. In their study, the amount of HES infused in the first 72 h was 6.0 ± 3.36 L (3 times higher than in our patients), and there was no difference in complications, urine output or serum creatinine. There was, however, a difference in the need for RRT and mortality, although not statistically significant. In particular, RRT was used in 25% of the patients treated with HES compared with 7.1% in the control group, and mortality was 43.8% in the patients treated with HES compared with 14.3% in the control (Table V). In our series, which included a larger number of patients (165 vs. 14 patients), we did not find an increase in AKI incidence; the AKI rate was similar to that described by Bèchir in the subgroup in which they did not use HES and much lower than that described in the subgroup in which they used HES. This may have been due to the use of ‘old’ HES (200/0.5/10%) in the previous study and ‘modern’ HES (130/0.4/6%) in our study, or because the use of colloids was much more restrictive in our study. In our patients, incidence of RRT was not higher than in other studies, although the criteria for RRT initiation were not the same. In many patients, RRT was initiated in the final phase of multi-organ failure, rather than after isolated renal failure. Therefore, initiation of RRT may not be accurately attributed to HES administration, and there are other predisposing factors, such as sepsis, severity, nephrotoxicity, previous surgeries and accumulated balance.
Bèchir et al., in a new study25 with ‘modern’ HES (130/0.4/6%), found that early renal function, incidence of ARDS, length of hospital stay and mortality were not negatively influenced by HES. However, they question the usefulness of this colloid. Similarly, our study did not show any unexpected increase in the incidence of AKI. Even patients who received HES in the first 12 h by hypotension and hypovolemia showed no more AKI than those who did not. This group would be expected to have a higher incidence of AKI. This may suggest the safety of using HES in the resuscitation of burn patients.
Although it appears that HES may have the same side effects as those described for sepsis, there are insufficient data to prove that the supplementation of HES in burn patients may cause renal dysfunction. Experts agree that HES could continue to be used in patients with hypovolaemia caused by acute blood loss where treatment with crystalloids alone is not considered to be sufficient. Perhaps the use of HES in the resuscitation of the severely burned patient with severe hypovolemia should also be allowed.
The main limitation of our study is that it was not designed to demonstrate the effectiveness and safety of HES and thus there is no control group. This prevents us from reaching conclusions about decreased fluid requirements and renal or respiratory dysfunction. In addition, there are many confounding factors; therefore, a larger number of patients would be needed to draw appropriate conclusions. Another limitation is that wound progression was not studied. Finally, the different criteria for the establishment of RRT may hamper comparisons with other studies and the interpretation of the study’s impact. Some clinicians begin RRT earlier than others and some patients received RRT by volume overload or respiratory dysfunction, but there are no specific criteria for this. Similarly, adequate resuscitation can resolve oliguria or creatinine elevation (after surgery or at the beginning of sepsis) and avoid the need for RRT techniques.
Conclusion
The incidence of AKI in burn patients using the AKIN/RIFLE criteria is high and is associated with more complications and higher mortality. Our study suggests that the use of HES at low doses does not seem to cause more AKI than those reported by studies in burn patients resuscitated without HES, but this requires further evaluation. We suggest that there are insufficient data to prove that supplementation of burn patients with HES may cause renal dysfunction. Further studies should be conducted to evaluate whether any dose of lowermolecular- weight HES can decrease the amount of fluid needed for resuscitation, without causing an increase in side effects.
Acknowledgments
Competing interests.The authors declare that they have no conflict of interest regarding any financial or personal relationships with the manufacturers or with any other people or organisations that could inappropriately influence or bias their work. The authors declare that they received no funding for this work.
References
- 1.Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs. the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004 doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metha RL, Kellum JA, Shah SV. Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve in acute kidney injury. Crit Care. 2007 doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coca SG, Bauling P, Schifftner T, Howard CS. Contribution of acute kidney injury toward morbidity and mortality in burns: a contemporary analysis. Am J Kidney Dis. 2007;49:517–523. doi: 10.1053/j.ajkd.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Baxter CR, Shires T. Physiological response to crystalloid resuscitation of severe burn. Ann NY AcadSci. 1968;150:874–894. doi: 10.1111/j.1749-6632.1968.tb14738.x. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich JB, Sullivan SR, Engrav LH. Is supra-Baxter resuscitation in burn patients a new phenomenon? Burns. 2004;30:464–466. doi: 10.1016/j.burns.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Oda J, Yamashita K, Inoue T. Resuscitation fluid volume and abdominal compartment syndrome in patients with major burns. Burn. 2006;32:151–154. doi: 10.1016/j.burns.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence A, Faraklas I, Watkins H. Colloid administration normalizes resuscitation ratio and ameliorates “fluid creep”. J Burn Care Res. 2010;31:40–47. doi: 10.1097/BCR.0b013e3181cb8c72. [DOI] [PubMed] [Google Scholar]
- 8.Atiyeh BS, Dibo AS, Ibrahim AE. Acute burn resuscitation and fluid creep: it is time for colloid rehabilitation. Ann Burns Fire Disasters. 2012;25:59–65. [PMC free article] [PubMed] [Google Scholar]
- 9.Westphal M, James MF, Kozek-Langenecker S. Hidroxyethyl starches: different products-different effects. Anesthesiology. 2009;111:187–202. doi: 10.1097/ALN.0b013e3181a7ec82. [DOI] [PubMed] [Google Scholar]
- 10.Vlachou E, Gosling P, Moiemen NS. Hydroxyethyl starch supplementation in burn resuscitation. A prospective randomised controlled trial. Burns. 2010;36:984–991. doi: 10.1016/j.burns.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Brunkhorst FM, Engel C, Bloos F. German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–39. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 12.Perner A, Haase N, Guttormsen AB. 6S Trial Group. Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367:124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 13.Myburgh J, Finder S, Bellomo R. Hydroxyethyl Starch or saline for fluid resuscitation in Intensive Care. NEJM. 2012;367:1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 14.Zarychanski R, Abou-Setta AM, Turgeon AF. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309:678–688. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 15.Gattas DJ, Dan A, Myburgh J. CHEST Management Committee. Fluid resuscitation with 6% hydroxyethyl starch (130/0.4) in acutely ill patients: an updated systematic review and meta-analysis. Anesth Analg. 2012;114:159–169. doi: 10.1213/ANE.0b013e318236b4d6. [DOI] [PubMed] [Google Scholar]
- 16.Guidet B, Martinet O, Boulain T. Assessment of hemodynamic efficacy and safety of 6% hydroxyethyl starch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS study. Critical Care. 2012;16:R94. doi: 10.1186/cc11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James MFM, Michell WL, Joubert IA. Resuscitation with hydroxyethyl starch improves renal function and lactate clearance in penetrating trauma in a randomized controlled study: the FIRST trial (Fluids in Resuscitation of Severe Trauma). British Journal of Anaesthesia. 2011;107:693–702. doi: 10.1093/bja/aer229. [DOI] [PubMed] [Google Scholar]
- 18.Brusselaers N, Monstrey S, Colpaert K, Decruyenaere J. Outcome of acute kidney injury in severe burns: A systematic review and metaanalysis. Intensive Care Med. 2010;36:915–925. doi: 10.1007/s00134-010-1861-1. [DOI] [PubMed] [Google Scholar]
- 19.Lopes JA, Jorge S, Neves F, Caneira M. An assessment of the rifle criteria for acute renal failure in severely burned patients. Nephrol Dial Transplant. 2007;22:285. doi: 10.1093/ndt/gfl468. [DOI] [PubMed] [Google Scholar]
- 20.Chung KK, Stewart IJ, Gisler C, Simmons JW. The Acute Kidney Injury Network (AKIN) criteria applied in burns. J Burn Care Res. 2012;33(4):183–190. doi: 10.1097/BCR.0b013e31825aea8d. [DOI] [PubMed] [Google Scholar]
- 21.Palmieri T, Lavrentieva A, Greenhalgh D. Acute kidney injury in critically ill burn patients. Risk factors, progression and impact on mortality. Burns. 2010;36:205–211. doi: 10.1016/j.burns.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Mosier M, Pham T, Klein M, Gibran N. Early acute kidney predicts progressive renal dysfunction and higher mortality in severely burned adults. J Burn Care Res. 2010;31:83–92. doi: 10.1097/BCR.0b013e3181cb8c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noshad H. Frequency and prognosis of Acute Kidney Injury in Burned Patients. Saudi J Kidney Dis Transpl. 2014;25:423–424. doi: 10.4103/1319-2442.128608. [DOI] [PubMed] [Google Scholar]
- 24.Bèchir M, Puhan M, Neff S. Early fluid resuscitation with hyperoncotic hydroxyethyl starch 200/0.5(10%) in severe burn injury. Critical Care. 2010;14:R123. doi: 10.1186/cc9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Béchir M, Puhan M, Fasshauer M, Schuepbach R. Erly fluid resuscitation with hydroxyethyl starch 130/0.4 (6%) in severe burn injury: a randomized, controlled, double-blind clinical trial. Critical Care. 2013;17:R299. doi: 10.1186/cc13168. [DOI] [PMC free article] [PubMed] [Google Scholar]


