Abstract
The legume family (Fabaceae) exhibits a high level of species diversity and evolutionary success worldwide. Previous phylogenetic studies of the genus Hedysarum L. (Fabaceae: Hedysareae) showed that the nuclear and the plastid topologies might be incongruent, and the systematic position of the Hedysarum sect. Stracheya clade was uncertain. In this study, phylogenetic relationships of Hedysarum were investigated based on the nuclear ITS, ETS, PGDH, SQD1, TRPT and the plastid psbA-trnH, trnC-petN, trnL-trnF, trnS-trnG, petN-psbM sequences. Both nuclear and plastid data support two major lineages in Hedysarum: the Hedysarum s.s. clade and the Sartoria clade. In the nuclear tree, Hedysarum is biphyletic with the Hedysarum s.s. clade sister to the Corethrodendron + Eversmannia + Greuteria + Onobrychis clade (the CEGO clade), whereas the Sartoria clade is sister to the genus Taverniera DC. In the plastid tree, Hedysarum is monophyletic and sister to Taverniera. The incongruent position of the Hedysarum s.s. clade between the nuclear and plastid trees may be best explained by a chloroplast capture hypothesis via introgression. The Hedysarum sect. Stracheya clade is resolved as sister to the H. sect. Hedysarum clade in both nuclear and plastid trees, and our analyses support merging Stracheya into Hedysarum. Based on our new evidence from multiple sequences, Hedysarum is not monophyletic, and its generic delimitation needs to be reconsidered.
Introduction
The legume family (Fabaceae) is the third largest flowering plant family with about 19,500 species in about 751 genera. The family exhibits a high level of species diversity and evolutionary success in various ecosystems worldwide [1]. The genus Hedysarum L. (Fabaceae: Hedysareae) consists of about 160 species of perennial herbs to rarely shrublets. It mainly distributes in temperate Eurasia, with a few species in North Africa and North America. Species of Hedysarum adapt to diverse habitats in temperate forests, steppes, alpine regions and the Tibetan plateau. This genus is generally characterized by brown membranous connate stipules, imparipinnate leaves, non-persistent corollas at fruit maturity, backward turning standard petal, right- to obtuse-angle-shaped keels and most prominently, lomented legumes with 2-several seeds [2, 3]. Some species of Hedysarum are good fodder plants, such as H. dahuricum Turcz. ex B.Fedtsch. and H. petrovii Yakovlev in arid regions, and H. neglectum Ledeb. in alpine regions [4, 5].
The generic delimitation of Hedysarum has been highly problematic. Established by Linnaeus [6], Hedysarum consisted of 54 species at his time [7]. However, Hedysarum sensu Linnaeus was unnatural with 51 species subsequently transferred to 16 other genera such as Alhagi Gagnebin, Desmodium Desv., Onobrychis Mill. and Sulla Medik. [2, 7]. With more species being discovered, Fedtschenko [8] divided Hedysarum into seven sections mainly based on their habits and morphology of stems and loments. Choi & Ohashi [2] revised Fedtschenko’s classification of Hedysarum based on their comprehensive morphological studies. They segregated the genera Corethrodendron Fischer & Basiner and Sulla from Hedysarum, and transferred the monotypic genus Stracheya Benth. [9] into Hedysarum as H. sect. Stracheya (Benth.) B.H.Choi & H.Ohashi. They also merged H. sect. Multicaulia (Boiss.) B.Fedtsch., H. sect. Crinifera (Boiss.) B.Fedtsch. and H. sect. Subacaulia (Boiss.) B.Fedtsch. into a broadly defined H. sect. Multicaulia s.l. [2].
Recent phylogenetic studies of Hedysareae [10, 11] did not support the monophyly of Hedysarum as circumscribed by Fedtschenko [8] nor by Choi & Ohashi [2]. Separations of the genera Corethrodendron and Sulla from Hedysarum were supported by molecular data [10, 11]. A new genus, Greuteria Amirahmadi & Kaz. Osaloo., was split from Hedysarum based on phylogenetic reconstruction [10, 11]. The monotypic Sartoria Boiss. & Heldr. was nested within Hedysarum sect. Multicaulia s.l. Therefore Sartoria hedysaroides Boiss. & Heldr. was transferred to Hedysarum as H. anatolicum Amirahmadi & Kaz. Osaloo [10]. After these treatments, Hedysarum was suggested to be monophyletic in the plastid trees [10, 11]. However, Hedysarum was not monophyletic in the nuclear ITS trees, and its relationship with other genera in Hedysareae was uncertain due to low support values of the ITS trees [10, 11].
With an extensive taxon sampling scheme and more molecular data, Duan et al. [11] recognized three main clades of Hedysarum: the H. sect. Hedysarum clade; the re-defined H. sect. Multicaulia s.l. clade excluding H. kumaonense Benth. ex Baker and H. lehmannianum Bunge, but including Sartoria hedysaroides; and the re-delimited H. sect. Stracheya clade consisting of H. tibeticum (Benth.) B.H.Choi & H.Ohashi, H. kumaonense and H. lehmannianum. The H. sect. Stracheya clade was sister to the H. sect. Multicaulia s.l. clade with very low support [11]. However, this relationship was not supported by Amirahmadi et al. [10]. The systematic position of the H. sect. Stracheya clade within Hedysarum was thus uncertain.
Previous phylogenetic hypotheses on Hedysarum and Hedysareae largely relied on plastid data since the nuclear ITS sequence showed limited resolution concerning the deep relationships [10, 11]. Sequencing more plastid genes may increase phylogenetic precision, however, accurate inference of the phylogenetic history of this group requires nuclear data [12, 13]. Nuclear genes are considered as an important complement or alternative to plastid ones due to their biparental inheritance, and are less vulnerable to hybridizations and introgressions than organelle genes [1, 14, 15].
To our knowledge, only the ribosomal internal transcribed spacer (ITS) sequence from the nuclear genome has been used to infer the phylogeny of Hedysarum. The ribosomal external transcribed spacer (ETS) has also been widely used in phylogenetic studies [13], and has an evolutionary rate at least as fast as the ITS sequence [16]. Furthermore, several studies have developed other nuclear sequences for phylogenetic reconstruction in various taxonomic groups of Fabaceae [12, 17, 18]. The SQD1 (UDP sulfoquinovose synthase gene) belongs to the low copy conserved ortholog set (COS) genes [19]. It has been used in a phylogenetic study of the caesalpinioid legumes [18]. The nuclear coding PGDH (putative phosphogluconate dehydrogenase gene) and TRPT (putative triosephosphate translocator gene) sequences are exon-derived, putative orthologs, single copy genes [12], and were used in a phylogenetic study of the Hologalegina legumes [17].
In the present study, we employed the nuclear ITS, ETS, PGDH, SQD1 and TRPT sequences and the plastid psbA-trnH, trnC-petN, trnL-trnF, trnS-trnG, and petN-psbM sequences to test (1) the monophyly of Hedysarum, and (2) the systematic position of the Hedysarum sect. Stracheya clade.
Materials and Methods
Ethics statement
The plant materials used in this study did not involve protected or endangered species. No specific permits were required for the collection of samples. Voucher information of all the samples was given in S1 Appendix.
Taxon sampling
A total of 58 accessions were included in this study (S1 Appendix), representing all genera in the tribe Hedysareae and all sections and major infra-sectional clades in Hedysarum as recognized by Duan et al. [11]. Hedysarum boveanum Bunge ex Basiner, H. denticulatum Regel, H. minjanense Rech.f., H. poncinsii Franch., and H. syriacum Boiss. were placed in sect. Multicaulia s.l. [2] based on morphological characters [20–22], and they were sampled for the first time to test their phylogenetic positions. Alhagi sparsifolia Shap. ex Keller & Shap. was selected as the outgroup based on previous results [10,11]. Some of the ITS, psbA-trnH, and trnL-trnF sequences used in this study were published by Duan et al. [11] and Amirahmadi et al. [10], while all other sequences were generated by the present study (S1 Appendix). Voucher information of DNA sequences was listed in S1 Appendix.
DNA extraction, PCR and sequencing
Total genomic DNAs were extracted from silica-gel dried leaf material or herbarium specimen using either the Plant DNA Extraction Kit AGP965/960 (AutoGen, Holliston, Massachusetts, USA) or the DNeasy Plant Mini Kit (Qiagen, Valencia, California, USA).
Primers used for amplification and sequencing were “ITS5a” and “ITS4” for ITS [23], “ETS-Hedy” (CCYTGWGCYRTTGTGCCTTGG, designed in this study) and “18S-IGS” [16] for ETS, forward and reverse primers for SQD1 [19], forward and reverse primers for PGDH and TRPT [12], “psbAF” and “trnHR” for psbA-trnH intergenic region [24], “trnC” and “petN 1R” for trnC-petN intergenic region [25], “trnS” [26] and “5’trnG2S” [27] for trnS-trnG intergenic region, “ycf6F” and “psbMR” [27] for the petN-psbM intergenic region (the ycf6 gene has been renamed as petN, see [28, 29]), “c” and “f” for trnL intron plus trnL-trnF intergenic region [30].
Polymerase chain reaction (PCR) was performed in a 25μl volume with the following components: 10× reaction buffer, 200μmol·L-1 of each dNTP, 10μg BSA, 0.4μmol·L-1 of each primer, 2.5mmol·L-1 MgCl2, 1U of BIOLASE DNA Polymerase (Bioline USA Inc., Taunton, Massachusetts), 1–7.5μL template DNA. The amplification conditions were 3 min at 95°C, followed by 36–40 cycles of 1 min at 94°C, 1 min at 50–60°C, and 1–1.5 min at 72°C, then a final extension at 72°C for 7–10 min. The PCR products were purified using ExoSAP-IT (USB Corporation, Cleveland, Ohio, USA) or the polyethylene glycol (PEG) precipitation procedure [31]. Cycle sequencing reactions were conducted in both directions using the amplification primers and the BigDye 3.1 reagents. After being cleaned up by the Sephadex columns, the sequencing products were run on an ABI 3730 automated sequencer (Applied Biosystems, Foster City, California, USA). Sequences were assembled using the program Geneious v.8.1.2 [32] (http://www.geneious.com/). All sequences have been deposited in GenBank and the accession numbers were listed in S1 Appendix.
Phylogenetic analysis
Multiple sequence alignments were conducted in Geneious v.8.1.2 using MUSCLE [33] with default settings, followed by manual adjustments. For the non-coding sequences, ambiguously aligned regions were removed from the matrix prior to phylogenetic analysis. Insertions and deletions (indels) in the data matrices were coded as binary characters using the program SeqState [34] according to the “simple coding” method [35]. The binary characters were combined with the DNA data as the additional partition of the matrix. SequenceMatrix [36] was employed to assemble combined datasets.
Single locus analysis
Phylogenetic reconstructions were first conducted by using single locus datasets. The best-fit nucleotide substitution model for each of the 10 individual sequences was determined using the Bayesian Information Criterion (BIC) in jModelTest v.2.1.7 [37]. For the ITS dataset, boundaries of the 5.8S, ITS1 and ITS2 regions were determined by comparing with the published 5.8S sequence of Vicia faba L. [38], and models for the ITS1, 5.8S and ITS2 regions were determined separately (see Table 1).
Table 1. Characteristics of individual datasets: alignment length, the number and percentage of constant, variable and potentially parsimony-informative (Pi) sites, the number of coded indel(s), and the best-fit nucleotide substitution model determined by BIC.
| Dataset | Length | Constant (%) | Variable (%) | Pi (%) | Indel | Model |
|---|---|---|---|---|---|---|
| ETS | 346 | 131 (37.9%) | 215 (62.1%) | 149 (43.1%) | 45 | HKY+G |
| ITS1 | 285 | 126 (44.2%) | 159 (55.8%) | 112 (39.3%) | 55 | TIM3ef+G |
| 5.8S | 165 | 152 (92.1%) | 13 (7.9%) | 6 (3.6%) | 2 | TPM3+I |
| ITS2 | 229 | 108 (47.2%) | 121 (52.8%) | 86 (37.6%) | 22 | TrNef+G |
| PGDH | 405 | 304 (75.1%) | 101 (24.9%) | 67 (16.5%) | 0 | K80+I |
| SQD1 | 273 | 212 (77.7%) | 61 (22.3%) | 41 (15.0%) | 0 | TPM1+G |
| TRPT | 330 | 215 (65.2%) | 115 (34.8%) | 65 (19.7%) | 7 | HKY+G |
| psbA-trnH | 403 | 300 (74.4%) | 103 (25.6%) | 58 (14.4%) | 31 | TPM1uf+I+G |
| trnC-petN | 1221 | 882 (72.2%) | 339 (27.8%) | 213 (17.4%) | 110 | TVM+G |
| trnL-trnF | 1068 | 881 (82.5%) | 187 (17.5%) | 110 (10.3%) | 60 | TVM+G |
| trnS-trnG | 692 | 485 (70.1%) | 207 (29.9%) | 142 (20.5%) | 69 | TPM1uf+G |
| petN-psbM | 1316 | 999 (75.9%) | 317 (24.1%) | 198 (15.0%) | 104 | TVM+G |
Bayesian inferences (BI) were conducted in MrBayes v.3.2.5 [39, 40]. When models (such as TIM3ef, TrNef, TPM1, TVM) determined by BIC were not directly available in MrBayes, we transformed a GTR model in MrBayes by fixing the six nucleotide substitution rates and four nucleotide state frequencies to the values as calculated by jModelTest for each dataset. The model applied to all the coded binary partitions was a default Standard Discrete Model in MrBayes [41]. For the ITS dataset, the partitions were done for ITS1, 5.8S and ITS2 separately. In the Bayesian inference, two independent analyses starting from different random trees with three heated and one cold chain were run for 10,000,000 generations, and trees were sampled every 1,000 generations (10,000 trees sampled in total). The first 2,500 trees (25%) were discarded as burn-in, and the remaining trees were used to construct a 50% majority-rule consensus tree and posterior probabilities (PP). Tree visualization was achieved in FigTree v1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/).
Phylogenetic reconstructions were also carried out with maximum parsimony (MP) and maximum likelihood (ML) analyses using PAUP* 4.0b10 [42] and RAxML v.8.2 [43], respectively. The MP bootstrap analysis was performed with the following settings: heuristic search, TBR branch-swapping, 1,000 bootstrap replicates, random addition sequence with 10 replicates, a maximum of 1,000 trees saved per round. The ML rapid bootstrap analysis was conducted with a random seed, 1,000 alternative runs, and the same partition scheme as in the Bayesian analysis. Model parameters were estimated and optimized for each partition of the dataset by RAxML with the GTRCAT commands. Bootstrap support values from the MP (PBS) and ML (LBS) analyses were labeled on the corresponding branches of the BI trees.
Concatenated data analysis
We conducted incongruence length difference (ILD) test [44] to assess whether different datasets could be concatenated for phylogenetic reconstruction. The ILD tests were performed in PAUP* 4.0b10 [42] by using only the informative characters [45] with heuristic search, 1,000 replicates, simple addition sequence (with a maximum of 1,000 trees saved per replicate), and tree-bisection-reconnection (TBR) branch-swapping algorithm. When the ILD test found a p value greater than 0.01, datasets were concatenated for phylogenetic analysis [46].
BI, MP and ML analyses were conducted for the concatenated datasets by using the same methods as in the single locus analyses. For BI and ML, the best partitioning scheme and substitution model were determined by using PartitionFinder v.1.1.1 [47] with the following settings: linked branch length, BIC metric for model and partitioning selection, and the greedy algorithm. Data blocks in PartitionFinder were set as the followings: the ETS, ITS1, 5.8S and ITS2 datasets, the first, second and third codon position of each of the PGDH, SQD1 and TRPT datasets, and the psbA-trnH, trnC-petN, trnL-trnF, trnS-trnG and petN-psbM datasets.
We performed the approximately unbiased (AU) tests [48] using CONSEL v.0.2 [49] to test statistical support of incongruence between nuclear and plastid topologies. Topological constraints were generated from the ML trees using MEGA v.6.0 [50]. Per site log likelihoods for each topology were estimated in RAxML v.8.2 [43] under the GTR + G model. The dataset partition scheme was the same as in the ML analysis.
Coalescent analysis
The Bayesian coalescent-based analyses for multi-locus data were performed using *BEAST [51] as implemented in the BEAST v.2.4.3 package [52]. Two sets of analyses were conducted: one with all five nuclear sequences, another with all five plastid sequences. The substitution model for each sequence was that used in the single locus analysis, and model parameters were unlinked among partitions. The lognormal relaxed clock model, the linear with constant root population function and the Yule speciation model were used. Four independent runs of 100,000,000 and 500,000,000 generations were performed for the nuclear data and the plastid data, respectively. Samples were stored every 5,000 generations. Tracer v.1.6 (http://beast.bio.ed.ac.uk/Tracer) was used to check the effective sample sizes (ESSs) of the parameters and the convergence of different runs. After removing the 10% burn-in of each run, the log and tree files from different runs were combined by using LogCombiner [52]. In the combined results, the ESSs of the sampled parameters all exceeded 200. The maximum clade credibility tree was annotated using TreeAnnotator [52].
Bayesian concordance analysis
We carried out the Bayesian Concordance Analysis (BCA) [53] for the five nuclear loci as well as the five plastid loci using the BUCKy v.1.1.4 package [54]. Gene trees of each locus were obtained from the single locus BI (.t files generated by MrBayes), and were summarized by using the program mbsum [54] with a 25% burn-in. The output files of mbsum were then used to construct the primary concordance tree and concordance factor (CF) in the program bucky [54]. The a priori level of discordance among loci (α) was set to 0.01, 1 and 100 in alternative runs to assess the effect of α on the BCA results. Each run was conducted with 10,000,000 updates, four replicates, one cold and three hot chains of the Metropolis coupled Markov chain Monte Carlo (MCMCMC). In MCMCMC, a swap was proposed once every 100 updates, and the α multiplier was set to 10. Different runs with α set to 0.01, 1 and 100 all recovered the same primary concordance tree topology and CFs, hence the BCA results obtained with the default α = 1 were reported. The primary concordance tree was visualized in FigTree, and branch labels were annotated as the sample-wide posterior mean CFs and their 95% credibility intervals.
Results
Nuclear data
Characteristics of the individual nuclear datasets were summarized in Table 1. Treating indels as missing data or binary characters did not change the topologies, but the analyses that included indels as characters increased support values in general. The results presented here were based on the datasets with indels treated as binary characters. Trees generated from each individual datasets supported the monophyly of each of the genera Ebenus L., Greuteria, Sulla, and Taverniera DC., and several small lineages within Hedysarum and Onobrychis. Hedysarum denticulatum and H. minjanense were nested with H. sect. Stracheya as redefined by Duan et al. [11] in all five nuclear trees. Deep relationships within Hedysareae were largely unsolved by single sequences. However, Hedysarum was not supported to be monophyletic in any of the five single-sequence trees.
In order to search trees with better resolution, we concatenated individual datasets in our analyses. To explore whether two or more nuclear datasets could be concatenated, we conducted 10 pairwise ILD tests for the five nuclear datasets. The p values were shown in Table 2. Congruence was detected between any pair in the ETS, ITS, PGDH and TRPT datasets (all with p > 0.01). We first concatenated the non-coding ribosomal ETS and ITS datasets (pairwise ILD: p = 0.13), as well as the coding PGDH and TRPT datasets (pairwise ILD: p = 0.061) for two separate analyses.
Table 2. P values of pairwise ILD tests for the nuclear ETS, ITS, PGDH, TRPT and SQD1 datasets.
| ITS | PGDH | TRPT | SQD1 | |
| ETS | 0.130 | 0.036 | 0.025 | 0.001 |
| ITS | 0.331 | 0.170 | 0.002 | |
| PGDH | 0.061 | 0.001 | ||
| TRPT | 0.003 |
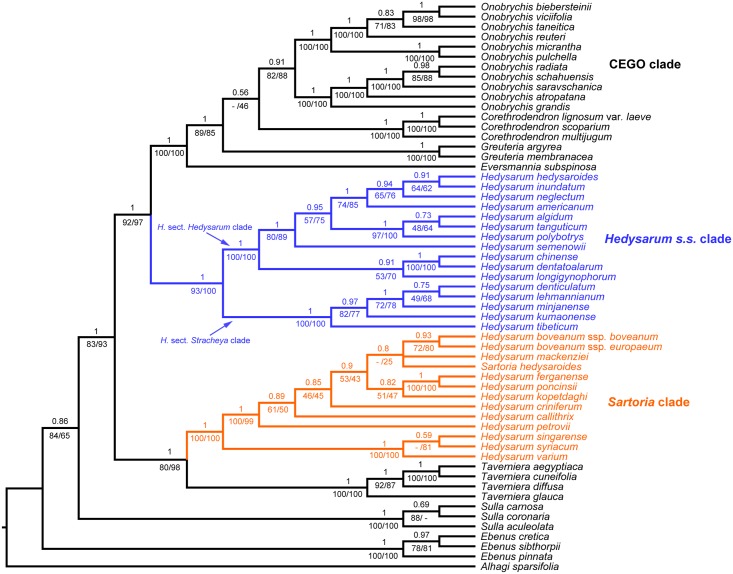
In the concatenated ETS + ITS tree (S1 Fig), Corethrodendron, Ebenus, Greuteria, Onobrychis, Sulla and Taverniera were each supported to be monophyletic. Corethrodendron, Eversmannia Bunge, Greuteria and Onobrychis together formed a well supported clade (the CEGO clade), with Bayesian posterior probabilitiy (PP) = 1, maximum parsimony bootstrap percent (PBS) = 100%, and maximum likelihood bootstrap percent (LBS) = 100%, but relationships among these four genera were poorly resolved. The Hedysarum sect. Hedysarum clade and the H. sect. Stracheya clade were sisters (the Hedysarum s.s. clade, PP = 1, PBS = 70%, LBS = 94%). Sartoria hedysaroides plus some members of the Hedysarum sect. Multicaulia s.l. formed a well supported clade (the Sartoria clade, PP = 1, PBS = 100%, LBS = 100%). The genus Hedysarum was not monophyletic, because the Hedysarum s.s. clade grouped with the CEGO clade, while the Sartoria clade formed a clade with Taverniera. However, these relationships had low support values in the ITS + ETS tree (not supported by the BI tree, poorly supported by the MP and ML trees).
In the concatenated PGDH + TRPT tree (S2 Fig), the Hedysarum sect. Hedysarum clade and the H. sect. Stracheya clade were supported to be sister to each other and constituted the Hedysarum s.s. clade (PP = 0.98, PBS = 54%, LBS = 80%). Additionally, the Hedysarum s.s. clade grouped with Corethrodendron, Eversmannia, Greuteria, and Onobrychis (PP = 0.96, PBS = 34%, LBS = 35%), but relationships within this clade were unclear. The Sartoria clade (PP = 1, PBS = 71%, LBS = 76%) and Taverniera were supported to be sisters with low support (PP = 0.8, PBS = 52%, LBS = 64%).
In both the ETS + ITS tree (S1 Fig) and the PGDH + TRPT tree (S2 Fig), Hedysarum was not monophyletic because the Hedysarum s.s. clade grouped with Corethrodendron, Eversmannia, Greuteria and Onobrychis, while the Sartoria clade was sister to the genus Taverniera. Although support values varied in these two trees, Hedysarum was shown to be biphyletic. Pairwise ILD test between the ETS + ITS dataset and the PGDH + TRPT dataset found p = 0.703, thus we concatenated these four sequences in our further analyses.
In the concatenated ETS + ITS + PGDH + TRPT tree (S3 Fig), Ebenus diverged first, followed by Sulla (PP = 0.71, PBS = 84%, LBS = 40%). Corethrodendron, Ebenus, Greuteria, Sulla, and Taverniera were each highly supported to be monophyletic (all with PP = 1, PBS = 100%, LBS = 100%). Onobrychis was weakly supported to be monophyletic (PP = 0.68, PBS = 60%, not supported by the ML tree) with two strongly supported subclades (both with PP = 1, PBS = 100%, LBS = 100%). The CEGO clade was strongly supported (PP = 1, PBS = 100%, LBS = 100%). Hedysarum was biphyletic with the Hedysarum s.s. clade sister to the CEGO clade (PP = 1, PBS = 86%, LBS = 94%), and the Sartoria clade forming a clade with Taverniera (PP = 0.97, PBS = 69%, LBS = 92%). The CEGO + Hedysarum s.s. clade was then sister to the Sartoria + Taverniera clade (PP = 1, PBS = 82%, LBS = 90%).
The SQD1 tree (S4 Fig) showed limited resolution as well as low support values concerning the deep relationship in Hedysareae. The SQD1 dataset has the smallest number (41) of parsimony-informative sites compared with the other four nuclear sequences (see Table 1). Hedysarum was, nevertheless, not monophyletic in the SQD1 tree. Members of the Sartoria clade grouped with Taverniera, Hedysarum longigynophorum C.C.Ni, H. dentatoalarum K.T.Fu and H. chinense (B.Fedtsch.) Hand.-Mazz., but the support values were low (PP = 0.85, PBS = 52%, LBS = 53%). Sulla, Ebenus, Eversmannia, Corethrodendron, Greuteria, Onobrychis and the rest of Hedysarum formed another clade (PP = 1, PBS = 62%, LBS = 68%). We could see that the SQD1 tree (S4 Fig) was not contrary to the ETS + ITS + PGDH + TRPT tree (S3 Fig) with regard to the non-monophyly of Hedysarum. Hence we tentatively concatenated the SQD1 dataset with the other four nuclear datasets.
In the concatenated ETS + ITS + PGDH + TRPT + SQD1 tree (Fig 1), relationships of the CEGO, Hedysarum s.s., Sartoria clades and Taverniera remained the same as in the concatenated ETS + ITS + PGDH + TRPT tree (S3 Fig). With the SQD1 dataset added, the support values were even higher (Fig 1): the Hedysarum s.s. clade was sister to the CEGO clade with PP = 1, PBS = 92%, LBS = 97% (compared with PP = 1, PBS = 86%, LBS = 94% in S3 Fig), and the Sartoria clade grouped with Taverniera with PP = 1, PBS = 80%, LBS = 98% (compared with PP = 0.97, PBS = 69%, LBS = 92% in S3 Fig).
Fig 1. Bayesian tree based on the concatenated nuclear ITS, ETS, PGDH, TRPT and SQD1 sequences.
The Bayesian posterior probabilities are above the branches, and the maximum parsimony (left) and maximum likelihood (right) bootstrap support values are below the branches. Dashes indicate branches that are not found in the maximum parsimony tree or the maximum likelihood tree.
The coalescent tree based on all five nuclear sequences (S5 Fig) revealed that the Hedysarum s.s clade was sister to the CEGO clade (PP = 0.95), and the Sartoria clade grouped with Taverniera (PP = 0.96). The CEGO, Hedysarum s.s., the Sartoria clade and Taverniera were each supported to be monophyletic (all with PP = 1).
The primary concordance tree based on all five nuclear sequences (S6 Fig) had similar topology compared with the concatenated data tree (Fig 1) and the coalescent tree (S5 Fig). It supported the sister relationship between the Hedysarum s.s and the CEGO clades (mean CF = 0.26), and that between the Sartoria clade and Taverniera (mean CF = 0.3).
Plastid data
Characteristics of plastid datasets were presented in Table 1. Trees based on individual plastid datasets had limited resolution. The 10 pairwise ILD tests for the five plastid datasets all found p > 0.01 (Table 3), therefore we concatenated these five plastid datasets in our analyses. Because there was incongruence between the plastid and nuclear trees, the plastid and nuclear datasets were not concatenated in our analysis.
Table 3. P values of pairwise ILD tests for the plastid psbA-trnH, trnC-petN, trnL-trnF, trnS-trnG and petN-psbM datasets.
| trnC-petN | trnL-trnF | trnS-trnG | petN-psbM | |
| psbA-trnH | 0.751 | 0.266 | 0.423 | 0.315 |
| trnC-petN | 0.143 | 0.192 | 0.894 | |
| trnL-trnF | 0.260 | 0.657 | ||
| trnS-trnG | 0.250 |
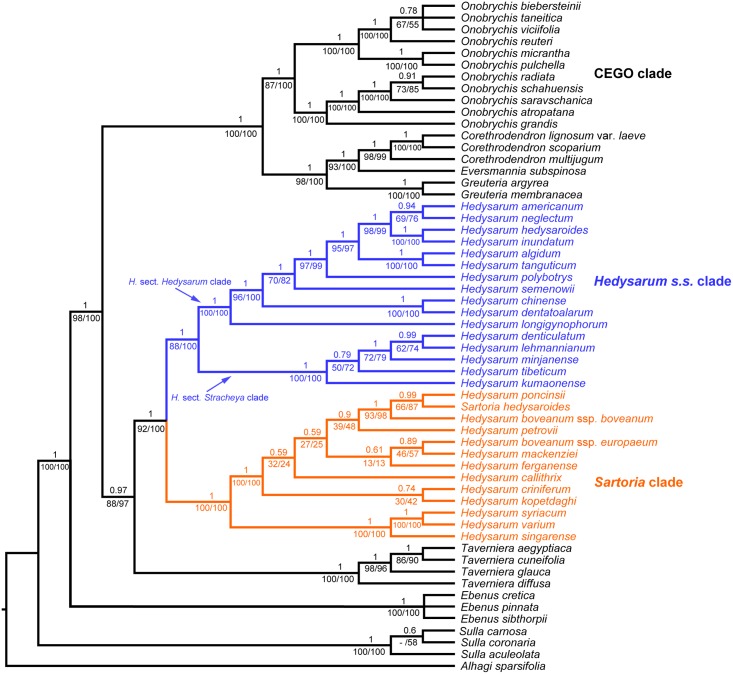
In the concatenated plastid tree based on psbA-trnH, trnC-petN, trnL-trnF, trnS-trnG and petN-psbM sequences (Fig 2), Sulla diverged first, followed by Ebenus with high support (PP = 1, PBS = 100%, LBS = 100%). Corethrodendron, Ebenus, Greuteria, Hedysarum, Onobrychis, Sulla, and Taverniera were each supported to be monophyletic. The CEGO clade was also highly supported (PP = 1, PBS = 100%, LBS = 100%), which was sister to the Hedysarum + Taverniera clade (PP = 1, PBS = 98%, LBS = 100%). Hedysarum was monophyletic (PP = 1, PBS = 92%, LBS = 100%), and was sister to Taverniera (PP = 0.97, PBS = 88%, LBS = 97%). Within Hedysarum, the Sartoria clade (PP = 1, PBS = 100%, LBS = 100%) and the Hedysarum s.s. clade (PP = 1, PBS = 88%, LBS = 100%) were each well supported. H. denticulatum and H. minjanense were nested within H. sect. Stracheya as redefined by Duan et al. [11]. The Hedysarum sect. Hedysarum clade was sister to the H. sect. Stracheya clade (PP = 1, PBS = 88%, LBS = 100%).
Fig 2. Bayesian tree based on the concatenated plastid psbA-trnH, trnC-petN, trnL-trnF, trnS-trnG, and petN-psbM sequences.
The Bayesian posterior probabilities are above the branches, and the maximum parsimony (left) and maximum likelihood (right) bootstrap support values are below the branches. A dash indicates a branch that is not found in the maximum parsimony tree.
The coalescent tree based on all five plastid sequences (S7 Fig) showed that Hedysarum was monophyletic (PP = 1), and was sister to Taverniera (PP = 0.83). The Hedysarum + Taverniera clade was then sister to the CEGO clade (PP = 1).
The primary concordance tree based on all five plastid sequences (S8 Fig) also had similar topology compared with the concatenated plastid data tree (Fig 2) and the coalescent tree (S7 Fig). Hedysarum was supported to be monophyletic (mean CF = 0.67) and was sister to Taverniera (mean CF = 0.35). The Hedysarum + Taverniera clade was then sister to the CEGO clade (mean CF = 0.56).
The AU tests of the concatenated nuclear ETS + ITS + PGDH + TRPT dataset against the concatenated five plastid sequence dataset were performed. The nuclear dataset rejected the plastid topology (p < 0.01), and the plastid dataset rejected the nuclear topology (p < 0.01) as well, suggesting incongruence between the nuclear and plastid datasets. The concatenated five nuclear sequence dataset (ETS + ITS + PGDH + TRPT + SQD1) also rejected the plastid topology (p < 0.01), and vice versa.
Discussion
Non-monophyly of Hedysarum
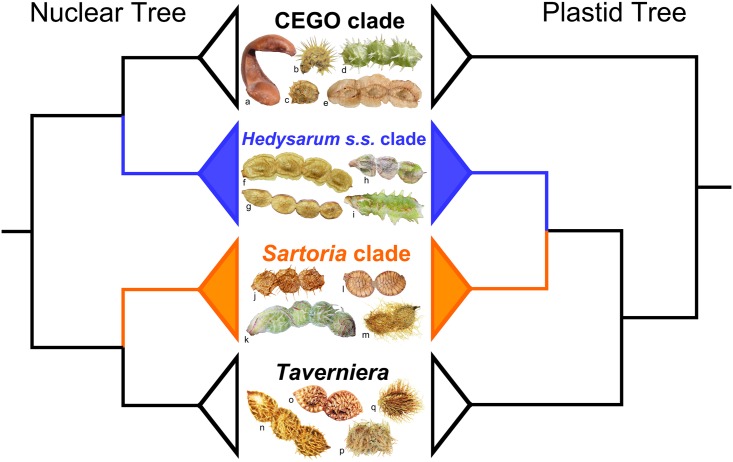
Our results revealed incongruence between the nuclear and the plastid trees in Hedysarum (Fig 3). In the nuclear trees (Figs 1 and 3), Hedysarum is biphyletic; the Sartoria clade is sister to Taverniera; and the Hedysarum s.s. clade is sister to the CEGO clade. On the other hand, Hedysarum is monophyletic and sister to Taverniera in the plastid tree (Figs 2 and 3). The topological incongruence concerning Hedysarum is robustly supported (Figs 1 and 2). The difference between the nuclear and the plastid topologies lies on the position of the Hedysarum s.s. clade, with the Hedysarum s.s. clade sister to the CEGO clade in the nuclear tree, in contrast with its sister relationship with the Sartoria clade in the plastid tree (Fig 3).
Fig 3. Summary of the incongruent position of the Hedysarum s.s. clade in the nuclear (left) and the plastid (right) trees, with selected legume species from each clade (middle).
The legumes: a, Eversmannia subspinosa B.Fedtsch.; b, Onobrychis atropatana Boiss.; c, O. viciifolia Scop.; d, Corethrodendron multijugum (Maxim.) B.H.Choi & H.Ohashi; e, Greuteria membranacea (Coss. & Balansa) Amirahm. & Kaz.Osaloo; f, Hedysarum semenowii Regel & Herder; g, H. americanum (Michx.) Britton; h, H. kumaonense Benth. ex Baker; i, H. tibeticum (Benth.) B.H.Choi & H.Ohashi; j, H. gremiale Rollins; k, H. petrovii Yakovlev; l, H. boveanum Bunge ex Basiner; m, H. callithrix Bunge ex Boiss.; n, Taverniera diffusa (Cambess.) Thulin; o, T. glauca Edgew.; p, T. lappacea DC.; q, T. longisetosa Thulin; photoed by PLL and LD.
Our trees based on concatenated nuclear and plastid data are well supported concerning the position of the Hedysarum s.s. clade (Figs 1 and 2). However, there is concern that data concatenation may lead to overconfidence in incorrect species tree [55]. Thus we also used the coalescent method and BCA to infer the phylogenetic relationships using multi-locus data. Our coalescent trees (S5 and S7 Figs) agree with the concatenated trees (Figs 1 and 2), and they are also well supported. In BCA, the concordance factor (CF) of a certain clade is the proportion of the genes or the genome for which that given clade is true, and should not be confused with a clade support value [53, 56]. In our BCA results (S6 and S8 Figs), CFs of many clades are below 1, which is likely due to the limited number of phylogenetic informative sites in each individual locus especially in the PGDH, SQD1, TRPT and psbA-trnH sequences (Table 1). The single locus trees show limited resolution with many polytomies, such as the SQD1 tree (S4 Fig). The nuclear sequences have fewer informative sites than the plastid ones (Table 1), and the CFs from the nuclear BCA are lower than those from the plastid BCA (S6 and S8 Figs). However, in the nuclear as well as in the plastid BCA results, the primary concordance tree is built from the clades with highest CFs and shows the relationships shared by a large proportion of the sampled genes [56]. It agrees with the concatenated data tree and the multi-locus coalescent tree. Closer inspection of the nuclear BCA result shows that in the list of clades which are not in the nuclear primary concordance tree but with estimated CF > 0.05, there is no such clade of a monophyletic Hedysarum as in the plastid phylogeny. Also, in the list of clades which are not in the plastid primary concordance tree but with estimated CF > 0.05, there is no contradictory clade of a biphyletic Hedysarum as in the nuclear phylogeny. In our analyses, various methods of phylogenetic reconstructions, i.e., BI, MP and ML based on concatenated data, multi-locus coalescent, and BCA, all uncover the same incongruent pattern between the nuclear and the plastid trees (Fig 3).
Various mechanisms have been proposed to explain topological incongruence between different gene trees, such as hybridization/introgression, lineage sorting, allopolyploidy, horizontal gene transfer, and many other factors [14, 57–59].
Insufficient data is probably responsible for the low resolution of the SQD1 tree, however it can be ruled out from the candidate mechanisms that explain the incongruent position of the Hedysarum s.s. clade because both the nuclear and plastid topologies are robustly supported (Figs 1 and 2, S3 Fig). Gene choice and rate of molecular evolution [58] can also be ruled out for the same reason. Sequencing error probably affects phylogenetic accuracy where there are a small number of informative sites [58]. In our trees, sequencing error at most only influences the closely related terminals where the support is low, but should not affect well supported deep relationships. Our sampling covers all genera of the tribe Hedysareae and all sections and major infra-sectional clades in Hedysarum as recognized by Duan et al. [11]. Furthermore the different sequences were generated from the same DNA extraction (except for the PGDH sequence of Sulla coronaria, see S1 Appendix) for each accession. Insufficient taxon sampling and sample misidentification are thus not likely to be the causes of the deep incongruence.
Incomplete homogenization of tandem repeats and the possibly uniparental inheritance of the nuclear ribosomal gene region, and orthology/paralogy conflation of the nuclear low or single copy genes, may affect the accuracy of phylogenetic reconstruction [13, 58, 60, 61]. These issues may lead to incongruence between different nuclear gene trees. We cannot fully rule out these issues in our nuclear data. However, the results based on ribosomal gene regions (S1 Fig) and low or single copy genes (S2 and S4 Figs) all support a biphyletic Hedysarum. Furthermore, the monophyly of Hedysarum is not detected in any of the nuclear trees. Therefore, the incongruence between the nuclear and the plastid trees cannot be simply explained by such mechanisms.
Allopolyploidy can be excluded from being considered as a mechanism to explain the incongruent position of the Hedysarum s.s. clade. We checked the chromosome number counts for Hedysareae in the Index to Plant Chromosome Numbers (IPCN, http://www.tropicos.org/Project/IPCN). Most species in Hedysarum as well as those of other genera in Hedysareae have been reported to have chromosome numbers 2n = 16 or 2n = 14, suggesting that they are diploid because their basic chromosome numbers are indicated as x = 8 or x = 7 [2, 62]. To summarize the available data from IPCN, deep lineages of Hedysareae are predominantly diploid, e.g., 2n = 2x = 16 for Alhagi, Sulla (species name still under Hedysarum in IPCN; with one report of 2n = 18), Taverniera, Corethrodendron (species name still under Hedysarum in IPCN), Eversmannia, the Hedysarum sect. Stracheya clade (H. minjanense and H. denticulatum), and 2n = 2x = 14 for Ebenus (with one report of 2n = 18). Most species in the Sartoria clade, the H. sect. Hedysarum clade and Onobrychis are diploid with chromosome numbers 2n = 14 or 16. Polyploidy in Hedysarum and Onobrychis occurs mostly as multiple cytotypes within one species (e.g., 2n = 14, 28 for H. hedysaroides Schinz & Thell., 2n = 16, 32 for H. mackenziei Richardson), with some being exclusively polyploid such as 2n = 28 for H. inundatum Turcz. and O. biebersteinii Širj. [63].
Occasionally horizontal gene transfer between species through nonsexual means may explain gene tree incongruence [58]. But the variational pattern in our study would require multiple horizontal transfers in the nuclear genes (ETS, ITS, PGDH, TRPT, SQD1) in a particular ancestor of Hedysarum, which simultaneously leads to discordant gene trees with the plastid tree. This hypothesis is viewed difficult, as many unlinked genes are not likely to be horizontally transferred in parallel [64]. Stegemann et al. [65] showed that an entire chloroplast genome could be horizontally transferred between sexually incompatible species through grafting. Their results were based on artificial grafting and selection process. It is not known whether chloroplast genome could be horizontally transferred through natural grafting. Additionally, unlike in woody plants, species of Hedysareae are not likely to form natural grafting because most species are herbaceous perennials.
Lineage sorting of ancestral polymorphisms can cause incongruent gene trees. The phylogenetic consequence of lineage sorting is usually difficult to distinguish from that of the other processes, such as hybridization/introgression [58, 66]. In general, lineage sorting is expected to be a possible cause of incongruence at lower taxonomic ranks often in population and/or species-level studies [58]. But the incongruence in our results involves deep relationships among different genera. Lineage sorting may contribute to incongruence at higher taxonomic ranks when polymorphisms are maintained by balancing selection through many speciation events [58]. Concerning the position of the Hedysarum s.s. clade, however, our tree based on coding genes (PGDH, TRPT) agrees with the tree based on non-coding sequences (ETS, ITS), which are thought to be neutral. Our tree based on the plastid non-coding data (Fig 2) also agrees with the tree based on the plastid coding matK gene in a previous study [10]. Hence lineage sorting may not be the best explanation for the incongruence in our results.
Our phylogenetic pattern may be best explained by a chloroplast capture hypothesis via introgression. In most angiosperm taxa, the plastid genome is uniparentally inherited, whereas the nuclear genome is biparentally inherited [13, 67]. After many generations of backcrossing through introgression, the introgressant inherited the plastid genome from one parent, and nearly all nuclear genes from the other parent. A significant feature of chloroplast capture is that plastid genome introgression is always not accompanied by nuclear introgression [14, 58, 65]. Our results are consistent with this scenario, i.e., multiple nuclear datasets are not supporting the monophyletic Hedysarum as suggested by the plastid tree. Chloroplast capture is probably one of the most common causes of phylogenetic incongruence [58], and has been reported in a wide range of taxa at various taxonomic levels [14, 15, 68–75].
Our phylogenetic analyses of the nuclear sequences show that Hedysarum is not monophyletic. Its generic delimitation needs to be reconsidered. The Sartoria clade and the Hedysarum s.s. clade can be distinguished from each other by several morphological characters. Most species in the Sartoria clade have grayish-green leaves, obscure lateral veins in the leaflets, biconvex and non-winged loments which always possess prickles, bristles, and ribs, whereas species in the Hedysarum s.s. clade always have bright-green leaves, visible lateral veins in the leaflets, compressed and more or less winged loments which are always unarmed [2, 3, 5]. Loment morphology of the Sartoria clade is very similar to that of Taverniera by its prickles, bristles, and ribs [2, 76], corresponding to the sister relationship between the Sartoria clade and Taverniera in the nuclear tree (Fig 3). The compressed, unarmed, more or less winged loment is also found in Greuteria and Eversmannia in the CEGO clade (Fig 3) [10]. Mironov [77] found that species of H. sect. Gamotion (members of the Hedysarum s.s. clade) differed from species of H. sect. Multicaulia (members of the Sartoria clade) in several pericarp anatomical characters that were thought to be of systematic significance.
Additionally, the Hedysarum s.s. clade and the Sartoria clade also show eco-geographical differentiation. In general, members of the Hedysarum s.s. clade adapt to mesic and/or psychric habitats in temperate montane forests, alpine and arctic regions of Eurasia and North America, whereas species of the Sartoria clade are distributed in xeric habitats in arid/semi-arid areas and steppes of central and western Asia, the Mediterranean region and western North America [11, 78].
Systematic position of the Hedysarum sect. Stracheya clade
Stracheya was originally established as a monotypic genus [9], which was then treated as a section of Hedysarum based on comprehensive morphological analyses [2, 3]. Hedysarum tibeticum was the sole member of H. sect. Stracheya when Choi and Ohashi first constructed this section in Hedysarum [2]. However, previous phylogenetic analyses showed different systematic positions of the H. sect. Stracheya clade in Hedysareae with low support values [10, 11]. With more sequence data, our nuclear and plastid trees resolved congruent sister relationship between the H. sect. Hedysarum clade and a clade of five species including H. tibeticum, H. kumaonense, H. lehmannianum, H. denticulatum, and H. minjanense (Figs 1 and 2, S3 Fig). Our analysis supports merging Stracheya with Hedysarum and expanding H. sect. Stracheya to include four additional species. These four species were previously treated as members of H. sect. Subacaulia because of their strongly reduced stems [5, 20]. However, they did not form a clade with other members of H. sect. Subacaulia such as H. petrovii, H. ferganense Korsh., and H. poncinsii (Figs 1 and 2, S3 Fig). Some species of H. sect. Hedysarum, for example, H. tanguticum B.Fedtsch. and H. pseudoastragalus Ulbr., also have reduced stems [5]. Thus, the strongly reduced stem is most likely a homoplasious convergence in Hedysarum. The dwarf habit of the H. sect. Stracheya lineage might be an adaptation to the cold and arid habitats in the pan-Himalayan region and the adjacent eastern part of central Asia [79, 80].
Supporting Information
Herbarium codes follow the Index Herbariorum (http://sweetgum.nybg.org/ih/). For each taxon, GenBank accession numbers of the ten sequences are given in the sequence of ETS, ITS, PGDH, SQD1, TRPT, trnL-trnF, psbA-trnH, trnS-trnG, trnC-petN, and petN-psbM. New sequences generated in this study are indicated by an asterisk (*). Missing sequences are indicated by a dash (-).
(XLS)
The Bayesian posterior probabilities are above the branches, and the maximum parsimony (left) and maximum likelihood (right) bootstrap support values are below the branches. Dashes indicate branches that are not found in the Bayesian tree or the maximum likelihood tree.
(TIF)
The Bayesian posterior probabilities are above the branches, and the maximum parsimony (left) and maximum likelihood (right) bootstrap support values are below the branches. Dashes indicate branches that are not found in the maximum parsimony tree or the maximum likelihood tree.
(TIF)
The Bayesian posterior probabilities are above the branches, and the maximum parsimony (left) and maximum likelihood (right) bootstrap support values are below the branches. Dashes indicate branches that are not found in the maximum parsimony or the maximum likelihood trees.
(TIF)
The Bayesian posterior probabilities are above the branches, and the maximum parsimony (left) and maximum likelihood (right) bootstrap support values are below the branches. Dashes indicate branches that are not found in the maximum parsimony or the maximum likelihood trees.
(TIF)
The posterior probabilities are above the branches.
(TIF)
The sample-wide posterior mean concordance factors are above the branches, and their 95% credibility intervals are below the branches.
(TIF)
The posterior probabilities are above the branches.
(TIF)
The sample-wide posterior mean concordance factors are above the branches, and their 95% credibility intervals are below the branches.
(TIF)
Acknowledgments
We thank curators of the United States National Herbarium (US) and the Field Museum (F) for permission to sample their specimens. Laboratory work was conducted in the Laboratories of Analytical Biology of the National Museum of Natural History, the Smithsonian Institution. We are grateful to Xue Yang, Ning Zhang, Gabriel Johnson, Matthew Kweskin and Jeffrey Hunt for their assistance in the experiments and data analyses, and Ashley Egan for helpful discussions in the early phase of the study.
Data Availability
All DNA sequences are available from the NCBI GenBank. Accession numbers are listed in Supporting Information S1 Appendix.
Funding Statement
This work was supported by the National Natural Science Foundation of China (www.nsfc.gov.cn, grant numbers 30270106, 30870155, to ZYC) and China Scholarship Council (www.csc.edu.cn, grant number 201406300108, to PLL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.LPWG (The Legume Phylogeney Working Group). Legume phylogeny and classification in the 21st century: progress, prospects and lessons for other species-rich clades. Taxon. 2013; 62: 217–248. [Google Scholar]
- 2.Choi B-H, Ohashi H. Generic criteria and an infrageneric system for Hedysarum and related genera (Papilionoideae-Leguminosae). Taxon. 2003; 52: 567–576. [Google Scholar]
- 3.Xu L-R, Choi B-H. Hedysarum L In: Wu Z-Y, Raven PH, Hong D-Y, editors. Flora of China. Beijing: Science Press, St. Louis: Missouri Botanical Garden Press; 2010. Volume 10: 514–525. [Google Scholar]
- 4.Sultanov B. Hedysarum neglectum Ldb. and Hedysarum austrosibiricum B.Fedtsch. in Flora of Kirghizia In: Nikitina EV, Sudnicyna IG, Aidarov RA, Sultanov RM, Arbaeva ZS, Sultanov B et al. , editors. Flora of Kirghiz SSR Supplement. Frunze: Ilim Publishing House; 1970. Volume 2: 31–34. [Google Scholar]
- 5.Xu L-R. Hedysarum L In: Cui H-B, editor. Flora Reipublicae Popularis Sinicae. Beijing: Science Press; 1998. Volume 42(2): 176–221. [Google Scholar]
- 6.Linnaeus C. Species plantarum. 1st ed Stockholm: Laurentii Salvii; 1753. [Google Scholar]
- 7.Jarvis C. Order out of Chaos: Linnaean Plant Names and their Types. 1st ed London: The Linnean Society of London & the Natural History Museum, London; 2007. [Google Scholar]
- 8.Fedtschenko B. Generis Hedysari revisio. Trudy Imp S-Peterburgsk Bot Sada. 1902; 19: 183–349. [Google Scholar]
- 9.Bentham G. On three new genera connected with the Indian Flora. Hooker’s J Bot Kew Gard Misc. 1853; 5: 304–309. [Google Scholar]
- 10.Amirahmadi A, Osaloo SK, Moein F, Kaveh A, Maassoumi AA. Molecular systematics of the tribe Hedysareae (Fabaceae) based on nrDNA ITS and plastid trnL-F and matK sequences. Plant Syst Evol. 2014; 300: 729–747. [Google Scholar]
- 11.Duan L, Wen J, Yang X, Liu P-L, Arslan E, Ertuğrul K, et al. Phylogeny of Hedysarum and tribe Hedysareae (Leguminosae: Papilionoideae) inferred from sequence data of ITS, matK, trnL-F and psbA-trnH. Taxon. 2015; 64: 49–64. [Google Scholar]
- 12.Choi H-K, Luckow MA, Doyle J, Cook DR. Development of nuclear gene-derived molecular markers linked to legume genetic maps. Mol Genet Genomics. 2006; 276: 56–70. 10.1007/s00438-006-0118-8 [DOI] [PubMed] [Google Scholar]
- 13.Zimmer EA, Wen J. Using nuclear gene data for plant phylogenetics: progress and prospects. Mol Phylogen Evol. 2012; 65: 774–785. [DOI] [PubMed] [Google Scholar]
- 14.Rieseberg LH, Soltis DE. Phylogenetic consequences of cytoplasmic gene flow in plants. Evol Trends Plants. 1991; 5: 65–84. [Google Scholar]
- 15.Soltis DE, Kuzoff RK. Discordance between nuclear and chloroplast phylogenies in the Heuchera group (Saxifragaceae). Evolution. 1995; 49: 727–742. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin BG, Markos S. Phylogenetic utility of the external transcribed spacer (ETS) of 18S-26S rDNA: congruence of ETS and ITS trees of Calycadenia (Compositae). Mol Phylogen Evol. 1998; 10: 449–463. [DOI] [PubMed] [Google Scholar]
- 17.Farruggia FT, Howard AJ. Examination of five nuclear markers for phylogenetic study of Hologalegina (Leguminosae). Brittonia. 2011; 63: 489–499. [Google Scholar]
- 18.Babineau M, Gagnon E, Bruneau A. Phylogenetic utility of 19 low copy nuclear genes in closely related genera and species of caesalpinioid legumes. S Afr J Bot. 2013; 89: 94–105. [Google Scholar]
- 19.Li M, Wunder J, Bissoli G, Scarponi E, Gazzani S, Barbaro E, et al. Development of COS genes as universally amplifiable markers for phylogenetic reconstructions of closely related plant species. Cladistics. 2008; 24: 727–745. [Google Scholar]
- 20.Fedtschenko B. Hedysarum L. In: Komarov VL, editor. Flora of USSR. Moskva-Leningrad: Izdatelstvo Akademii Nauk SSSR; 1948. Volume 13: 259–319. [Google Scholar]
- 21.Hedge IC. Hedysarum L In: Davis PH, editor. Flora of Turkey and the East Aegean Islands. Edinburgh: Edinburgh University Press; 1970. Volume 3: 549–560. [Google Scholar]
- 22.Valdés B. Hedysarum L In: Talavera S, Aedo C, Castroviejo S, Herrero A, Romero Zarco C, Salgueiro FJ, et al. , editors. Flora Iberica. Madrid: Real Jardín Botánico, CSIC; 2000. Volume 7(2): 943–955. [Google Scholar]
- 23.Stanford AM, Harden R, Parks CR. Phylogeny and biogeography of Juglans (Juglandaceae) based on matK and ITS sequence data. Am J Bot. 2000; 87: 872–882. [PubMed] [Google Scholar]
- 24.Sang T, Crawford DJ, Stuessy TF. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am J Bot. 1997; 84: 1120–1136. [PubMed] [Google Scholar]
- 25.Lee C, Wen J. Phylogeny of Panax using chloroplast trnC–trnD intergenic region and the utility of trnC–trnD in interspecific studies of plants. Mol Phylogen Evol. 2004; 31: 894–903. [DOI] [PubMed] [Google Scholar]
- 26.Shaw J, Lickey EB, Schilling EE, Small RL. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Am J Bot. 2007; 94: 275–288. 10.3732/ajb.94.3.275 [DOI] [PubMed] [Google Scholar]
- 27.Shaw J, Lickey EB, Beck JT, Farmer SB, Liu W, Miller J, et al. The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am J Bot. 2005; 92: 142–166. 10.3732/ajb.92.1.142 [DOI] [PubMed] [Google Scholar]
- 28.Hager M, Biehler K, Illerhaus J, Ruf S, Bock R. Targeted inactivation of the smallest plastid genome-encoded open reading frame reveals a novel and essential subunit of the cytochrome b6f complex. EMBO J. 1999; 18: 5834–5842. 10.1093/emboj/18.21.5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarz EN, Ruhlman TA, Sabir JSM, Hajrah NH, Alharbi NS, Al-Malki AL, et al. Plastid genome sequences of legumes reveal parallel inversions and multiple losses of rps16 in papilionoids. J Syst Evol. 2015; 53: 458–468. [Google Scholar]
- 30.Taberlet P, Gielly L, Pautou G, Bouvet J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol. 1991; 17: 1105–1109. [DOI] [PubMed] [Google Scholar]
- 31.Hiraishi A, Kamagata Y, Nakamura K. Polymerase chain reaction amplification and restriction fragment length polymorphism analysis of 16S rRNA genes from methanogens. J Ferment Bioeng. 1995; 79: 523–529. [Google Scholar]
- 32.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012; 28: 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004; 32: 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller K. SeqState: primer design and sequence statistics for phylogenetic DNA datasets. Appl Bioinformatics. 2005; 4: 65–69. [DOI] [PubMed] [Google Scholar]
- 35.Simmons MP, Ochoterena H. Gaps as characters in sequence-based phylogenetic analyses. Syst Biol. 2000; 49: 369–381. [PubMed] [Google Scholar]
- 36.Vaidya G, Lohman DJ, Meier R. SequenceMatrix: concatenation software for the fast assembly of multi-gene dataset with character set and codon information. Cladistics. 2011; 27: 171–180. [DOI] [PubMed] [Google Scholar]
- 37.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012; 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokota Y, Kawata T, Iida Y, Kato A, Tanifuji S. Nucleotide sequences of the 5.8S rRNA gene and internal transcribed spacer regions in carrot and broad bean ribosomal DNA. J Mol Evol. 1989; 29: 294–301. [DOI] [PubMed] [Google Scholar]
- 39.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003; 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 40.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012; 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ronquist F, Huelsenbeck J, Teslenko M. MrBayes version 3.2 manual: tutorials and model summaries. 2011. http://mrbayes.sourceforge.net/mb3.2_manual.pdf
- 42.Swofford DL. PAUP*: phylogenetic analysis using parsimony (*and other methods) Version 4.0b10. Sunderland, Massachusetts: Sinauer Associates; 2002. [Google Scholar]
- 43.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014; 30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farris JS, Källersjö M, Kluge AG, Bult C. Testing significance of incongruence. Cladistics. 1995; 10: 315–319. [Google Scholar]
- 45.Lee MSY. Uninformative characters and apparent conflict between molecules and morphology. Mol Biol Evol. 2001; 18: 676–680. [DOI] [PubMed] [Google Scholar]
- 46.Cunningham CW. Can three incongruence tests predict when data should be combined? Mol Biol Evol. 1997; 14: 733–740. [DOI] [PubMed] [Google Scholar]
- 47.Lanfear R, Calcott B, Ho SYW, Guindon S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 2012; 29: 1695–1701. 10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- 48.Shimodaira H. An approximately unbiased test of phylogenetic tree selection. Syst Biol. 2002; 51: 492–508. 10.1080/10635150290069913 [DOI] [PubMed] [Google Scholar]
- 49.Shimodaira H, Hasegawa M. CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics. 2001; 17: 1246–1247. [DOI] [PubMed] [Google Scholar]
- 50.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013; 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heled J, Drummond AJ. Bayesian inference of species trees from multilocus data. Mol Biol Evol. 2010; 27: 570–580. 10.1093/molbev/msp274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu C-H, Xie D. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput Biol. 2014; 10: e1003537 10.1371/journal.pcbi.1003537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ané C, Larget B, Baum DA, Smith SD, Rokas A. Bayesian estimation of concordance among gene trees. Mol Biol Evol. 2007; 24: 412–426. 10.1093/molbev/msl170 [DOI] [PubMed] [Google Scholar]
- 54.Larget BR, Kotha SK, Dewey CN, Ané C. BUCKy: Gene tree/species tree reconciliation with Bayesian concordance analysis. Bioinformatics. 2010; 26: 2910–2911. 10.1093/bioinformatics/btq539 [DOI] [PubMed] [Google Scholar]
- 55.Edwards SV. Is a new and general theory of molecular systematics emerging? Evolution. 2009; 63: 1–19. 10.1111/j.1558-5646.2008.00549.x [DOI] [PubMed] [Google Scholar]
- 56.Baum DA. Concordance trees, concordance factors, and the exploration of reticulate genealogy. Taxon. 2007; 56: 417–426. [Google Scholar]
- 57.Doyle JJ. Gene trees and species trees: molecular systematics as one-character taxonomy. Syst Bot. 1992; 17: 144–163. [Google Scholar]
- 58.Wendel JF, Doyle JJ. Phylogenetic incongruence: window into genome history and molecular evolution In: Soltis DE, Soltis PS, Doyle JJ, editors. Molecular systematics of plants II: DNA sequencing. Norwell: Kluwer Academic Publishers; 1998. pp. 265–296. [Google Scholar]
- 59.Zou X-H, Ge S. Conflicting gene trees and phylogenomics. J Syst Evol. 2008; 46: 795–807. [Google Scholar]
- 60.Álvarez I, Wendel JF. Ribosomal ITS sequences and plant phylogenetic inference. Mol Phylogen Evol. 2003; 29: 417–434. [DOI] [PubMed] [Google Scholar]
- 61.Blattner FR. Phylogenetic analysis of Hordeum (Poaceae) as inferred by nuclear rDNA ITS sequences. Mol Phylogen Evol. 2004; 33: 289–299. [DOI] [PubMed] [Google Scholar]
- 62.Goldblatt P. Cytology and the phylogeny of Leguminosae In: Polhill RM, Raven PH, editors. Advances in Legume Systematics, part 2. London: Royal Botanic Gardens, Kew; 1981. pp. 427–463. [Google Scholar]
- 63.Doyle JJ. Polyploidy in Legumes In: Soltis PS, Soltis DE, editors. Polyploidy and genome evolution. Berlin Heidelberg: Springer-Verlag; 2012. pp. 147–180. [Google Scholar]
- 64.Sang T, Zhong Y. Testing hybridization hypotheses based on incongruent gene trees. Syst Biol. 2000; 49: 422–434. [DOI] [PubMed] [Google Scholar]
- 65.Stegemann S, Keuthe M, Greiner S, Bock R. Horizontal transfer of chloroplast genomes between plant species. Proc Natl Acad Sci USA. 2012; 109: 2434–2438. 10.1073/pnas.1114076109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maureira-Butler IJ, Pfeil BE, Muangprom A, Osborn TC, Doyle JJ. The reticulate history of Medicago (Fabaceae). Syst Biol. 2008; 57: 466–482. 10.1080/10635150802172168 [DOI] [PubMed] [Google Scholar]
- 67.Wicke S, Schneeweiss GM, de Pamphilis CW, Müller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 2011; 76: 273–297. 10.1007/s11103-011-9762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolfe AD, Elisens WJ. Evidence of chloroplast capture and pollen-mediated gene flow in Penstemon sect. Peltanthera (Scrophulariaceae). Syst Bot. 1995; 20: 395–412. [Google Scholar]
- 69.Okuyama Y, Fujii N, Wakabayashi M, Kawakita A, Ito M, Watanabe M, et al. Nonuniform concerted evolution and chloroplast capture: heterogeneity of observed introgression patterns in three molecular data partition phylogenies of Asian Mitella (Saxifragaceae). Mol Biol Evol. 2005; 22: 285–296. 10.1093/molbev/msi016 [DOI] [PubMed] [Google Scholar]
- 70.Fehrer J, Gemeinholzer B, Chrtek J, Bräutigam S. Incongruent plastid and nuclear DNA phylogenies reveal ancient intergeneric hybridization in Pilosella hawkweeds (Hieracium, Cichorieae, Asteraceae). Mol Phylogen Evol. 2007; 42: 347–361. [DOI] [PubMed] [Google Scholar]
- 71.Acosta MC, Premoli AC. Evidence of chloroplast capture in South American Nothofagus (subgenus Nothofagus, Nothofagaceae). Mol Phylogen Evol. 2010; 54: 235–242. [DOI] [PubMed] [Google Scholar]
- 72.Xu B, Wu N, Gao X-F, Zhang L-B. Analysis of DNA sequences of six chloroplast and nuclear genes suggests incongruence, introgression, and incomplete lineage sorting in the evolution of Lespedeza (Fabaceae). Mol Phylogen Evol. 2012; 62: 346–358. [DOI] [PubMed] [Google Scholar]
- 73.Deng T, Nie Z-L, Drew BT, Volis S, Kim C, Xiang C-L, et al. Dose the Arcto-Tertiary biogeographic hypothesis explain the disjunct distribution of northern hemisphere herbaceous plants? The case of Meehania (Lamiaceae). PLoS ONE. 2015; 10: e0117171 10.1371/journal.pone.0117171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yi T-S, Jin G-H, Wen J. Chloroplast capture and intra- and inter-continental biogeographic diversification in the Asian—New World disjunct plant genus Osmorhiza (Apiaceae). Mol Phylogen Evol. 2015; 85: 10–21. [DOI] [PubMed] [Google Scholar]
- 75.Duan L, Yang X, Liu P, Johnson G, Wen J, Chang Z. A molecular phylogeny of Caraganeae (Leguminosae, Papilionoideae) reveals insights into new generic and infrageneric delimitations. PhytoKeys. 2016; 70: 111–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thulin M. Revision of Taverniera (Leguminosae-Papilionoideae). Symb Bot Upsal. 1985; 25: 43–95. [Google Scholar]
- 77.Mironov YM. Pericarp anatomy of East European species of the genus Hedysarum L. (Papilionaceae): sections Gamotion and Multicaulia. Bull Mosc Soc Nat Ser Biol. 2000; 105: 50–53. [Google Scholar]
- 78.Xu L-R. The ecological differentiation of the Hedysarum L. and geographical distribution in China. Acta Bot Bor-Occ Sinica. 1985; 4: 275–285. [Google Scholar]
- 79.Wen J, Zhang J-Q, Nie Z-L, Zhong Y, Sun H. Evolutionary diversifications of plants on the Qinghai-Tibetan Plateau. Front Genet. 2014; 5: 4 10.3389/fgene.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meng H-H, Gao X-Y, Huang J-F, Zhang M-L. Plant phylogeography in arid Northwest China: retrospectives and perspectives. J Syst Evol. 2015; 53: 33–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Herbarium codes follow the Index Herbariorum (http://sweetgum.nybg.org/ih/). For each taxon, GenBank accession numbers of the ten sequences are given in the sequence of ETS, ITS, PGDH, SQD1, TRPT, trnL-trnF, psbA-trnH, trnS-trnG, trnC-petN, and petN-psbM. New sequences generated in this study are indicated by an asterisk (*). Missing sequences are indicated by a dash (-).
(XLS)
The Bayesian posterior probabilities are above the branches, and the maximum parsimony (left) and maximum likelihood (right) bootstrap support values are below the branches. Dashes indicate branches that are not found in the Bayesian tree or the maximum likelihood tree.
(TIF)
The Bayesian posterior probabilities are above the branches, and the maximum parsimony (left) and maximum likelihood (right) bootstrap support values are below the branches. Dashes indicate branches that are not found in the maximum parsimony tree or the maximum likelihood tree.
(TIF)
The Bayesian posterior probabilities are above the branches, and the maximum parsimony (left) and maximum likelihood (right) bootstrap support values are below the branches. Dashes indicate branches that are not found in the maximum parsimony or the maximum likelihood trees.
(TIF)
The Bayesian posterior probabilities are above the branches, and the maximum parsimony (left) and maximum likelihood (right) bootstrap support values are below the branches. Dashes indicate branches that are not found in the maximum parsimony or the maximum likelihood trees.
(TIF)
The posterior probabilities are above the branches.
(TIF)
The sample-wide posterior mean concordance factors are above the branches, and their 95% credibility intervals are below the branches.
(TIF)
The posterior probabilities are above the branches.
(TIF)
The sample-wide posterior mean concordance factors are above the branches, and their 95% credibility intervals are below the branches.
(TIF)
Data Availability Statement
All DNA sequences are available from the NCBI GenBank. Accession numbers are listed in Supporting Information S1 Appendix.