Abstract
Thrombin-induced secondary injury is mediated through its receptor, protease activated receptor-1 (PAR-1), by "biased agonism." Activated protein C (APC) acts through the same PAR-1 receptor but functions as an anti-coagulant and anti-inflammatory protein, which counteracts many of the effects of thrombin. Although the working mechanism of PAR-1 is becoming clear, the functional role of PAR-1 and its correlation with APC in the injured spinal cord remains to be elucidated. Here we investigated if PAR-1 and APC are determinants of long-term functional recovery after a spinal cord contusive injury using PAR-1 null and wild-type mice. We found that neutrophil infiltration and disruption of the blood-spinal cord barrier were significantly reduced in spinal cord injured PAR-1 null mice relative to the wild-type group. Both locomotor recovery and ability to descend an inclined grid were significantly improved in the PAR-1 null group 42 days after injury and this improvement was associated with greater long-term sparing of white matter and a reduction in glial scarring. Wild-type mice treated with APC acutely after injury showed a similar level of improved locomotor recovery to that of PAR-1 null mice. However, improvement of APC-treated PAR-1 null mice was indistinguishable from that of vehicle-treated PAR-1 null mice, suggesting that APC acts through PAR-1. Collectively, our findings define a detrimental role of thrombin-activated PAR-1 in wound healing and further validate APC, also acting through the PAR-1 by biased agonism, as a promising therapeutic target for spinal cord injury.
Introduction
Spinal cord injury results in direct vascular damage, followed by an inflammatory response and a cascade of events that further disrupt the blood-spinal cord barrier. One of the earliest factors produced following injury is the serine protease thrombin [1, 2], which is a 36 kDa protein comprised of two chains, A and B, that are linked by a disulfide bond [3]. Thrombin primarily contributes to coagulation by the conversion of fibrinogen to fibrin and the activation of platelets and several coagulation factors [4–6]. It is also known to mediate inflammation and endothelial permeability [7, 8].
Thrombin acts through at least four different receptor subtypes, called protease activated receptors (PARs), that are designated PAR-1, -2, -3, and -4 [9–12]. In the CNS, PAR-1 is expressed on motoneurons [13, 14], astrocytes, and cerebral endothelial cells [15, 16]. When activated by thrombin, PAR-1 has multiple detrimental effects, including death of motor neurons, neurite retraction, and astrogliosis [15]. Moreover, thrombin-activated PAR-1 in the endothelium not only induces pro-inflammatory signaling but also alters the cell shape, increases vascular permeability, and modulates leukocyte trafficking [17, 18]. In fact, PAR-1 induces several adhesion molecules such as P-selectin in endothelial cells, which contribute to the recruitment of inflammatory cells and subsequent secondary damage to vascular tissue [19].
Thrombin is modulated by several factors including activated protein C (APC), which is created when thrombomodulin binds to thrombin and protein C. Unlike thrombin, APC functions as an anti-coagulant but acts through the same PAR-1 receptor. [20–24]. However, subsequent signaling of APC results in opposite effects to that of thrombin. Convergent evidence indicates that APC reduces coagulation and inflammation, stabilizes the vascular barrier, enhances neuroprotection and neurological recovery in experimental models and clinical trials such as stroke, traumatic brain injury, and compressive spinal cord injury [25–29].
The diverse effects of thrombin and APC may reflect “biased agonism” [12] whereby activation of PAR-1 by thrombin or APC involves distinct intracellular signaling cascades [30, 31]. In endothelium, for example, thrombin-PAR-1 activates GTPase RhoA, but not the related protein Rac1 [32], a cascade known to disrupt endothelial barrier integrity [33]. In contrast, APC-PAR-1 activates Rac1, but not RhoA [32] thus enhancing barrier integrity [12, 34].
Here we consider the concept of biased agonism and test the hypothesis that thrombin-PAR-1 activation impedes wound healing and recovery after spinal cord injury, while the same receptor activated by APC improves functional recovery. We compared the leukocyte infiltration, vascular barrier disruption, glial scar formation, axonal sparing, and locomotor recovery between PAR-1 null and wild-type mice. Our results not only implicate PAR-1 in early secondary injury, but also confirm the potential of APC as an effective treatment for spinal cord injury, suggesting that biased agonism can be favorably exploited to improve outcome after spinal cord injury.
Materials and Methods
All procedures involving animals were approved by the UCSF Institutional Animal Care and Use Committee and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. PAR-1 null mice were a generous gift from Dr. Sean Coughlin (Cardiovascular Research Institute, University of California, San Francisco) and bred on a C57Bl/6 background. Adult PAR-1 null mice have been extensively characterized and demonstrate normal physiology [35, 36]. All efforts were made to minimize animal suffering.
Surgery and animal care
Adult male mice (n = 100) weighing between 30 and 35g were anesthetized with 250 mg/kg body weight of 2.5% tribromoethanol. Body temperature was maintained during surgery by means of a warming blanket. Animals were subjected to a moderate spinal cord contusive injury at the mid-thoracic level as we have previously described [37]. Briefly, a laminectomy was performed at the T8 vertebra to expose the spinal cord. The animal was then positioned in a modified contusion device with forceps attached to the spinous processes of T7 and T9. An impactor tip was carefully positioned on the surface of the exposed cord and a 3 g weight was dropped 5 cm onto the impactor tip. Animals, subjected to laminectomy only, served as sham controls. Postoperative care included subcutaneous administration of antibiotics sulfamethoxizole-trimethoprim (0.9 mg/ml) and manual expression of the bladder twice per day.
Histology
Mice were re-anesthetized and transcardially perfused with 4% paraformaldehyde in phosphate buffered saline (PBS) at either 1 or 42 days after injury. The vertebral column was removed and post-fixed in 4% paraformaldehyde for 3 hours at 4°C. Then the spinal cord was extracted and cryoprotected in 20% sucrose in PBS for 3 days at 4°C. A 15-mm length of cord, centered over the impact site was divided into 3 segments of equal length. Each segment was embedded in tissue freezing medium and cut transversely at 20-μm in thickness on a cryostat.
Sections were prepared for immunofluorescence or stained with luxol fast blue. Neutrophils were immunolocalized using a 1:1600 dilution of rat anti-mouse neutrophil antibody (GR1, Caltag, Burlingame, CA) and visualized by a CY3 conjugated goat anti-rat secondary antibody. Astrocytes were localized with mouse anti-glial fibrillary acidic protein (GFAP 1:400, clone G-A-5, G3893; Sigma, St. Louis, MO) and CY3-conjugated mouse anti-GFAP (1:400, clone G-A-5, C9205; Sigma, St. Louis, MO). Endothelium of blood vessels were localized using rat anti-platelet/endothelial cell adhesion molecule-1 (PECAM-1, a.k.a. CD31, 1:400, clone MEC13.3, 550274; BD Biosciences, San Jose, CA). PAR-1 was immunolocalized with goat anti-human PAR-1, recognizing the amino and carboxy termini (1:100, Santa Cruz Biotechnology, Santa Cruz, CA), followed by a 1:200 dilution of biotinylated rabbit anti-goat secondary antibody (Jackson Immunoresearch, Westgrove, PA). Binding was visualized with Fluorescein Avidin D or Texas red-avidin D (1:100 in PBS; Vector Laboratories, Burlingame, CA).
Quantification of neutrophils
Infiltration of neutrophils was assessed at 24 hours after spinal cord injury in each of the genotypes (n = 28). The epicenter of injury was defined by dark field microscopy. Five cross sections of the spinal cord were prepared from each of the 3 regions including the epicenter, 3 mm rostral to the epicenter, and 3 mm caudal to the epicenter. After neutrophil immunostaining, each of these sections was photographed with a 4× objective using a Leica microscope equipped with a CCD camera (SPOT software, model 1.3.0; Diagnostic Instruments, Inc. Sterling Heights, MI) and imported into Stereo Investigator (MicroBrightField, Williston, VT). A contour was drawn closely around the entire section. An observer, blinded to the experimental condition, counted all neutrophils in the section. For accuracy of counting, the images were enlarged to 40× to better identify clustered cells.
Luciferase assay
At 24 hours after surgery, mice (n = 8/group) were re-anesthetized and the integrity of the vascular barrier was quantified as we have previously described [37]. Each mouse was injected with luciferase (Sigma, St. Louis, MO) through the jugular vein (100 μl/30 g body weight). At 25 minutes after the injection, 20 μl of venous blood was obtained and diluted in 80 μl of PBS. At 30 minutes after the injection, each animal was intracardially perfused with PBS and the cord was extracted. Both the cord and blood sample were quickly frozen on dry ice.
A 9 mm length of cord, centered over the lesion epicenter, was divided into 3 segments of equal length and weighed. Each segment was then homogenized in cell lysis buffer at a 1:50 dilution. The homogenate was subjected to centrifugation (8 minutes at 12,000 rpm) and 15 μl of supernatant was collected and incubated in 1,400 μl of cell lysis buffer at room temperature for one hour. Then 15 μl of sample was added to 100 μl of substrate from the Luciferase Assay System (Promega, Madison, WI) and mixed by pipette. The luminosity of the reaction was measured in a TD 20/20 luminometer (Turner Designs, Sunnyvale, CA). Values were expressed as percent of the luminosity, as determined in the blood sample.
Functional assessment
Several tests were used to assess recovery of function in spinal cord injured animals (n = 10/group). These included quantitative assessment of locomotion in an open field and ability to traverse a grid, and qualitative assessment of hindlimb paw position. A trained observer, who was blinded to the animal identity, assessed motor recovery in an open field using the Basso Mouse Scale for locomotion [38] at 1 and 3 days after injury and weekly thereafter for six weeks. Each mouse was videotaped during testing.
For three sequential days starting 35 days after injury, each mouse was tested on a grid of 20 parallel bars with 20-mm spacing and a 30-degree plane of inclination [39]. The mouse was videotaped and evaluated in slow motion by 2 blinded observers who counted each time the mouse intentionally gripped the bar while descending.
Analysis of hindlimb placement was studied in those animals that scored at 4 or higher on the open field testing paradigm. At 42 days after injury, the hind feet were painted with non-toxic paint. The mouse then traversed a Lucite tunnel (60 cm in length) lined with paper. Impressions of pawprints were obtained for each experimental group.
Assessment of white matter sparing
Sparing of white matter, a correlate of motor recovery [40], was quantified in animals euthanized at 42 days post injury (n = 7/group). Every fifth section from the lesion epicenter was stained with 0.1% luxol fast blue in 0.5% acetic acid at 60°C for 3 hours, followed by 0.05% lithium carbonate for 3 minutes to visualize the white matter. The sections were then photographed at 100× magnification and their contours were delineated around the residual white matter as well as the circumference of the cross section to measure the area using the Neurolucida imaging system (MicroBrightField, Williston, VT). The section with the least residual white matter was defined as the epicenter of the injury.
Assessment of the astrocytic scar
Astrocytic scarring was evaluated in sections immunolabeled for GFAP at 42 days post-injury (n = 6/group). The severity of the glial scarring was analyzed by a semi-quantitative method first described by Hsu et al. [40]. Briefly, the transverse section of the injured spinal cord was subdivided into 12 sectors by superimposing a grid over the entire digitized image of the section using Photoshop CS (Adobe Systems Inc., San Jose, CA). In each sector, the complexity of the astrocytic scar was evaluated based on the distribution of astrocytes, arborization and organization of astrocytic processes, astrocytic hypertrophy, and the intensity of GFAP immunoreactivity. Then a score ranging from 0 to 3 was given for each sector. A score of 0 indicates no evidence of glial scar formation, whereas a score of 1 to 3 represents increasing complexity of the glial scarring. Three serial sections at intervals of 480 μm were sampled from the lesion epicenter in each animal. In each sample section, the score obtained from all 12 sectors represented the severity (complexity and/or extent) of the glial scarring and the total score of 3 sample sections was tallied for subsequent comparison between mouse groups.
Administration of APC
Each of the wild-type and PAR-1 null groups was further divided into two subgroups. Based on the dosage of a previous study [41], one subgroup (n = 7) was given recombinant (r) APC (2 mg/kg body weight, Eli Lilly, Indianapolis, IN) dissolved in saline as a single 0.3-ml bolus intravenously 20 minutes after injury, followed immediately by an additional 0.3-ml subcutaneous dose. The other subgroup (n = 7) received saline vehicle the same way to serve as the control group. Motor recovery for mice treated with rAPC or control vehicle was assessed using the Basso Mouse Scale as described above.
Statistical analyses
Limited numbers of animals died during the course of experiments. Two mice died in each of the saline-treated wild-type, APC-treated wild-type, and saline-treated PAR-1 null groups 1 days after the injury. One mouse died in the saline-treated PAR-1 null group 3 days after the injury and in the APC-treated PAR-1 null group 1 day and 3 days, respectively, after the injury. Data obtained from deceased animals were excluded from statistical analyses. To reduce bias, all quantitative measurements were assessed by designated observers blinded to the animal identity, genotypes, and treatments. Then a second researcher knowing the keys that identify animal subjects collected the data for further statistical analyses. Comparisons between PAR-1 null and wild-type groups were made by unpaired Student's t tests to analyze neutrophil infiltration, luciferase permeability, glial scarring, residual white matter, and grid walking, whereas repeated 2-way ANOVA, followed by Bonferroni's post hoc test, was performed for multiple comparisons between groups to evaluate locomotor recovery. The mean values ± SEM were reported. Statistical significance was defined at P ≤ 0.05.
Results
PAR-1 is expressed by neurons and endothelial cells in uninjured cord and is up-regulated in astrocytes after injury
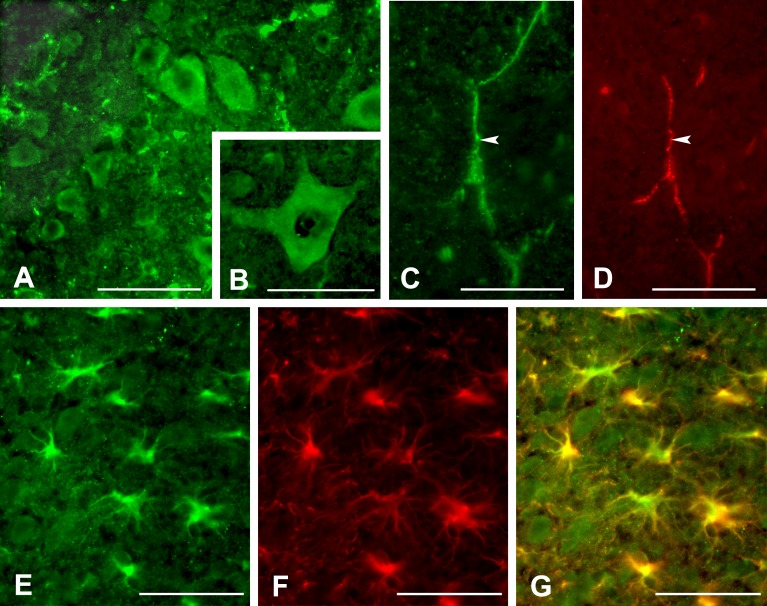
We first identified the cell types that express PAR-1 in the spinal cord of wild-type mice. PAR-1 was immunoexpressed in ventral horn motoneurons and co-localized with PECAM-1, a vascular marker, in the uninjured spinal cord (Fig 1A–1D). Specificity of the anti-PAR-1 antibody was confirmed using both a blocking antibody as well as omission of the primary antibody (data not shown). While PAR-1 was not localized to astrocytes in the uninjured cord, it was expressed in reactive astrocytes, featured by increased GFAP immunoreactivity and hypertrophic cell bodies, at the lesion epicenter by 24 hours post injury (Fig 1E–1G).
Fig 1. Immunolocalization of PAR-1 in the spinal cord of the wild-type mice.
PAR-1 is expressed by neurons in the ventral horn in the uninjured spinal cord (A). At higher magnification, the PAR-1-positive neuron exhibits typical multipolar morphology of the spinal motor neurons (B). PAR-1 (arrow, C) also co-localizes with PECAM-1-positive capillaries (arrow, D) in the uninjured cord. After spinal cord injury, PAR-1 is expressed by reactive astrocytes 24 hours post-injury (E). These reactive astrocytes in the lesion show increased expression of GFAP and hypertrophic morphology (F) as demonstrated in the digitally merged image (G). Scale bars = 100 μm for A, C, D; 50 μm for B, E, F, G.
PAR-1 null mice show reduced neutrophil infiltration and improved vascular integrity after spinal cord injury
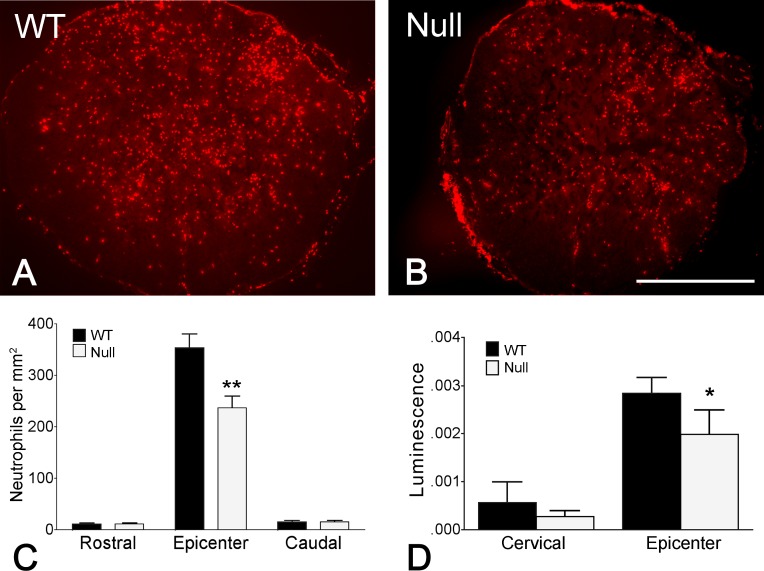
To evaluate the role of PAR-1 in early inflammatory responses and the integrity of the vascular barrier after spinal cord injury, we examined the extent to which neutrophils infiltrated into the cord parenchyma and the permeability of blood vessels in the lesion using both PAR-1 null and wild-type mice. Neutrophils were immunolocalized in the injured cord at 24 hours post injury and were most prominent within the lesion epicenter in both groups of mice (Fig 2A and 2B). The numbers of neutrophils were quantified at the epicenter as well as segments both rostral and caudal to the epicenter. We found that the number of infiltrated neutrophils was significantly higher in the epicenter than in the rostral and caudal segments in both groups of mice (Fig 2C). Remarkably, PAR-1 null mice showed significantly fewer neutrophils within the epicenter as compared to the wild-type group (Fig 2C).
Fig 2. Reduced infiltration of neutrophils and disruption of vascular barrier in the injured spinal cord in PAR-1 null mice.
Aggregation of immunolabled GR1-positive neutrophils is evident in the lesion epicenter in both wild-type (A) and PAR-1 null mice (B) 24 hours after injury. Quantitative analysis reveals that the number of infiltrated neutrophils within the lesion epicenter is significantly lower in the PAR-1 null mice than in the wild-type mice (C). Such a difference in number between the 2 groups of mice is not apparent in segments rostral or caudal to the epicenter. The luciferase luminescence, which inversely represents the integrity of the blood-spinal cord barrier, is significantly decreased at the lesion epicenter in PAR-1 null mice as compared to that of the wild-type mice. (n = 7/genotype, means ± SEM, unpaired Student's t-test, *p < 0.05, **p < 0.01).
We have previously shown that spinal cord injury results in a profound disruption of the blood-spinal cord barrier to the protein luciferase [37]. To determine if PAR-1 is a determinant of this abnormal vascular permeability, we compared barrier disruption to luciferase in wild-type and PAR-1 null mice 24 hours after injury. Luminescence, indicative of blood-spinal cord barrier disruption to luciferase, was greatest within the injury epicenter, as compared to segments rostral to the epicenter in both groups of mice (Fig 2D). Moreover, PAR-1 null mice had significantly lower luminescence in the epicenter than wild-type mice (Fig 2D). Our findings suggest a detrimental role of PAR-1 in promoting early inflammation and vascular barrier breakdown after spinal cord injury.
PAR-1 null mice show better locomotor recovery, more spared white matter, and reduced glial scarring
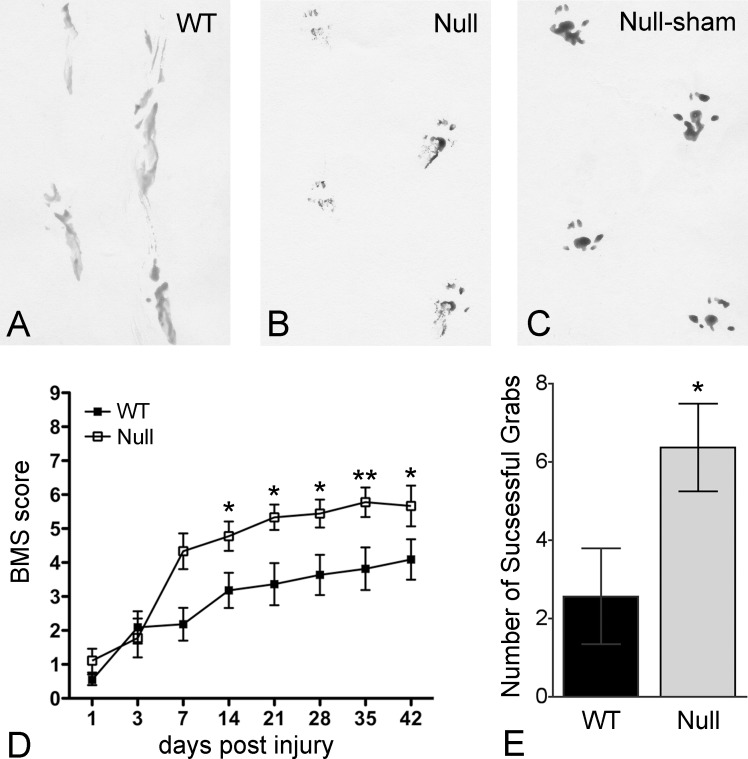
As reduction in infiltrated neutrophils and early stabilization of the vascular barrier has been associated with long-term neurological improvement, we next examined functional recovery after spinal cord injury in both PAR-1 null and wild-type mice using a series of behavioral tests including a pawprint analysis to study the stepping pattern, an open-field testing paradigm, the Basso Mouse Scale [38], to assess locomotion, and an inclined grid to evaluate accurate fine motor control in descending the grid (Fig 3). PAR-1 null mice displayed an improved gait pattern with weight-bearing stepping and coordination as compared to wild-type mice, which showed consistent dragging of hindlimbs in pawprint analysis at 42 days after injury (Fig 3A–3C). Moreover, PAR-1 null mice showed a marked improvement in locomotion beginning 14 days post injury and for the remainder of the testing period (Fig 3D). Performance on an inclined grid was likewise significantly improved in the PAR-1 null group (6.3 ± 1.1 successful grabs) as compared to wild-type group (2.5 ± 1.2 successful grabs) 35 days after injury (Fig 3E).
Fig 3. Improved motor function recovery in PAR-1 null mice after spinal cord injury.
Representative pawprints show the walking patterns of the injured wild-type (A) and PAR-1 null mice (B) 42 days post injury as well as that of the uninjured PAR-1 null mice (C). After spinal cord injury, dragging of the hindlimbs with poor coordination is evident in the wild-type mice, whereas considerable improvement with weight-bearing stepping and slight external rotation of the hindpaws is consistent in the PAR-1 null mice. Injured PAR-1 null mice also show significant locomotor recovery, assessed by the Basso Mouse Scale, as compared to the wild-type mice (D). This result parallels the better performance of PAR-1 null mice on the inclined grid (E). (n = 10/genotype, means ± SEM, 2-way ANOVA for locomotor assessment, unpaired Student's t-test for inclined grid, *p < 0.05, **p < 0.01).
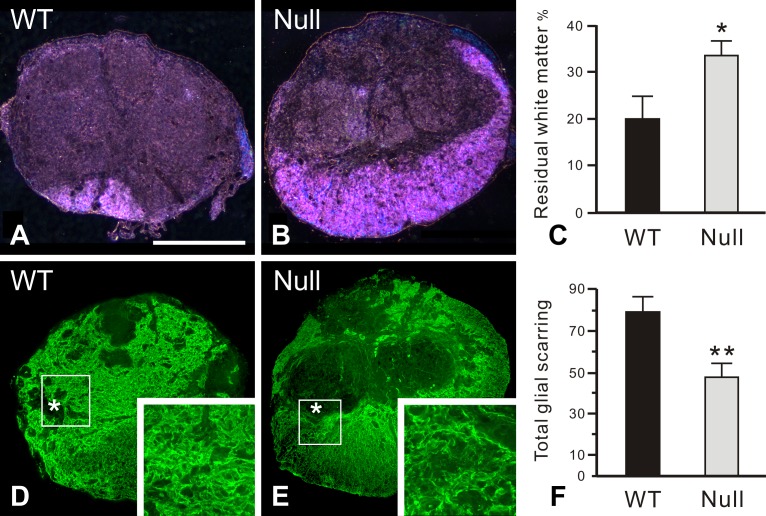
At the completion of the behavioral tests, we further determined the size of the cross-sectional area of the spinal cord and the area of residual white matter stained by luxol fast blue at the lesioned epicenter. Although the cross-sectional areas of the injured cords were comparable between the 2 groups, PAR-1 null mice possessed significantly more residual white matter (34 ± 4%) than the wild-type mice (20 ± 6%) 42 days after injury (Fig 4A–4C).
Fig 4. Enhanced white matter sparing and reduced glial scar formation at the lesion epicenter in PAR-1 null mice 42 days post injury.
Residual white matter is visualized by luxol fast blue staining using dark-field microscopy. Wild-type mice (A) have less residual white matter, mainly located at the ventral-most part of the spinal cord cross section, than the PAR-1 null mice (B). Such a difference in the size of spared white matter is statistically significant (C). The glial scar, characterized by intense GFAP immunoreactivity, is more widespread at the lesion epicenter in the wild-type mice (D) relative to the PAR-1 null mice (E). Boxed areas enclose part of the glial limitans, an interface separating the GFAP-quiescent areas (asterisks) in the lesion epicenter from the residual cord tissue. At higher magnification, more densely entangled astrocytic processes are apparent in the wild-type mice than in the PAR-1 null mice as demonstrated in the insets. As described in Materials and Methods, the quantitative analysis demonstrates that the total score of the glial scarring, which represents the severity of glial scar formation, is significantly lower in the PAR-1 null mice than in the wild-type controls (F). Scale bar = 500 μm. (n = 7 and 5/genotype for the measurement of spared white matter and glial scarring, respectively, means ± SEM, unpaired Student's t-test, *p < 0.05, **p < 0.01).
As PAR-1 was found to be expressed in reactive astrocytes in the injured cord, we next examined the severity of glial scar formation at 42 days after injury by evaluating the complexity and extent of the astrocytic scarring using a semi-quantitative scale. Reactive astrocytes, demonstrated by GFAP immunostaining, aggregated to form a glial scar along the lesion border in both groups of mice (Fig 4D and 4E). In each mouse, scores obtained from 3 sample sections of the spinal cord at intervals of 480 um across the lesion epicenter were totaled for comparison. The result showed that glial scarring was significantly reduced in injured PAR-1 null mice (47 ± 8) than in the wild-type mice (80 ± 9) (Fig 4F). Collectively, our results indicate that PAR-1 deficiency facilitates favorable wound healing after spinal cord injury, evidenced by improved motor function recovery, preservation of white matter, and decreased glial scarring in the lesion.
While treatment with rAPC after spinal cord injury improves locomotor recovery in wild-type mice no change is seen in PAR-1 null mice
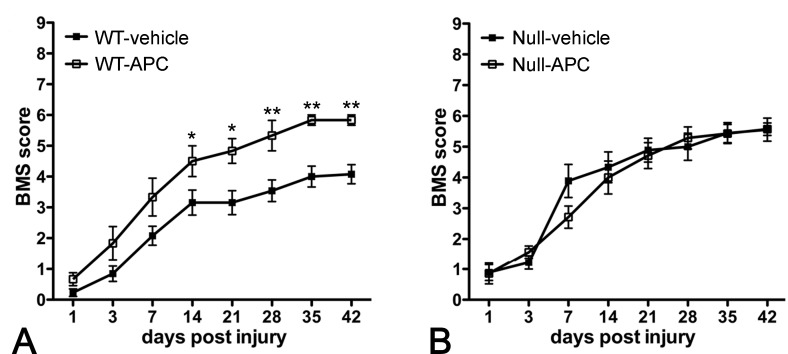
We subsequently asked whether rAPC, given 20 minutes after injury, influences long-term locomotor recovery. We found that administration of rAPC to spinal cord-injured wild-type mice significantly improved locomotor recovery from 14 days after injury onwards as compared to vehicle-treated wild-type controls (Fig 5A). To determine if such benefit, afforded by rAPC treatment, is dependent upon the PAR-1 receptor, we examined locomotor recovery in spinal cord-injured PAR-1 null mice treated with or without rAPC. The results showed that the locomotor scores were comparable between these 2 groups, providing further evidence that PAR-1 is involved in APC-induced functional improvements (Fig 5B). Our findings suggest that APC facilitates locomotor recovery by counteracting PAR-1-mediated secondary injury after spinal cord injury.
Fig 5. Improved locomotor recovery after the treatment with rAPC in spinal cord-injured wild-type mice.
Administration of rAPC 20 minutes after the injury significantly improves locomotor performance of wild-type mice (A), starting from 14 days post injury, to an extent comparable to that of the injured PAR-1 null mice receiving no APC treatment. However, APC shows no additional benefit of functional improvements in injured PAR-1 null mice (B), suggesting that the effectiveness of APC is associated with PAR-1. (n = 10/genotype, means ± SEM, 2-way ANOVA,*p < 0.05, **p < 0.01).
Discussion
The thrombin receptor PAR-1 is critical to a number of wound healing events after spinal cord injury. We demonstrate that spinal cord-injured PAR-1 null mice display reduced leukocyte infiltration, vascular barrier disruption, glial scar formation, and improved white matter sparing and locomotor recovery. We further find that injured wild-type mice treated with rAPC, presumably acting through the same PAR-1 receptor, improves neurological recovery to a similar degree to that of the injured PAR-1 null mice, suggesting that APC is acting through PAR-1 by the property of biased agonism. Together, these findings suggest that the PAR-1 receptor is an effective target for therapeutic intervention after spinal cord injury.
Roles of PAR-1 in wound healing
Here we show that PAR-1 is expressed in neurons and blood vessels within the uninjured adult mouse spinal cord. Neuronal expression, particularly within alpha motoneurons and rat primary spinal afferent neurons, has been reported by others [42, 43]. PAR-1 has likewise been localized in blood vessels in the human brain [44]. Immunoreactivity of PAR-1 increased acutely within 24 hours after spinal cord injury, which is consistent with a rapid 12-fold increase in PAR-1 mRNA in the contused rat spinal cord [13]. This injury-induced PAR-1 immunoreactivity was primarily located in reactive astrocytes, suggesting PAR-1 has a functional role in response to neuropathological states.
Spinal cord injury is characterized by a breakdown in the blood-spinal cord barrier [37], resulting in an exodus of inflammatory cells and indiscriminate extravasation of molecules including plasma proteins to the cord parenchyma, which further damages the neuronal tissue [45, 46]. We have demonstrated reduced disruption of the blood-spinal cord barrier to the protein luciferase following spinal cord contusion in mice lacking the PAR-1 receptor. The dependency of barrier disruption on PAR-1 is mainly linked to thrombin. Research has shown that thrombin-triggered activation of PAR-1 results in disassembly of endothelial adhering junctions, altered endothelial morphology, and formation of inter-endothelial gaps [8, 47], giving rise to compromised barrier to circulating proteins and the evolution of vasogenic edema and neutrophil infiltration [18, 48].
Infiltration of neutrophils signals the early inflammatory response after spinal cord injury, which in turn is detrimental to the vasculature and spinal cord parenchyma, resulting in poor long-term locomotor recovery [28, 49]. This acute inflammation is mediated through PAR-1 via a variety of mechanisms. In addition to disruption of vascular architecture, PAR-1 increases neutrophil infiltration by promoting endothelial expression of surface P-selectin and subsequent leukocyte rolling in venules [50, 51]. PAR-1 also facilitates the release of inflammatory cytokines such as monocyte chemoattractant protein-1 (MCP-1) [52], TNF-alpha, interleukin-6 (IL-6), and IL-8 [53, 54].
After mechanical impact to the spinal cord, acute inflammation soon develops into prolonged secondary injuries, including macrophage infiltration, cell apoptosis, oxidative stress, demyelination, and axonal loss, which are more widespread than the original insult and finally lead to permanent functional disabilities [55, 56]. In injured PAR-1 null mice, reduced neutrophil infiltration early after the injury was closely associated with greater white matter sparing at the chronic stage of the injury, evidenced by the staining of myelinated axons with luxol fast blue. Therefore, significant attenuation of neutrophil infiltration and consequently better preserved white matter axons in PAR-1 null mice confirm the critical role of PAR-1 in de-stabilizing the blood-spinal cord barrier, which enhances early inflammation and ultimately contributes to white matter loss and locomotor deficits. Moreover, research has revealed that PAR-1 is a major suppressor of myelination during development and in adults. Deletion of PAR-1 enhances myelination as a result of elevated extracellular signal-regulated kinase (ERK)1/2 and AKT signaling and consequently more production of myelin basic protein, thicker myelin sheaths, and more myelinated axons in the spinal cord [57].
Whether reduced cell death in motor neurons contributes to better recovery of function in PAR-1 null mice is an intriguing question. Research has showed that the pro-apoptotic marker BCL2-interacting mediator of cell death is significantly lower at the lesion epicenter in PAR-1 null mice than in the wild-type mice 30 days after spinal cord injury [58]. However, more preserved neurons are observed in PAR-1 null mice only in the ventral horn at spinal segments rostral to the lesion epicenter, but not at the epicenter or caudal spinal segments [58]. This finding suggests that motor neurons in the ventral horn at the lesion epicenter are probably equally vulnerable to the contusion and consequent apoptosis in both PAR-1 null and wild-type mice. Therefore, the better recovery of motor function observed in injured PAR-1 null mice is more likely attributable to more spared white matter at the epicenter as demonstrated by our luxol fast blue staining, which has been showed to be the best single measurement for the severity of injury and predictive of motor recovery after contusive spinal cord injury in mice [59].
We found that PAR-1 was not expressed in astrocytes in the uninjured cord, but was up-regulated after a contusion injury primarily in reactive astrocytes with enhanced GFAP expression. After spinal cord injury, reactive astrocytes form a glial scar, which not only physically blocks regenerative axons but also biochemically arrests neurite outgrowth by its expression of growth inhibitory proteoglycans, leading to regenerative failure and defective locomotor function [60]. Here we show that PAR-1 null mice have reduced glial scar formation in the more chronically injured spinal cord, which is consistent with a reduced astrogliotic response to cortical stab wound [61] and spinal cord compression injury [58] in PAR-1 null mice, suggesting that PAR-1 is a determinant of glial scar formation.
In the injured spinal cord, the glial scar is formed by the migration and proliferation of reactive astrocytes around the lesion [62, 63]. Activation of PAR-1 not only mediates astrocytic secretion of anti-apoptotic cytokines in vitro [64] but also stimulates astrocyte proliferation and eventually astrogliosis after brain injury [61, 65]. The mechanisms underlying PAR-1-mediated astrocyte proliferation involve its coupling to multiple G-protein-linked signaling cascades, including Rho kinase, that induce sustained ERK activation [61, 66]. This sustained activation of ERK up-regulates astrocytic cyclin D1, a cell cycle modulator that controls cell proliferation [61]. Moreover, PAR-1 is known to mediate thrombin-induced expression of matrix metalloproteinase-9 [67], a zinc- and calcium-dependent endopeptidase that facilitates cell translocation by proteolytic remodeling of extracellular matrix molecules [68]. We have previously demonstrated that matrix metalloproteinase-9 not only enhances neutrophil infiltration and vascular breakdown [69] but also promotes astrocyte migration and glial scar formation after spinal cord injury [70]. Furthermore, astrocyte reactivity and migration in response to CNS insults is regulated by signal transducer and activator of transcription 3 (STAT3), which is activated by a number of cytokines including IL-6 [71, 72]. Recent research suggests that PAR-1 promotes astrocytic expression of IL-6, which in turn triggers STAT3 signaling cascade, causing enhanced migration of reactive astrocytes and astrogliosis after spinal cord injury [58]. Although the glial scar ultimately becomes an inhibitory barrier that blocks axonal regeneration at the chronic stage of the injury, its formation is considered beneficial at the acute stage by confining inflammation to the lesion epicenter and thus preventing the uninjured neuronal tissue from secondary damage [71, 73, 74]. The expression of PAR-1 in astrocytes, therefore, is conceivable to mediate both glial scar formation and inflammatory response during wound healing after spinal cord injury.
PAR-1, APC, and motor function recovery
PAR-1 null mice showed significant locomotor improvement after spinal cord injury. This result highlights the importance of thrombin-PAR-1 as an upstream modulator of effects that adversely influence locomotor recovery. The mechanisms for improved locomotor recovery in spinal cord-injured PAR-1 null mice are likely significantly decreased inflammation, blood-spinal cord barrier breakdown, and/or glial scarring. As discussed above, the attenuation of early inflammation is possibly the most potent effect improving locomotor recovery because prolonged recruitment of neutrophils is associated with breakdown of the blood-brain barrier and a loss of the myelin sheaths from axons [75]. Similar improvements in both locomotion and myelin sparing have been demonstrated in spinal cord contusion models where early inflammation has been suppressed by anti CD 11d monoclonal antibodies [76]. Moreover, reduced glial scarring tempers the physical and biochemical barriers of astrogliosis to neuronal regeneration. Thus, absence of PAR-1 conceivably nullifies thrombin-induced neurological deficits, leading to substantially improved motor function. However, more research is needed to further explore other possible mechanisms underlying improved functional outcome when PAR-1 is eliminated.
For the first time in the setting of contusive spinal cord injury, we further demonstrated improvement in locomotor function after rAPC treatment in injured wild-type mice. APC and APC analogs have been proven effective in a number of disease states including spinal cord compression [28], spinal cord ischemia [77], traumatic brain injury [78], amyotrophic lateral sclerosis [79, 80], stroke [24, 81–83], murine sepsis [84], ischemic and reperfusion injury of heart [85], kidney, and liver [86], pulmonary, renal, and gastrointestinal inflammation [87], diabetes [88], and lethal body radiation [89]. However, until recently, the mechanism by which APC produced these beneficial effects across diverse pathologic states has not been entirely comprehended.
APC is effective against sepsis because it reduces inflammatory response by decreasing cytokine production, protecting endothelium against injury, and thus inhibiting neutrophil infiltration in mice [20, 90]. In endothelial cells and monocytes, APC reduces the expression of pro-inflammatory cytokines and apoptotic mediators by modulating nuclear factor-κB [91]. Moreover, the cytoprotective actions of APC require its ability to activate PAR-1 [30]. APC-PAR-1-mediated signaling is implicated in direct neuronal protection by inhibiting both the caspase-9 dependent and p53-mediated apoptotic pathways [26, 92]. In stroke models where the thrombolytic therapy of tissue plasminogen activator (tPA) is given, APC improves barrier function and blocks the complication of hemorrhage by inhibiting the activity of matrix metalloproteinase-9 [29, 93], which proteolytically degrades the vascular basement membrane and proteins associated with the blood-brain barrier [94].
We found that APC-treated wild-type mice showed better locomotor function than vehicle-treated wild-type mice, whereas APC-treated PAR-1 null mice exhibited comparable improvement to vehicle-treated PAR-1 null mice after spinal cord injury. These results demonstrated that APC acts on PAR-1, giving rise to beneficial outcome and, therefore, APC renders no additional improvement if PAR-1 is knocked out. Intriguingly, however, deletion of PAR-1 to block thrombin-mediated activation is also beneficial to functional recovery, suggesting a dual, ligand-dependent role of PAR-1 in wound healing after spinal cord injury.
The signaling mechanism by which APC uses the same PAR-1 receptor as thrombin to generate opposite effects has created tremendous interest. APC requires PAR-1 biased signaling to exhibit protective effects in endothelial cells, neurons, and microglia [24, 95]. PAR-1 signaling is initiated by different tethered N-terminal sequences, either the essential thrombin cleavage site Arg41 or the novel APC site Arg46 [96]. APC cleavage of PAR-1 signals B-arrestin-2, phosphatidylinositol 3-kinase/Akt, and Rac1, causing barrier protective and anti-inflammatory effects [30]. In endothelium, APC bound to endothelial protein C receptor (EPCR) uses PAR-1 to activate sphingosine 1-phosphate receptor 1 (S1P1). Cross-activation of S1P1 activates Rac1, leading to Rac1-dependent stabilization of the cytoskeleton [34]. This effect is opposite to the cleavage of PAR-1 by thrombin, which initiates GTPase RhoA and ERK1/2 signaling cascade, causing endothelial barrier disruption and proinflammatory effects [30, 32].
Therefore, biased agonism may explain what initially appears to be conflicting roles for PAR-1 observed in our study [30, 96, 97]. APC-PAR-1 signaling differs from thrombin-PAR-1 signaling in that thrombin cleaves and activates PAR-1 with at least 3 times greater efficiency [23]. Thus, PAR-1 signaling in the injured spinal cord is theoretically biased towards the abundant post-injury thrombin with consequent deleterious effects. When APC is administered following trauma, however, APC prevails and most likely becomes the dominant biased PAR-1 agonist, resulting in better protection of endothelial barrier and improved neurological outcome [98].
Further studies are needed to investigate the protective mechanism of APC-PAR-1 downstream signaling and to evaluate the optimal therapeutic window and pharmokinetics of APC treatment for spinal cord injury.
Conclusion
In conclusion, we have demonstrated a detrimental role for thrombin-activated PAR-1 in wound healing and locomotor recovery after spinal cord injury. Administration of APC counteracts thrombin-PAR-1-induced adverse effects and provides protection. APC has been shown to exert beneficial effects in a spinal cord compression model in the rat [28] as well as in spinal cord ischemia in the rat and rabbit [77, 99]. Together with the current study, APC has now been independently validated in three species using different models of spinal cord injury. Understanding the mechanisms underlying the efficacy of APC provides opportunity for its further refinement with the goal of optimizing long-term neurological outcomes and translating this effort to human clinical trials.
Acknowledgments
The authors are grateful to Dr. Sean Coughlin for providing PAR-1 null mice, Samuel Beckerman for data analyses, and Ilona Garner for editorial assistance.
Data Availability
All relevant data are contained within the paper.
Funding Statement
This study was supported by the U.S. National Institutes of Health/National Institute of Neurological Disorders and Stroke (http://www.ninds.nih.gov) NS39278 (LN), NS43302 (WW), the Roman Reed Fund of California (http://romanreedfoundation.com/) (WW), and the Ministry of Science and Technology, Taiwan (https://www.most.gov.tw/en/public) 101-2320-B-006-008-MY3 (JYH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Krenzlin H, Lorenz V, Danckwardt S, Kempski O, Alessandri B. The Importance of Thrombin in Cerebral Injury and Disease. Int J Mol Sci. 2016;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham DD, Pulliam L, Vaughan PJ. Protease nexin-1 and thrombin: injury-related processes in the brain. Thromb Haemost. 1993;70(1):168–71. Epub 1993/07/01. [PubMed] [Google Scholar]
- 3.Mann KG. Prothrombin and Thrombin In: Colman R, Hirsh J, Marder V, Salzman E, editors. Hemostasis and Thrombosis. 3rd ed. Philadelphia PA, USA: J.B. Lippincott Co.; 1994. p. 184–99. [Google Scholar]
- 4.Francis CW, Markham RE, Barlow GH, Florack TM, Dobrzynski DM, Marder VJ. Thrombin Activity of Fibrin Thrombi and Soluble Plasmic Derivatives. J Lab Clin Med. 1983;102(2):220–30. [PubMed] [Google Scholar]
- 5.Liu CY, Nossel HL, Kaplan KL. Binding of Thrombin by Fibrin. J Biol Chem. 1979;254(20):421–5. [PubMed] [Google Scholar]
- 6.Wilner GD, Danitz MP, Mudd MS, Hsieh KH, Fenton JW. Selective Immobilization of Alpha-Thrombin by Surface-Bound Fibrin. J Lab Clin Med. 1981;97(3):403–11. [PubMed] [Google Scholar]
- 7.Ma L, Dorling A. The roles of thrombin and protease-activated receptors in inflammation. Semin Immunopathol. 2012;34(1):63–72. 10.1007/s00281-011-0281-9 [DOI] [PubMed] [Google Scholar]
- 8.Malik AB, Fenton JW 2nd. Thrombin-mediated increase in vascular endothelial permeability. Semin Thromb Hemost. 1992;18(2):193–9. 10.1055/s-2007-1002425 [DOI] [PubMed] [Google Scholar]
- 9.Coughlin SR. How the protease thrombin talks to cells. P Natl Acad Sci USA. 1999;96(20):11023–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vu TKH, Hung DT, Wheaton VI, Coughlin SR. Molecular-Cloning of a Functional Thrombin Receptor Reveals a Novel Proteolytic Mechanism of Receptor Activation. Cell. 1991;64(6):1057–68. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3(8):1800–14. 10.1111/j.1538-7836.2005.01377.x [DOI] [PubMed] [Google Scholar]
- 12.Adams MN, Ramachandran R, Yau MK, Suen JY, Fairlie DP, Hollenberg MD, et al. Structure, function and pathophysiology of protease activated receptors. Pharmacol Therapeut. 2011;130(3):248–82. [DOI] [PubMed] [Google Scholar]
- 13.Citron BA, Smirnova IV, Arnold PM, Festoff BW. Upregulation of neurotoxic serine proteases, prothrombin, and protease-activated receptor 1 early after spinal cord injury. J Neurotrauma. 2000;17(12):1191–203. 10.1089/neu.2000.17.1191 [DOI] [PubMed] [Google Scholar]
- 14.Hirschberg DL, Yoles E, Belkin M, Schwartz M. Inflammation after Axonal Injury Has Conflicting Consequences for Recovery of Function—Rescue of Spared Axons Is Impaired but Regeneration Is Supported. J Neuroimmunol. 1994;50(1):9–16. [DOI] [PubMed] [Google Scholar]
- 15.Luo W, Wang YF, Reiser G. Protease-activated receptors in the brain: Receptor expression, activation, and functions in neurodegeneration and neuroprotection. Brain Res Rev. 2007;56(2):331–45. 10.1016/j.brainresrev.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 16.Bartha K, Domotor E, Lanza F, Adam-Vizi V, Machovich R. Identification of thrombin receptors in rat brain capillary endothelial cells. J Cerebr Blood F Met. 2000;20(1):175–82. [DOI] [PubMed] [Google Scholar]
- 17.Minami T, Sugiyama A, Wu SQ, Abid R, Kodama T, Aird WC. Thrombin and phenotypic modulation of the endothelium. Arterioscl Throm Vas. 2004;24(1):41–53. [DOI] [PubMed] [Google Scholar]
- 18.Alabanza LM, Bynoe MS. Thrombin induces an inflammatory phenotype in a human brain endothelial cell line. J Neuroimmunol. 2012;245(1–2):48–55. 10.1016/j.jneuroim.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirano K, Kanaide H. Role of protease-activated receptors in the vascular system. J Atheroscler Thromb. 2003;10(4):211–25. [DOI] [PubMed] [Google Scholar]
- 20.Schuepbach RA, Feistritzer C, Fernandez JA, Griffin JH, Riewald M. Protection of vascular barrier integrity by activated protein C in murine models depends on protease-activated receptor-1. Thromb Haemostasis. 2009;101(4):724–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiyagarajan M, Fernandez JA, Lane SM, Griffin JH, Zlokovic BV. Activated Protein C Promotes Neovascularization and Neurogenesis in Postischemic Brain via Protease-Activated Receptor 1. Journal of Neuroscience. 2008;28(48):12788–97. 10.1523/JNEUROSCI.3485-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guitton C, Cottereau A, Gerard N, Quillard T, Chauveau A, Devalliere J, et al. Protective cross talk between activated protein C and TNF signaling in vascular endothelial cells: implication of EPCR, noncanonical NF-kappaB, and ERK1/2 MAP kinases. American journal of physiology Cell physiology. 2011;300(4):C833–42. Epub 2011/01/14. 10.1152/ajpcell.00003.2010 [DOI] [PubMed] [Google Scholar]
- 23.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296(5574):1880–2. 10.1126/science.1071699 [DOI] [PubMed] [Google Scholar]
- 24.Griffin JH, Fernandez JA, Lyden PD, Zlokovic BV. Activated protein C promotes neuroprotection: mechanisms and translation to the clinic. Thromb Res. 2016;141:S62–S4. 10.1016/S0049-3848(16)30368-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Zhao Z, Rege SV, Wang M, Si G, Zhou Y, et al. 3K3A-activated protein C stimulates postischemic neuronal repair by human neural stem cells in mice. Nat Med. 2016. Epub 2016/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, et al. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nature Medicine. 2003;9(3):338–42. 10.1038/nm826 [DOI] [PubMed] [Google Scholar]
- 27.Petraglia AL, Marky AH, Walker C, Thiyagarajan M, Zlokovic BV. Activated Protein C Is Neuroprotective and Mediates New Blood Vessel Formation and Neurogenesis After Controlled Cortical Impact. Neurosurgery. 2010;66(1):165–71. 10.1227/01.NEU.0000363148.49779.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taoka Y, Okajima K. Role of leukocytes in spinal cord injury in rats. J Neurotraum. 2000;17(3):219–29. [DOI] [PubMed] [Google Scholar]
- 29.Mosnier LO, Zlokovic BV, Griffin JH. Cytoprotective-selective activated protein C therapy for ischaemic stroke. Thromb Haemostasis. 2014;112(5):883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood. 2015;125(19):2898–907. 10.1182/blood-2015-02-355974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenakin T, Miller LJ. Seven Transmembrane Receptors as Shapeshifting Proteins: The Impact of Allosteric Modulation and Functional Selectivity on New Drug Discovery. Pharmacol Rev. 2010;62(2):265–304. 10.1124/pr.108.000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo A, Soh UJK, Paing MM, Arora P, Trejo J. Caveolae are required for protease-selective signaling by protease-activated receptor-1. P Natl Acad Sci USA. 2009;106(15):6393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Science's STKE: signal transduction knowledge environment. 2007;2007(412):re8 Epub 2007/11/15. 10.1126/stke.4122007re8 [DOI] [PubMed] [Google Scholar]
- 34.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105(8):3178–84. 10.1182/blood-2004-10-3985 [DOI] [PubMed] [Google Scholar]
- 35.Connolly AJ, Ishihara H, Kahn ML, Farese RV, Coughlin SR. Role of the thrombin receptor In development and evidence for a second receptor. Nature. 1996;381(6582):516–9. 10.1038/381516a0 [DOI] [PubMed] [Google Scholar]
- 36.Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, et al. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. P Natl Acad Sci USA. 2003;100(22):13019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whetstone WD, Hsu JYC, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: Relation to revascularization and wound healing. J Neurosci Res. 2003;74(2):227–39. 10.1002/jnr.10759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso mouse scale for locomotion detects differences in recovery after spinal cord in ury in five common mouse strains. J Neurotraum. 2006;23(5):635–59. [DOI] [PubMed] [Google Scholar]
- 39.Fiore C, Inman DM, Hirose S, Noble LJ, Igarashi T, Compagnone NA. Treatment with the neurosteroid dehydroepiandrosterone promotes recovery of motor behavior after moderate contusive spinal cord injury in the mouse. J Neurosci Res. 2004;75(3):391–400. 10.1002/jnr.10821 [DOI] [PubMed] [Google Scholar]
- 40.Hsu JYC, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. Journal of Neuroscience. 2006;26(39):9841–50. 10.1523/JNEUROSCI.1993-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gresele P, Momi S, Berrettini M, Nenci GG, Schwarz HP, Semeraro N, et al. Activated human protein C prevents thrombin-induced thromboembolism in mice—Evidence that activated protein C reduces intravascular fibrin accumulation through the inhibition of additional thrombin generation. J Clin Invest. 1998;101(3):667–76. 10.1172/JCI575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Festoff BM, D'Andrea MR, Citron BA, Salcedo RM, Smirnova IV, Andrade-Gordon P. Motor neuron cell death in wobbler mutant mice follows overexpression of the G-protein-coupled, protease-activated receptor for thrombin. Mol Med. 2000;6(5):410–29. [PMC free article] [PubMed] [Google Scholar]
- 43.Niclou SP, Suidan HS, Pavlik A, Vejsada R, Monard D. Changes in the expression of protease-activated receptor 1 and protease nexin-1 mRNA during rat nervous system development and after nerve lesion. Eur J Neurosci. 1998;10(5):1590–607. [DOI] [PubMed] [Google Scholar]
- 44.Domotor E, Benzakour O, Griffin JH, Yule D, Fukudome K, Zlokovic BV. Activated protein C alters cytosolic calcium flux in human brain endothelium via binding to endothelial protein C receptor and activation of protease activated receptor-1. Blood. 2003;101(12):4797–801. 10.1182/blood-2002-12-3680 [DOI] [PubMed] [Google Scholar]
- 45.Noble LJ, Wrathall JR. Distribution and Time Course of Protein Extravasation in the Rat Spinal-Cord after Contusive Injury. Brain Research. 1989;482(1):57–66. [DOI] [PubMed] [Google Scholar]
- 46.Popovich PG, Horner PJ, Mullin BB, Stokes BT. A quantitative spatial analysis of the blood spinal cord barrier .1. Permeability changes after experimental spinal contusion injury. Experimental Neurology. 1996;142(2):258–75. 10.1006/exnr.1996.0196 [DOI] [PubMed] [Google Scholar]
- 47.Kouklis P, Konstantoulaki M, Vogel S, Broman M, Malik AB. Cdc42 regulates the restoration of endothelial barrier function. Circ Res. 2004;94(2):159–66. 10.1161/01.RES.0000110418.38500.31 [DOI] [PubMed] [Google Scholar]
- 48.de Garavilla L, Vergnolle N, Young SH, Ennes H, Steinhoff M, Ossovskaya VS, et al. Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br J Pharmacol. 2001;133(7):975–87. 10.1038/sj.bjp.0704152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trivedi A, Olivas AD, Noble-Haeusslein LJ. Inflammation and Spinal Cord Injury: Infiltrating Leukocytes as Determinants of Injury and Repair Processes. Clinical neuroscience research. 2006;6(5):283–92. Epub 2007/12/07. PubMed Central PMCID: PMCPMC1864937. 10.1016/j.cnr.2006.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmerman BJ, Paulson JC, Arrhenius TS, Gaeta FCA, Granger DN. Thrombin Receptor Peptide-Mediated Leukocyte Rolling in Rat Mesenteric Venules—Roles of P-Selectin and Sialyl-Lewis-X. Am J Physiol. 1994;267(3):H1049–H53. [DOI] [PubMed] [Google Scholar]
- 51.Sugama Y, Malik AB. Thrombin Receptor-14 Amino-Acid Peptide Mediates Endothelial Hyperadhesivity and Neutrophil Adhesion by P-Selectin Dependent Mechanism. Circulation Research. 1992;71(4):1015–9. [DOI] [PubMed] [Google Scholar]
- 52.Colotta F, Sciacca FL, Sironi M, Luini W, Rabiet MJ, Mantovani A. Expression of Monocyte Chemotactic Protein-1 by Monocytes and Endothelial-Cells Exposed to Thrombin. Am J Pathol. 1994;144(5):975–85. [PMC free article] [PubMed] [Google Scholar]
- 53.Lang R, Song PI, Legat FJ, Lavker RM, Harten B, Kalden H, et al. Human corneal epithelial cells express functional PAR-1 and PAR-2. Invest Ophth Vis Sci. 2003;44(1):99–105. [DOI] [PubMed] [Google Scholar]
- 54.Vergnolle N, Hollenberg MD, Wallace JL. Pro- and anti-inflammatory actions of thrombin: a distinct role for proteinase-activated receptor-1 (PAR(1)). Brit J Pharmacol. 1999;126(5):1262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papastefanaki F, Matsas R. From demyelination to remyelination: the road toward therapies for spinal cord injury. Glia. 2015;63(7):1101–25. Epub 2015/03/04. 10.1002/glia.22809 [DOI] [PubMed] [Google Scholar]
- 56.Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of Secondary Spinal Cord Injury. Frontiers in cellular neuroscience. 2016;10:98 Epub 2016/05/06. PubMed Central PMCID: PMCPMC4829593. 10.3389/fncel.2016.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoon H, Radulovic M, Drucker KL, Wu JM, Scarisbrick IA. The Thrombin Receptor Is a Critical Extracellular Switch Controlling Myelination. Glia. 2015;63(5):846–59. 10.1002/glia.22788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radulovic M, Yoon H, Wu J, Mustafa K, Scarisbrick IA. Targeting the thrombin receptor modulates inflammation and astrogliosis to improve recovery after spinal cord injury. Neurobiology of disease. 2016;93:226–42. Epub 2016/05/05. PubMed Central PMCID: PMCPMC4930708. 10.1016/j.nbd.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noble LJ, Wrathall JR. Spinal-Cord Contusion in the Rat—Morphometric Analyses of Alterations in the Spinal-Cord. Experimental Neurology. 1985;88(1):135–49. [DOI] [PubMed] [Google Scholar]
- 60.Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. Epub 2014/01/16. PubMed Central PMCID: PMCPMC3951813. 10.1016/j.expneurol.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicole O, Goldshmidt A, Hamill CE, Sorensen SD, Sastre A, Lyuboslavsky P, et al. Activation of protease-activated receptor-1 triggers astrogliosis after brain injury. Journal of Neuroscience. 2005;25(17):4319–29. 10.1523/JNEUROSCI.5200-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–56. Epub 2004/01/22. 10.1038/nrn1326 [DOI] [PubMed] [Google Scholar]
- 63.Rhodes KE, Moon LD, Fawcett JW. Inhibiting cell proliferation during formation of the glial scar: effects on axon regeneration in the CNS. Neuroscience. 2003;120(1):41–56. [DOI] [PubMed] [Google Scholar]
- 64.Wang YF, Luo WB, Stricker R, Reiser G. Protease-activated receptor-1 protects rat astrocytes from apoptotic cell death via JNK-mediated release of the chemokine GRO/CINC-1. J Neurochem. 2006;98(4):1046–60. 10.1111/j.1471-4159.2006.03950.x [DOI] [PubMed] [Google Scholar]
- 65.Traynelis SF, Trejo J. Protease-activated receptor signaling: new roles and regulatory mechanisms. Curr Opin Hematol. 2007;14(3):230–5. 10.1097/MOH.0b013e3280dce568 [DOI] [PubMed] [Google Scholar]
- 66.Wang H, Ubl JJ, Stricker R, Reiser G. Thrombin (PAR-1)-induced proliferation in astrocytes via MAPK involves multiple signaling pathways. Am J Physiol-Cell Ph. 2002;283(5):C1351–C64. [DOI] [PubMed] [Google Scholar]
- 67.Yang CC, Hsiao LD, Yang CM, Lin CC. Thrombin Enhanced Matrix Metalloproteinase-9 Expression and Migration of SK-N-SH Cells via PAR-1, c-Src, PYK2, EGFR, Erk1/2 and AP-1. Molecular neurobiology. 2016. Epub 2016/05/18. [DOI] [PubMed] [Google Scholar]
- 68.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. 10.1146/annurev.cellbio.17.1.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22(17):7526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsu JY, Bourguignon LY, Adams CM, Peyrollier K, Zhang H, Fandel T, et al. Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J Neurosci. 2008;28(50):13467–77. 10.1523/JNEUROSCI.2287-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herrmann JE, Imura T, Song BB, Qi JW, Ao Y, Nguyen TK, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. Journal of Neuroscience. 2008;28(28):7231–43. 10.1523/JNEUROSCI.1709-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nature Medicine. 2006;12(7):829–34. 10.1038/nm1425 [DOI] [PubMed] [Google Scholar]
- 73.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. Journal of Neuroscience. 2004;24(9):2143–55. 10.1523/JNEUROSCI.3547-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferrari CC, Depino AM, Prada F, Muraro N, Campbell S, Podhajcer O, et al. Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Pathol. 2004;165(5):1827–37. 10.1016/S0002-9440(10)63438-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gris D, Marsh DR, Oatway MA, Chen YH, Hamilton EF, Dekaban GA, et al. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. Journal of Neuroscience. 2004;24(16):4043–51. 10.1523/JNEUROSCI.5343-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamauchi T, Sakurai M, Abe K, Takano H, Sawa Y. Neuroprotective effects of activated protein C through induction of insulin-like growth factor-1 (IGF-1), IGF-1 receptor, and its downstream signal phosphorylated serine-threonine kinase after spinal cord ischemia in rabbits. Stroke. 2006;37(4):1081–6. 10.1161/01.STR.0000206280.30972.21 [DOI] [PubMed] [Google Scholar]
- 78.Walker CT, Marky AH, Petraglia AL, Ali T, Chow N, Zlokovic BV. Activated protein C analog with reduced anticoagulant activity improves functional recovery and reduces bleeding risk following controlled cortical impact. Brain Research. 2010;1347:125–31. 10.1016/j.brainres.2010.05.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhong ZH, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119(11):3437–49. 10.1172/JCI38476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma QY, Zuniga E, et al. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. P Natl Acad Sci USA. 2014;111(11):E1035–E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang YM, Zhao Z, Chow N, Ali T, Griffin JH, Zlokovic BV. Activated protein C analog promotes neurogenesis and improves neurological outcome after focal ischemic stroke in mice via protease activated receptor 1. Brain Research. 2013;1507:97–104. 10.1016/j.brainres.2013.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo H, Singh I, Wang YM, Deane R, Barrett T, Fernandez JA, et al. Neuroprotective activities of activated protein C mutant with reduced anticoagulant activity. Eur J Neurosci. 2009;29(6):1119–30. 10.1111/j.1460-9568.2009.06664.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Wang YM, Thiyagarajan M, Chow N, Singh I, Guo H, Davis TP, et al. Differential Neuroprotection and Risk for Bleeding From Activated Protein C With Varying Degrees of Anticoagulant Activity. Stroke. 2009;40(5):1864–9. 10.1161/STROKEAHA.108.536680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant-activated protein C. J Exp Med. 2007;204(10):2439–48. 10.1084/jem.20070404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loubele STBG, Spek CA, Leenders P, van Oerle R, Aberson HL, Hamulyak K, et al. Activated Protein C Protects Against Myocardial Ischemia/Reperfusion Injury via Inhibition of Apoptosis and Inflammation. Arterioscl Throm Vas. 2009;29(7):1087–U159. [DOI] [PubMed] [Google Scholar]
- 86.Park SW, Chen SWC, Kim M, D'Agati VD, Lee HT. Human activated protein C attenuates both hepatic and renal injury caused by hepatic ischemia and reperfusion injury in mice. Kidney Int. 2009;76(7):739–50. 10.1038/ki.2009.255 [DOI] [PubMed] [Google Scholar]
- 87.Esmon CT. Protein C anticoagulant system-anti-inflammatory effects. Semin Immunopathol. 2012;34(1):127–32. 10.1007/s00281-011-0284-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nature Medicine. 2007;13(11):1349–58. 10.1038/nm1667 [DOI] [PubMed] [Google Scholar]
- 89.Geiger H, Pawar SA, Kerschen EJ, Nattamai KJ, Hernandez I, Liang HPH, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nature Medicine. 2012;18(7):1123–+. 10.1038/nm.2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lay AJ, Donahue D, Tsai MJ, Castellino FJ. Acute inflammation is exacerbated in mice genetically predisposed to a severe protein C deficiency. Blood. 2007;109(5):1984–91. 10.1182/blood-2006-07-037945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joyce DE, Grinnell BW. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappa B. Crit Care Med. 2002;30(5):S288–S93. [DOI] [PubMed] [Google Scholar]
- 92.Liu D, Cheng T, Guo H, Fernandez JA, Griffin JH, Song XM, et al. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nature Medicine. 2004;10(12):1379–83. 10.1038/nm1122 [DOI] [PubMed] [Google Scholar]
- 93.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12(11):1278–85. 10.1038/nm1498 [DOI] [PubMed] [Google Scholar]
- 94.Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36(9):1954–9. 10.1161/01.STR.0000177517.01203.eb [DOI] [PubMed] [Google Scholar]
- 95.Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34(4):198–209. 10.1016/j.tins.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mosnier LO, Sinha RK, Burnier L, Bouwens EA, Griffin JH. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood. 2012;120(26):5237–46. 10.1182/blood-2012-08-452169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schuepbach RA, Madon J, Ender M, Galli P, Riewald M. Protease-activated receptor-1 cleaved at R46 mediates cytoprotective effects. J Thromb Haemost. 2012;10(8):1675–84. 10.1111/j.1538-7836.2012.04825.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schuepbach RA, Feistritzer C, Brass LF, Riewald M. Activated protein C-cleaved protease activated receptor-1 is retained on the endothelial cell surface even in the presence of thrombin. Blood. 2008;111(5):2667–73. 10.1182/blood-2007-09-113076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hirose K, Okajima K, Taoka Y, Uchiba M, Tagami H, Nakano K, et al. Activated protein C reduces the ischemia/reperfusion-induced spinal cord injury in rats by inhibiting neutrophil activation. Ann Surg. 2000;232(2):272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are contained within the paper.