Abstract
Asian dust or yellow sand events in East Asia are a major issue of environmental contamination and human health, causing increasing concern. A high amount of dust particles, especially called as particulate matter 10 (PM10), is transported by the wind from the arid and semi-arid tracks to the Korean peninsula, bringing a bacterial population that alters the terrestrial and atmospheric microbial communities. In this study, we aimed to explore the bacterial populations of Asian dust samples collected during November–December 2014. The dust samples were collected using the impinger method, and the hypervariable regions of the 16S rRNA gene were amplified using PCR followed by pyrosequencing. Analysis of the sequencing data were performed using Mothur software. The data showed that the number of operational taxonomic units and diversity index during Asian dust events were higher than those during non-Asian dust events. At the phylum level, the proportions of Proteobacteria, Actinobacteria, and Firmicutes were different between Asian dust and non-Asian dust samples. At the genus level, the proportions of the genus Bacillus (6.9%), Arthrobacter (3.6%), Blastocatella (2%), Planomicrobium (1.4%) were increased during Asian dust compared to those in non-Asian dust samples. This study showed that the significant relationship between bacterial populations of Asian dust samples and non-Asian dust samples in Korea, which could significantly affect the microbial population in the environment.
Introduction
Asian dust events in East Asia, including Korea and Japan, are a seasonal phenomenon, mostly in the early spring that influences the airborne environment and human health problems [1–3]. Asian dust is known to originate from several arid regions of China, including the Gobi desert and the Taklamakan desert, and the dust particles affect the surrounding countries, including China, Japan, and Korea [4–6], as well as Greenland and North America through the global transport of Asian dust particles [7–9]. Moreover, Asian dust events have increased from 77 days containing 8 days during winter (Dovember to February) in 1990s to 122 days containing 21 days during winter in 2000s (Korea meteorological administrate, KMA).
During Asian dust events, several hundred times the normal level of dust particles are present in the airborne environment, and the dust particles contain various chemical components as well as microorganisms [10–13]. Previous studies have shown that an increasing concentration of the dust particles is associated with various human health problems and several chemical components or heavy metals found in the dust particles are known to contribute various human diseases [14, 15]. However, a report by Kim et al. showed that the airborne Hg concentration was not different during Asian dust events [16], and He et al. showed that heat-treated Asian dust samples did not induce allergic lung inflammation compared to normal dust samples, suggesting that unknown microbial materials could contribute some risk to human health [17]. In recent years, the chemical compositions of Asian dust particles have been identified comparatively, but the microbial community structure of the dust particles is not yet understood. Only limited studies have reported that the bacterial communities in an airborne environment were altered during Asian dust events, and exposure to some of the microorganisms could cause some human health problems [18–20]. These studies have provided a biological feature of Asian dust particles, and are useful researches to investigate environmental effects of Asian dust events. However, since an Asian dust event is a seasonal phenomenon and bacterial communities in Asian dust particles are influenced by a variety of conditions, including origin region, sampling method, sampling method, and period, an accumulation of bacterial information through a continuous monitoring is important to understand an alteration of bacterial community structure during Asian dust events.
In this study, we aimed to investigate an alteration of bacterial communities during Asian dust and non-Asian dust events in December 2014, South Korea, and focused to determine a detailed taxonomic feature. For the characterization of airborne bacterial structure, we performed a metagenomics analysis using the next-generation sequencing method to overcome the limitations of culture-based techniques, which can only analyze culturable bacteria in a specific kind of medium. We analyzed bacterial communities and diversity features from the Asian dust and non-Asian dust events, and statistically investigated different phyla and genera between the two events. These results will be used to understand the alteration of bacterial communities by transported dust particles during Asian dust events and to provide information on airborne bacteria during the period November to December, 2014 in South Korea.
Materials and Methods
Collection of air samples
Asian dust and non-Asian dust event samples were collected on the rooftop of Dongguk University in Seoul, South Korea (GPS; 37.557 N, 127.002 E). No other commercial buildings surround the sampling building and it is free of the influence of other pollutant contamination. A liquid impinger connected with an aspirator was used to collect the dust samples on the rooftop (25 m height) of the building. The dust samples were collected in 300 ml of phosphate-buffered saline (PBS) for 10 to 24 hours (flow rate is 20 L/min). Asian dust event samples were collected from December 1 to December 2, 2014 and the non-Asian dust event samples were collected on November 26 and December 3, 2014 for comparison. The water-insoluble bioaerosols were filtered using a polycarbonate filter membrane and used for the analysis [21].
Environmental information and backward trajectories of air during the sampling period
Meteorological information, including humidity, temperature, and particulate matter 10 (PM10) concentration, was obtained from the Korea meteorological administration (KMA). For the backward trajectory analysis of transported dust particles, the hybrid single-particle Lagrangian integrated trajectory (HYSPLIT) model was used [22, 23]. Global data assimilation system (GDAS) data provided by national centers for environmental prediction (NCEP) were used as meteorological prediction information for the trajectory analysis. The starting point of the backward trajectory analysis was the sampling location (GPS; 37.557 N, 127.002 E), and the starting time was 15 UTC (00 Korea standard time). To track the source of dust particles during the Asian dust and non-Asian dust events, 48 h backward trajectories were calculated at the height of 500 m.
DNA isolation
After filtering the collected bioaerosol through a polycarbonate filter membrane, half of the membrane was used to extract genomic DNA. The genomic DNA was extracted by a previously described method [24]. In brief, half of the membrane was mixed with DNA extraction buffer (100 mM Tris-Cl, pH 8.0; 100 mM sodium-EDTA, pH 8.0; 100 mM sodium phosphate, pH 8.0; 1.5 M NaCl; 1% CTAB] and 1 mg of proteinase K, followed by incubation with shaking at 37°C for 30 min. Then, the mixture was incubated with 2% SDS at 65°C for 30 min. After centrifugation, the supernatants were mixed with an equal volume of chloroform:isoamylalcohol (24:1, v/v). The aqueous layer was mixed with 70% isopropanol and the precipitated DNA pellet was washed with 70% ethanol. The genomic DNA pellet was dried and resuspended with distilled water.
Pyrosequencing and analysis
For amplification of the 16S rRNA gene region from the extracted DNA, a set of forward and reverse primers was constructed according to a previous report [25]. The forward primer sequence is 517F of the 16S rRNA gene region, GCCAGCAGCCGCGGTAAT, and the reverse primer sequence is 896R of the 16S rRNA gene region, CCGTACTCCCCAGGCGG. The region contains the fourth and fifth hypervariable regions of 16S rRNA gene sequences. For pyrosequencing, proper adaptor and multiplex identifier tag sequences provided by the manufacturer’s instructions (Macrogen, Korea) were conjugated with primer sequences. PCR was performed by following conditions: pre-denaturation at 95°C for 3 min, 35 cycles of denaturation at 95°C for 3 s, and annealing and extension at 64°C for 15 s. After amplification of the 16S rRNA gene region, equal amounts of each PCR product (30 ng) were collected in a new tube and pyrosequencing processes were performed by a GS FLX system (Roche, Switzerland). The sequences were submitted into the INSDC SRA (S1 File, BioProject and SRA accession numbers are PRJNA360153 and SRP096119, respectively). For analysis of the pyrosequencing results, we performed a standard procedure using Mothur software [26]. Briefly, trim.seq command was used to remove short and low quality sequences. Since the length of amplified PCR product had more than 300 bp, a threshold length of sequence was determined as more than 200 bp. A threshold of quality score value was determined by a default value in Mothur software (qwindowaverage value in trim.seq command is 35). After trimming the unnecessary sequences, chimera.uchime command was used to remove potentially chimeric sequences. The total read numbers for November 26 and December 1 to December 3 were 7269, 7119, 7873, and 8483, respectively. Bacterial classification and statistical analysis were performed using the SILVA database (release 119) [27] and Metastats software, respectively [28]. For calculating diversity indices, including OTU [29], Chao [30], and inversed simpson index [31], a taxonomic data-based phylotype analysis at species level was performed using Mothur software. Briefly, the bacterial classification data (taxonomy files in Mothur software) analyzed by the SILVA database were used to process phylotype command. We used a phylotype analysis instead of cutoff-based clustering, because several sequences were classified into a same taxonomic name even though the sequences had a different OTU at cutoff value of 0.03. In the present study, since we focused the respective taxonomic information for identifying a taxonomic characteristic between Asian dust and non-Asian dust events, the taxonomic data-based phylotype analysis was used. After producing shared files at species level using phylotype command, the files were used to analyze diversity indices, including OTU, Chao, and inversed simpson index. Bacterial proportion results and statistical data were analyzed using shared files at phylum and genus level.
Results and Discussion
Environmental conditions during the sampling period
In order to determine any alterations to the bacterial community during Asian dust events, we collected airborne samples on November 26 and from December 1 to December 3. The relative humidity during the sampling period varied randomly around 57.6–79.3% and the temperature was about -2.7–6°C. No precipitation occurred during sampling period. The backward trajectory analysis showed that the air mass on November 26, 2014 originated from the East Sea. Meanwhile the air mass during December 1 to December 3 came from the desert area of Mongolia, passed over China, and arrived at the Korean peninsula for 2 days (HYSPLIT, data not shown). As shown in Fig 1, the PM10 concentration increased from December 1, and was more than 200 μg/m3 at 03:00–07:00 (Korea standard time, KST, UTC + 09) on December 2. Based on the hourly averaged PM10 concentration obtained from KMA and backward trajectory analysis, the collected airborne samples were classified into two group, Asian dust (December 1 and December 2) and non-Asian dust (November 26 and December 3). In this study, there are some different sampling durations between December 2 and other days. We, however, cautiously suggests that the different duration may not affect the following results since there are a similar biological characteristic between December 1 and 2. In addition, we performed SourceTracker software to confirm whether the samples were contaminated by other sources during sampling or following processes. Human microbiome project (HMP) data was used to compare a proportion of each sample under different source condition. In case of non-Asian dust as source, Asian dust samples had a relative high proportions, 0.8081 (December 1) and 0.7419 (December 2), but HMP data revealed clearly low proportion (0.1048). Moreover, the proportions of non-Asian dust samples (0.9007 in November 26) and 0.9564 in December 3) are clearly higher than HMP data (0.1876) in case of Asian dust as source.
Fig 1. Concentration of PM10 during Asian dust and non-Asian dust events.
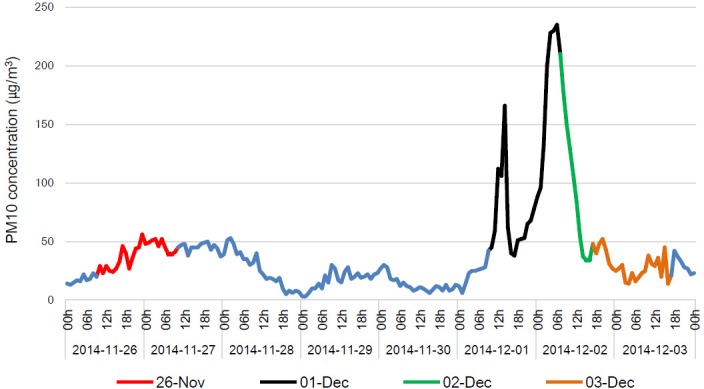
Indicated date presents sampling time and the time is Korea standard time, KST (UTC + 09).
Diversity analysis of the bacterial communities in the Asian dust and non-Asian dust event samples
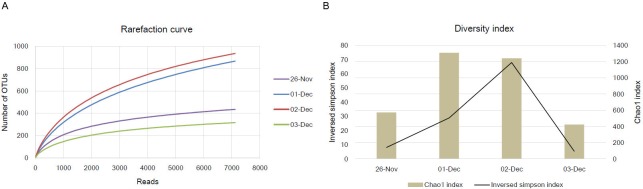
After pyrosequencing of amplified 16S rRNA gene products obtained from November 26 and December 1 to December 3, we analyzed the sequences using the standard operating procedure of Mothur software. For normalization of each sample, we randomly selected 7119 sequences presented the lowest sequences among each sample. To analyze an alteration of the diversity index caused by newly transported dust particles containing variable microorganisms during the Asian dust events, we first determined operational taxonomic units (OTUs) of each sample at the species level. As shown in Fig 2A, the number of OTUs in the Asian dust sample were higher than those in the non-Asian dust sample (t-test, P = 0.02). In addition, the Chao1 index and the inversed Simpson index, which present a diversity index, were also increased in the Asian dust event samples (Fig 2B). Each diversity index was 2.55 times (t-test, P = 0.044) and 7.22 times (t-test, P = 0.13) higher than that for the non-Asian dust event samples. In a previous study, Jeon et al. also show that the diversity index, Chao1, of Asian dust event samples was 2.63 times higher than non-Asian dust event samples [32]. Indeed, while we expected that both richness (OTUs and Chao1) and evenness (Simpson) indices had a significantly difference value between bacterial communities of Asian dust and non-Asian dust, only richness indices were significantly higher in the Asian dust samples. However, we observed a consistent result in the other period (in submission), suggesting that while various kind of microorganisms were transported by Asian dust particles, several taxonomic groups were presented as a relatively higher proportion in the dust particles. Beside the concerned issue, these results mean that newly transported microorganisms are present in the airborne environment during Asian dust events, leading to an alteration of the airborne microbial community compared to its normal status.
Fig 2. Rarefaction curves and diversity index of the Asian dust and non-Asian dust event samples.
Characterization of bacterial community at the phylum level
Through the diversity analysis, we confirmed that the airborne microbial environment was affected by transported dust particles, which probably contain various microorganisms, during Asian dust events. We therefore classified the sequences obtained from pyrosequencing data according to the SILVA database. As a result, a total of 19 phyla were classified and several predominant phyla (more than 1% of total bacterial community) are presented in Fig 3 and Table 1. From the results, we confirmed that the proportion of phylum Proteobacteria in the non-Asian dust samples was 1.6 times higher than the Asian dust samples. Meanwhile, the proportions of various phyla, including Actinobacteria, Acidobacteria, Firmicutes, and Gemmatimonadetes, were increased compared to those in the non-Asian dust event samples. To determine which phyla have a statistical difference between the two groups, we performed Metastats analysis using Mothur software. As shown in Table 2, the proportions of phyla Proteobacteria, Actinobacteria, and Firmicutes were significantly different between the two groups. In the Asian dust event samples, the phylum Proteobacteria was 0.62 times lower than in the non-Asian dust samples, and phyla Actinobacteria and Firmicutes were increased by 2.8 times and 5.6 times, respectively. These results mean that the proportion of the predominantly presented phylum, Proteobacteria, in the airborne environment was reduced with an increasing influx of newly transported microorganisms during Asian dust events. The influx of phyla Actinobacteria and Firmicutes during Asian dust events was also reported in previous work [32, 33]. As similar with this results, the phylum Proteobacteria was predominantly presented during non-Asian dust events (53.1%), while Firmicutes presented as a relatively low proportion. The proportions of phyla Proteobacteria and Firmicues were changed into 15% and 53%, but the proportion of Actinobacteria was rarely altered during Asian dust events [32]. In addition, Yamaguchi et al. showed that the phylum Actinobacteria was largely present in the Asian dust event samples as well as in several source soils of Asian dust, including the Gobi desert and the Taklamakan desert [34]. Although there are several difference between these studies, Proteobacteria is one of predominant phylum during normal days, and proportions of Firmicutes and Actinobacteria were mainly altered during Asian dust events. However, since environmental conditions, sampling methods, and regions are different in each research, it is important to accumulate various database for understanding biological features of Asian dust particles.
Fig 3. Bacterial communities (%) at the phylum level in the Asian dust and non-Asian dust event samples.
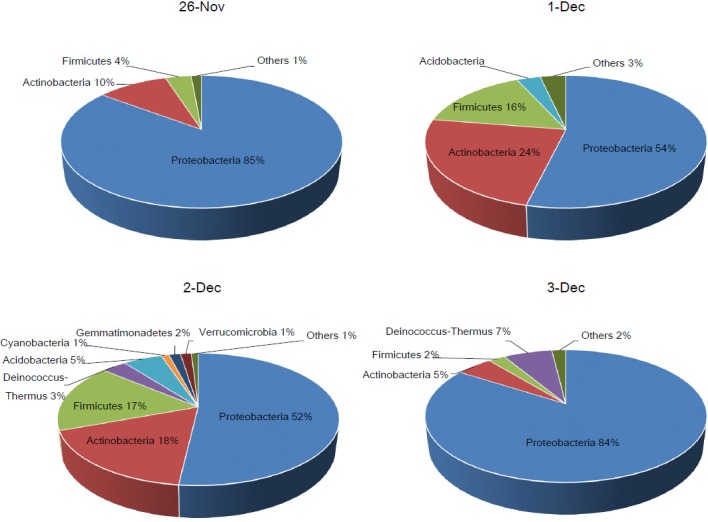
Other phyla present lower than 1% of total bacteria community.
Table 1. Bacterial communities (%) at the phylum level in the Asian dust and non-Asian dust event samples.
| Phylum | Bacterial communities (%) | |||
|---|---|---|---|---|
| 26-Nov (non-Asian dust) | 1-Dec (Asian dust) | 2-Dec (Asian dust) | 3-Dec (non-Asian dust) | |
| Proteobacteria | 85.29371 | 53.7716 | 51.83539 | 84.13297 |
| Actinobacteria | 9.739992 | 23.75334 | 17.50286 | 4.962867 |
| Firmicutes | 3.549319 | 15.69041 | 16.84237 | 2.298715 |
| Deinococcus-Thermus | Tr | Tra | 3.391337 | 6.695744 |
| Acidobacteria | Tr | 3.258885 | 5.334688 | Tr |
| Cyanobacteria | Tr | Tr | 1.079639 | Tr |
| Gemmatimonadetes | Tr | Tr | 1.587705 | Tr |
| Verrucomicrobia | Tr | Tr | 1.486092 | Tr |
| Others | 1.416976 | 3.525776 | 0.939921 | 1.909702 |
a Tr, trace, indicates a proportion lower than 1%.
Table 2. Metastats analysis at the phylum level in the Asian dust and non-Asian dust event samples.
| Phylum | 1-Dec and 2-Dec (Asian dust) | 26-Nov and 3-Dec | P-valueb | ||||
|---|---|---|---|---|---|---|---|
| Averagea | Variance | Std. error | Averagea | Variance | Std. error | ||
| Proteobacteria | 52.8035 | 1.87 | 0.9681 | 84.7133 | 0.67 | 0.5804 | 0.000167 |
| Actinobacteria | 20.6281 | 19.53 | 3.1252 | 7.3514 | 11.41 | 2.3886 | 0.036333 |
| Firmicutes | 16.2664 | 0.66 | 0.576 | 2.924 | 0.78 | 0.6253 | 0.017222 |
a Average proportion of the phylum during indicated date.
b P<0.05 means that the proportion of indicated phylum has statistically difference.
Characterization of bacterial community at the genus level
Next, we characterized the bacterial community at the genus level in the collected samples. For the analysis, we classified the pyrosequencing data at the genus level and confirmed that a total of 646 genera were present. Among them, 12 genera were found to make up more than 2% of the total proportion, as shown in Fig 4 and Table 3. During the non-Asian dust events, genus Sphingomonas belong to phylum Proteobacteria was predominantly present at the proportion of 45%, and genera Acinetobacter, Comamonas, Deinococcus, and Diaphorobacter were present at proportions of 7.8%, 5.4%, 3.3%, and 2.9%, respectively. Although the genus Sphingomonas was still present predominantly in the Asian dust event samples, the proportion in the total genera was largely decreased to 14.6%. Instead of the decrement, genera Bacillus, Arthrobacter, Microbacterium, and Methylobacterium were increased by 6.8, 4.8, 2.9, and 17.6 times, respectively, in the Asian dust event samples. To determine the significantly different genera in their proportions between the two groups, we performed Metastats analysis. As a result, genera Bacillus and Arthrobacter were found to be significantly increased by 6.8 and 4.8 times, respectively (Table 4). In addition, among various genera that have proportion of lower than 2%, genera Blastocatella (1.99% of the Asian dust event samples) and Planomicrobium (1.43% of the Asian dust event samples) were significantly increased by 16.2 and 6.9 times, respectively. These results reveal that genera Bacillus and Planomicrobium belonging to the phylum Firmicutes and Arthrobacter belonging to the phylum Actinobacteria were major parts of the bacterial community transported with dust particles in South Korea during the December 2014 Asian dust event. In particular, these genera have been known to find arid or semi-arid regions, suggesting that the feature of habitat may contribute a relatively high proportion of the genera in the Asian dust samples [35–37]. Among the identified genera, Bacillus has been reported to induce severe diseases such as anthrax and food poisoning caused by Bacillus anthracis and Bacillus cereus, respectively [38–41]. In our results, the most abundant Bacillus species is Bacillus circulans (2.6%, data not shown). Although there are a few cases which reveal Bacillus circulans-associated diseases, a fatal sepsis case in an immunocompromised patient is reported [42, 43]. A relationship between disease and the other genera identified in this result is not well understood, but several reports have showed that the genus Arthrobacter is found in clinical samples [44, 45]. An accumulation of bacterial information by continuous monitoring during Asian dust and non-Asian dust events should be necessary for establishing a relationship between human health and bacterial community during Asian dust events.
Fig 4. Bacterial communities (%) at the genus level in the Asian dust and non-Asian dust event samples.
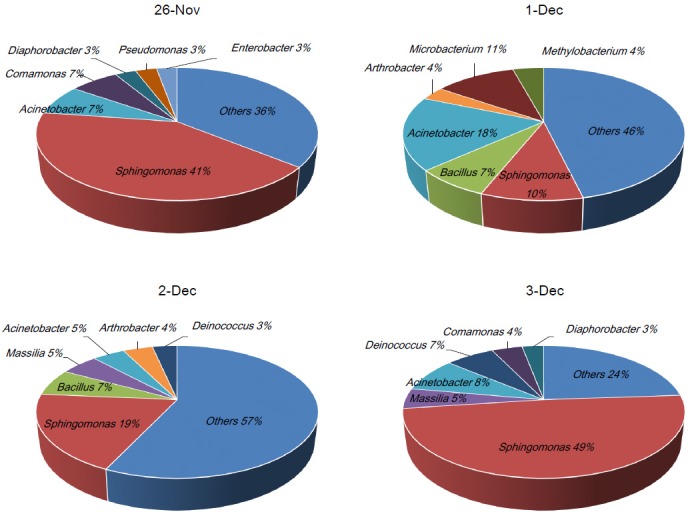
Other genera present lower than 2% of total bacteria community.
Table 3. Bacterial communities (%) at the genus level in the Asian dust and non-Asian dust event samples.
| Genus | Bacterial communities (%) | |||
|---|---|---|---|---|
| 26-Nov (non-Asian dust) | 1-Dec (Asian dust) | 2-Dec (Asian dust) | 3-Dec (non-Asian dust) | |
| Sphingomonas | 41.44999 | 9.832842 | 19.3573 | 48.96853 |
| Bacillus | Tra | 7.262256 | 6.541344 | Tr |
| Massilia | Tr | Tr | 5.182269 | 4.962867 |
| Acinetobacter | 7.373779 | 17.98005 | 4.458275 | 8.322527 |
| Arthrobacter | Tr | 3.286979 | 4.064524 | Tr |
| Deinococcus | Tr | Tr | 3.378636 | 6.695744 |
| Microbacterium | Tr | 11.1673 | Tr | Tr |
| Methylobacterium | Tr | 4.228122 | Tr | Tr |
| Comamonas | 6.699684 | Tr | Tr | 4.196629 |
| Diaphorobacter | 2.888981 | Tr | Tr | 2.970647 |
| Pseudomonas | 2.916495 | Tr | Tr | Tr |
| Enterobacter | 2.820195 | Tr | Tr | Tr |
| Others | 35.85087 | 46.24245 | 57.01766 | 23.88306 |
a Tr, trace, indicates a proportion lower than 1%.
Table 4. Metastats analysis at the genus level in the Asian dust and non-Asian dust event samples.
| Genus | 1-Dec and 2-Dec (Asian dust) | 26-Nov and 3-Dec | P-valueb | ||||
|---|---|---|---|---|---|---|---|
| Averagea | Variance | Std. error | Averagea | Variance | Std. error | ||
| Bacillus | 6.9018 | 0.26 | 0.3605 | 1.0209 | 0.11 | 0.231 | 0.025584 |
| Arthrobacter | 3.6758 | 0.3 | 0.3888 | 0.7595 | 0.04 | 0.1347 | 0.039053 |
| Blastocatella | 1.9934 | 0.05 | 0.1532 | 0.1228 | 0.01 | 0.054 | 0.027008 |
| Planomicrobium | 1.4293 | 0.02 | 0.0949 | 0.2063 | 0.03 | 0.1238 | 0.036915 |
a Average proportion of the genus during indicated date.
b P<0.05 means that the proportion of indicated genus has statistically difference.
Conclusions
In this study, we characterized the airborne bacterial community during Asian dust and non-Asian dust events from November to December 2014. As a result, we found that the diversity of the airborne bacterial environment was increased by newly transported dust particles, which contain various microorganisms, during the Asian dust event, leading to a decrement of the proportion of the predominant bacteria in the airborne environment. During the Asian dust event on December 2014, the proportion of predominantly presented phylum Proteobacteria was significantly reduced compared to the non-Asian dust event in November and December 2014. Meanwhile, phyla Actinobacteria and Firmicutes, especially genera Bacillus, Arthrobacter, and Planomicrobium, were newly introduced with dust particles. In addition, although the proportion was comparatively lower than the microorganisms mentioned above, various microorganisms were also transported during the Asian dust event. Indeed, the small proportion of newly transported microorganisms cannot be disregarded because the total amount (16S rRNA gene copy number) of microorganisms was dramatically increased (several hundreds to thousands) in the airborne environment during the Asian dust event [33]. In a further study, continuous monitoring of Asian dust and non-Asian dust event particles should be performed to determine the biological characterization of Asian dust particles. Moreover, various environmental effects, including soil environment, human health, and industrial problems, affected by the precipitated microorganisms should also be evaluated.
Supporting Information
Files named by Nov 26 and Dec 3 are non-Asian events, and files named by Dec 1 and Dec 2 are Asian dust events. The files were submitted into the INSDC SRA (BioProject and SRA accession numbers are PRJNA360153 and SRP096119, respectively).
(ZIP)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Institute of Environmental Research, Republic of Korea. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kar A, Takeuchi K. Yellow dust: an overview of research and felt needs. J Arid Environ. 2004;59(1):167–87. [Google Scholar]
- 2.Kim YH, Kim KS, Kwak NJ, Lee KH, Kweon SA, Lim Y. Cytotoxicity of yellow sand in lung epithelial cells. J Biosciences. 2003;28(1):77–81. [DOI] [PubMed] [Google Scholar]
- 3.Sun JM, Liu TS, Lei ZF. Sources of heavy dust fall in Beijing, China on April 16, 1998. Geophys Res Lett. 2000;27(14):2105–8. [Google Scholar]
- 4.Kwon HJ, Cho SH, Chun YS. The effects of the Asian dust events on daily mortality in Seoul, Korea. Epidemiology. 2000;11(4):S88–S. [DOI] [PubMed] [Google Scholar]
- 5.Kim KH, Kim MY. The effects of Asian Dust on particulate matter fractionation in Seoul, Korea during spring 2001. Chemosphere. 2003;51(8):707–21. 10.1016/S0045-6535(03)00036-5 [DOI] [PubMed] [Google Scholar]
- 6.Mori I, Nishikawa M, Tanimura T, Quan H. Change in size distribution and chemical composition of kosa (Asian dust) aerosol during long-range transport. Atmos Environ. 2003;37(30):4253–63. [Google Scholar]
- 7.Bory AJM, Biscaye PE, Grousset FE. Two distinct seasonal Asian source regions for mineral dust deposited in Greenland (NorthGRIP). Geophys Res Lett. 2003;30(4). [Google Scholar]
- 8.Duce RA, Unni CK, Ray BJ, Prospero JM, Merrill JT. Long-range atmospheric transport of soil dust from Asia to the tropical north pacific: temporal variability. Science. 1980;209(4464):1522–4. 10.1126/science.209.4464.1522 [DOI] [PubMed] [Google Scholar]
- 9.Kellogg CA, Griffin DW. Aerobiology and the global transport of desert dust. Trends Ecol Evol. 2006;21(11):638–44. 10.1016/j.tree.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 10.Kim KH, Choi GH, Kang CH, Lee JH, Kim JY, Youn YH, et al. The chemical composition of fine and coarse particles in relation with the Asian Dust events. Atmos Environ. 2003;37(6):753–65. [Google Scholar]
- 11.Nakano T, Yokoo Y, Nishikawa M, Koyanagi H. Regional Sr-Nd isotopic ratios of soil minerals in northern China as Asian dust fingerprints. Atmos Environ. 2004;38(19):3061–7. [Google Scholar]
- 12.Cheng TT, Lu DR, Wang GC, Xu YF. Chemical characteristics of Asian dust aerosol from Hunshan Dake sandland in Northern China. Atmos Environ. 2005;39(16):2903–11. [Google Scholar]
- 13.Hua NP, Kobayashi F, Iwasaka Y, Shi GY, Naganuma T. Detailed identification of desert-originated bacteria carried by Asian dust storms to Japan. Aerobiologia. 2007;23(4):291–8. [Google Scholar]
- 14.Reichhardt T. Weighing the health risks of airborne particulates. Environ Sci Technol. 1995;29(8):360A–4A. 10.1021/es00008a744 [DOI] [PubMed] [Google Scholar]
- 15.Pritchard RJ, Ghio AJ, Lehmann JR, Winsett DW, Tepper JS, Park P, et al. Oxidant generation and lung injury after particulate air pollutant exposure increase with the concentrations of associated metals. Inhal Toxicol. 1996;8(5):457–77. [Google Scholar]
- 16.Kim MY, Shin JY, Cho SC, Kim J, Lee GW, Kim KH. Environmental Mobilization Characteristics of Total Gaseous Mercury in the Western Coast of Korea During the Yellow Sand Period, 2001. Journal of Korean Earth Science Society. 2001;22(6):480–90. [Google Scholar]
- 17.He M, Ichinose T, Yoshida S, Nishikawa M, Mori I, Yanagisawa R, et al. Airborne Asian sand dust enhances murine lung eosinophilia. Inhal Toxicol. 2010;22(12):1012–25. 10.3109/08958378.2010.510151 [DOI] [PubMed] [Google Scholar]
- 18.Choi DS, Park YK, Oh SK, Yoon HJ, Kim JC, Seo WJ, et al. Distribution of airborne microorganisms in yellow sands of Korea. J Microbiol. 1997;35(1):1–9. [Google Scholar]
- 19.Lee S, Choi B, Yi SM, Ko G. Characterization of microbial community during Asian dust events in Korea. Science of the Total Environment. 2009;407(20):5308–14. 10.1016/j.scitotenv.2009.06.052 [DOI] [PubMed] [Google Scholar]
- 20.Maki T, Puspitasari F, Hara K, Yamada M, Kobayashi F, Hasegawa H, et al. Variations in the structure of airborne bacterial communities in a downwind area during an Asian dust (Kosa) event. Science of the Total Environment. 2014;488:75–84. 10.1016/j.scitotenv.2014.04.044 [DOI] [PubMed] [Google Scholar]
- 21.Whon TW, Kim MS, Roh SW, Shin NR, Lee HW, Bae JW. Metagenomic characterization of airborne viral DNA diversity in the near-surface atmosphere. J Virol. 2012;86(15):8221–31. 10.1128/JVI.00293-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Draxier RR, Hess GD. An overview of the HYSPLIT_4 modelling system for trajectories, dispersion and deposition. Aust Meteorol Mag. 1998;47(4):295–308. [Google Scholar]
- 23.Draxler RR. The use of global and mesoscale meteorological model data to predict the transport and dispersion of tracer plumes over Washington, D. C. Weather Forecast. 2006;21(3):383–94. [Google Scholar]
- 24.Islam MR, Sultana T, Joe MM, Cho JC, Sa TM. Comparisons of direct extraction methods of microbial DNA from different paddy soils. Saudi J Biol Sci. 2012;19(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasileiadis S, Puglisi E, Arena M, Cappa F, Cocconcelli PS, Trevisan M. Soil Bacterial Diversity Screening Using Single 16S rRNA Gene V Regions Coupled with Multi-Million Read Generating Sequencing Technologies. Plos One. 2012;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl Environ Microb. 2009;75(23):7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(D1):D590–D6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White JR, Nagarajan N, Pop M. Statistical Methods for Detecting Differentially Abundant Features in Clinical Metagenomic Samples. Plos Comput Biol. 2009;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sneath RR, Sokal PHA. Numerical Taxonomy, San Francisco: W.H. Freeman; 1973 [Google Scholar]
- 30.Chao A. Non-parametric estimation of the number of classes in a population. Scand J Statist. 1984;11:265–270 [Google Scholar]
- 31.Simpson EH. Measurement of diversity. Nature. 1949;163:688–688 [Google Scholar]
- 32.Jeon EM, Kim HJ, Jung K, Kim JH, Kim MY, Kim YP, et al. Impact of Asian dust events on airborne bacterial community assessed by molecular analyses. Atmos Environ. 2011;45(25):4313–21. [Google Scholar]
- 33.Yamaguchi N, Park J, Kodama M, Ichijo T, Baba T, Nasu M. Changes in the Airborne Bacterial Community in Outdoor Environments following Asian Dust Events. Microbes Environ. 2014;29(1):82–8. 10.1264/jsme2.ME13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi N, Ichijo T, Sakotani A, Baba T, Nasu M. Global dispersion of bacterial cells on Asian dust. Sci Rep-Uk. 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foesel BU, Rohde M, Overmann J. Blastocatella fastidiosa gen. nov., sp. nov., isolated from semiarid savanna soil—The first described species of Acidobacteria subdivision 4. Syst Appl Microbiol. 2013;36:82–89. 10.1016/j.syapm.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 36.Kellogg CA, Griffin DW, Garrison VH, Peak K, Royall N, Smith RR, Shinn EA. Characterization of aerosolized bacteria and fungi from desert dust events in Mali, West Africa. Aerobiologia. 2004;20(2):99–110. [Google Scholar]
- 37.Kwaasi AA, Parhar RS, al-Mohanna FA, Harfi HA, Collison KS, al-Sedairy ST. Aeroallergens and viable microbes in sandstorm dust. Potential triggers of allergic and nonallergic respiratory ailments. Allergy. 1998;53(3):255–65. [DOI] [PubMed] [Google Scholar]
- 38.Dixon TC, Meselson M, Guillemin J, Hanna PC. Medical progress: anthrax. N. Engl. J. Med. 1999;341:815–826. 10.1056/NEJM199909093411107 [DOI] [PubMed] [Google Scholar]
- 39.Dixon TC, Fahd A, Koehler T, Swanson J, Hanna PC. Early events in anthrax pathogenesis: intracellular survival of B. anthracis and its escape from RAW264.7 macrophages. Cell Microbiol. 2000;2:453–463. [DOI] [PubMed] [Google Scholar]
- 40.Ehling-Schulz M, Fricker M, Scherer S. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol Nutr Food Res. 2004;48(7):479–487. 10.1002/mnfr.200400055 [DOI] [PubMed] [Google Scholar]
- 41.Guinebretière MH, Broussolle V, Nguyen-The C. Enterotoxigenic Profiles of Food-poisoning and Food-borne Bacillus cereus Strains. J Clin Microbiol. 2002;40(8):3053–3056. 10.1128/JCM.40.8.3053-3056.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alebouyeh M, Orimi P, Azimi-rad M, Tajbakhsh M, Tajeddin E, Sherafat S, Nazemalhosseini mojarad E, Zali MR. Fatal sepsis by Bacillus circulans in an immunocompromised patient. Iran J Microbiol. 2011;3(3):156–158. [PMC free article] [PubMed] [Google Scholar]
- 43.Logan NA, Old DC, Dick HM. Isolation of Bacillus circulans from a wound infection. J Clin Pathol. 1985;38(7):838–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funke G, Hutson RA, Bernard KA, Pfyffer GE, Wauters G, Collins MD. Isolation of Arthrobacter spp. from clinical specimens and description of Arthrobacter cumminsii sp. nov. and Arthrobacter woluwensis sp. nov. J Clin Microbiol. 1996;34:2356–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernasconi E, Valsangiacomo C, Peduzzi R, Carota A, Moccetti T, Funke G. Arthrobacter woluwensis subacute infective endocarditis: case report and review of the literature. Clin Infect Dis. 2004;38(4):e27–31. 10.1086/381436 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Files named by Nov 26 and Dec 3 are non-Asian events, and files named by Dec 1 and Dec 2 are Asian dust events. The files were submitted into the INSDC SRA (BioProject and SRA accession numbers are PRJNA360153 and SRP096119, respectively).
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.