Abstract
Hormone replacement therapy (HRT) consists of highly effective prescription medications for treating menopausal symptoms; however, these agents have exhibited side effects including the risk of estrogen-induced carcinogenesis. Therefore, interest in phytotherapy-based materials as a natural source of alternatives to estrogen therapy has increased. However, some of these herbal medicines have been reported to increase the risk of estrogen-induced cancer. Herbal formulations composed of a combination of Cynanchum wilfordii Hemsley (CW), Phlomis umbrosa Turczaninow (PU), and Angelica gigas Nakai (AG) extracts (CPAE) have been used for treating menopausal symptoms. Therefore, in this study, we aimed to examine the safety of CPAE by determining its potential adverse estrogenic activity using the Organization for Economic Cooperation and Development (OECD) test guideline 455 (TG455) in a stably transfected transcriptionally activated human estrogen receptor α (hERα)-HeLa9903 cell model. We found that CPAE did not how any estrogenic activity or stimulate promoters containing estrogen response elements in MCF-7 cells. In addition, CPAE showed no significant selective activity against hERα and hERβ, non-selective activity against the ER, or effects on ER target gene expression. Furthermore, CPAE did not significantly induce MCF-7 cell proliferation and uterine weight increase in ovariectomized rats. These results demonstrate that CPAE can be used as beneficial herbal drug for prevention and therapeutic intervention of estrogen carcinogenesis in menopausal women.
Keywords: Cynanchum wilfordii Hemsley, Phlomis umbrosa Turczaninow, Angelica gigas Nakai, Estrogenic activity, Estrogen receptor
INTRODUCTION
In 1992, the American Medical Association (AMA) published a recommendation that menopausal women should consider the use of hormone replacement therapy (HRT) for treating menopausal symptoms caused by low estrogen (specifically estradiol, E2) levels (1). Since then, HRT has been considered the best alternative for improving menopausal women’s health, and its clinical use has drastically increased. However, the Women’s Health Initiative performed a multi-center, randomized, double-blind, placebo-controlled study with approximately 16,000 post-menopausal women for 5 years and reported that HRT increased the number of cases of breast cancer, heart disease, and stroke by 26, 29, and 41%, respectively (2).
There has been an increasing interest in developing natural compounds with high efficacy and low risk of adverse effects, such as isoflavones, black cohosh, and pomegranate, as alternative phytotherapies. Unfortunately, the National Institutes of Health reported that genistein, an aglycone of isoflavone from soybean, shows carcinogenic potential through its binding with estrogen receptors (ER) α and β (3,4). Although the genistein contents in isoflavone-related ingredients may not be high enough to cause cancer, further investigations are needed to determine the possible side effects of phytoestrogens.
Cynanchum wilfordii Hemsley (CW), Phlomis umbrosa Turczaninow (PU), and Angelica gigas Nakai (AG) have been used for centuries as traditional herbal treatments and are classified as raw materials for food ingredients by the Ministry of Food and Administration Safety (MFDS). The use of these traditional herbs was described in the Dong-Eui-Bo-Gam, the most famous book on Korean traditional medicine, which is an encyclopedic guideline of medical knowledge and treatment techniques first compiled by Joon Heo in 1613. These herbal treatments have shown various beneficial activities including anti-inflammation, antioxidant, anticancer, and anti-atherosclerosis. Recently, the extracts of CW, PU, and AG were reported to have beneficial effects as an alternative HRT (5–8).
The effectiveness of an herbal formulation composed of a combination of CW, PU, and AG extracts (CPAE; brand name, EstroG-100™) has been proven in several animal studies and two randomized, double-blind, placebo-controlled clinical studies. The positive data obtained from the 1-year clinical study with 48 patients revealed that the formulation significantly ameliorated various menopausal symptoms in South Korea. At the same time, it did not show any serious side effects or increase body weight, body mass index (BMI), and serum levels of E2 and follicle stimulating hormone (FSH) (9). In addition, a randomized, double-blinded, placebo-controlled clinical study was performed in non-Asian American women at Friends Medical Group and Shady Canyon Medical Group in CA, USA. In this study, the CPAE-treated group showed a more significant improvement in various menopausal symptoms such as hot flashes and night sweats, paresthesia, insomnia, nervousness, melancholia, vertigo, fatigue, rheumatic pain, and vaginal dryness than the placebo group did. Furthermore, no adverse effects or significant changes were observed in body weight, BMI, serum E2 and FSH levels, and liver enzymes that showed changes following treatment with HRT and other phytoestrogens (10).
However, to further confirm the safety of CPAE in terms of its estrogenic activity, it has been tested in estrogen-mimicking activity assays according to the Organization for Economic Cooperation and Development (OECD) guideline 455 (11).
MATERIALS AND METHODS
Plant material and preparation of CPAE
To prepare the CPAE, CW and PU were purchased from Jirisan Farming Association (Sancheong, Gyeongsangnam-do, Korea) and Baocheng Chinese Herbal Med Co., Ltd. (Hunan, China), in July 2015, respectively, while AG was provided by Yeongdong Herb Medicine Farming Association (Jecheon, Chungcheongbuk-do, Korea) in October 2015. CW, PU, and AG were mixed at a ratio of 1 : 1 : 1.08, extracted with distilled water (1 : 8 w/v) by boiling under reflux for 8 hr, cooled to room temperature for 30 min, and filtered using Whatman # 4 filter paper (Whatman, Maidstone, MA, USA). Then, the filtrate was subsequently evaporated using a rotary evaporator (R-215, Buchi, Switzerland) under reduced pressure until 40°Brix, lyophilized using a freeze drier (DW-86L728, Haier, Qingdao, China), and then the dry residues were dissolved in 40% dimethyl sulfoxide (DMSO) to a concentration of 40 mg/mL for the in vitro assays.
Cells and culture medium
The human estrogen receptor α (hERα)-expressing HeLa-9903 cell line (HeLa9903), which is stably transfected with a human ERα gene containing a luciferase reporter gene, was provided by the Chemicals Evaluation and Research Institute (CERI), Japan. The human breast cancer MCF-7 cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in a humidified 5% CO2 incubator at 37°C with 10% dextran-coated charcoal-treated fetal bovine serum (FBS) and phenol-free Dulbecco’s modified Eagle’s medium (DMEM).
Stably transfected transcriptional activation (STTA) assay
The luciferase activities were determined in accordance with the OECD TG 455 method (11). HeLa9903 cells were exposed to reference chemicals and the serially diluted test extract, followed by incubation for 20~24 hr (at 37°C in an atmosphere of 5% CO2). The cell preparations were then lysed, centrifuged (12,000 rpm, 10 min), and the supernatant was assayed for luciferase activity. Each compound was tested in three independent experiments in triplicate and all the plates contained E2 as a positive control.
Cell-based ER-mediated luciferase activity
MCF-7 cells were transfected with 1 μg human estrogen response element (ERE)-Luc reporter plasmid (Addgene plasmid no. 11354), 0.2 μg pCMV-β-gal (Addgene plasmid no. 155), or both per well using Lipofectamine™ 2000 (Invitrogen, Paisley, UK). Then, 6 hr after transfection, the cells were maintained for at least 24 hr in DMEM without phenol red (supplemented with 10% FBS previously treated with charcoal dextran), followed by incubation with CPAE, E2, ICI 182,780 (ICI, ER antagonist), or a combination of these compounds for 24 hr. Luciferase and β-galactosidase activities were subsequently measured in the cellular extracts as described previously (12).
Estrogen ligand-binding affinity assay
The binding ability of the test compound to the control or recombinant full-length hERα was assessed as described previously (13). Briefly, hERα (2088 pmol/mg estrogen binding activity; Invitrogen Corp., Carlsbad, CA, USA) as diluted to a concentration of 5 nM with a binding buffer containing 10 mM Tris (pH 7.5), 10% glycerol, 1 mM dithiothreitol (DTT), and 1 mg/mL bovine serum albumin (BSA). hERα was labeled with [3H]-E2 (3 nM) in the presence or absence of 10−6 M unlabeled E2 or CPAE (10 or 100 μg/mL). For the hERβ binding assay, hERβ (4500 pmol/mg estrogen binding activity; Invitrogen Corp.) was diluted to a concentration of 10 nM with the binding buffer and labeled with [3H]-E2 (10 nM) in the presence or absence of 10−6 M unlabeled E2 or CPAE. After incubation for 2 hr at 27°C, the reactions were terminated by rapid filtration through glass fiber filters (Packard Instrument B.V.-Chemical Operations, Groningen, The Netherlands) presoaked with ice-cold 0.05% polyethylenimine using a filter mate harvester (PerkinElmer Life and Analytical Sciences, Boston, MA, USA). The filters were washed five times with ice-cold buffer, dried, and then placed in scintillation vials containing 3 mL Packard Ultima Gold scintillation cocktail (Canberra Packard; Frankfurt, Germany). After shaking the vials and allowing them to equilibrate overnight, the radioactivity trapped in each filter was measured using a Packard 2000CA liquid scintillation counter. Non-specific [3H]-E2 binding was determined in the presence of 10−6 M unlabeled E2. The specific binding percentage of each ER was determined as follows: [(d.p.m. sample) − (d.p.m. nonspecific binding)]/[(d.p.m. control) − (d.p.m. non-specific binding)] × 100%.
Estrogen-related gene expression
One-Step RT-PCR System (Invitrogen) as described previously (14). The product formation was monitored continuously during the polymerase chain reaction (PCR) using the Sequence Detection System software (ver. 1.7, Applied Biosystems, Foster City, CA, USA). The accumulated PCR products were detected directly by monitoring the increase in SYBR® reporter dye fluorescence. The mRNA expression levels of E2-inducible genes (progesterone receptor [PGR] and trefoil factor 1 [TFF1]) in the MCF-7 cells treated with CPAE, E2, ICI, or a combination of these compounds were compared to those in the control cells at each time point using the comparative cycle threshold (Ct) method. The following primer sequences were used: hPGR, forward, 5′-GAC GTG GAG GGC GCA TAT-3′ and reverse, 5′-GCA GTC CGC TGT CCT TTT CT3′; hTFF1, forward, 5′-CCC TCC CAG TGT GCA AAT A-3′ and reverse, 5′-CTG GAG GGA CGT CGA TGG TA-3′; and hβ-ACTIN, forward, 5′-TGG CAC CCA GCA CAA TGA A-3′ and reverse, 5′-CTA AGT CAT AGT CCG CCT AGA AGC A-3′. The quantity of each transcript was calculated as described in the instrument manual and normalized to the amount of β-ACTIN, which was the housekeeping gene.
Cell proliferation assay (E-screen assay)
The viability of MCF-7 cells was assessed using the water-soluble tetrazolium (WST)-1 (Roche Molecular Biochemicals, Mannheim, Germany). The cells (10,000 cells/well) were seeded in 96-well plates for 24 hr, followed by treatment with CPAE, E2, ICI, or a combination of these compounds and then they were incubated for 3 days. Based on the manufacturer’s instructions, we added 10 μL of the WST-1 cell proliferation reagent to each well, incubated the plates for 2 hr at 37°C, and then the cell viability was quantified by measuring the absorbance at 440 nm using a BioTek Synergy HT microplate reader (BioTek Instruments, Winooski, USA).
Animals and experimental design
All the animal experiments were performed in accordance with the guidelines of the MFDS. Specific-pathogen-free (SPF), 10-week-old female Sprague-Dawley rats were obtained from KOATECH Korea. The rats were acclimatized for at least 1 week prior to use, and were housed in an air-conditioned room with a 12-hr light/dark cycle at a temperature of 22 ± 2°C and 50 ± 15.0% humidity. The rats were anesthetized with tribromoethanol (Avertin), and either sham-operated (SHAM, controls, n = 7) or bilaterally ovariectomized (OVX, n = 28) surgically. One week after surgery, the OVX rats were divided into the following four groups of seven rats each and treated as indicated: OVX control group, E2 (10 μg/kg), and low- and high-dose CPAE (125 and 250 mg/kg, respectively) groups. The CPAE was dissolved in saline prior to administration for 12 weeks, and the SHAM and OVX control rats were orally administered the same volume of saline alone. After the treatment period, the rat uteri were quickly removed, the connective tissue was excised, and then they were nicked, blotted, and weighed. The effects of CPAE on the uterus wet weight were determined by normalizing the uterine absolute and wet weights to the respective body weights of each rat. The animal experiments were conducted after approval (WJIACUC20160120-1-01) by WOOJUNG Life Science Research Center (WOOJUNGBSC, Suwon, Korea).
Statistical analysis
All experiments were repeated at least thrice, and a one-way analysis of variance (ANOVA) was used to determine the significance of differences between the treatment groups. The Newman-Keuls test was used for multi-group comparisons and a p-value < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Estrogenic activity of CPAE in STTA assay
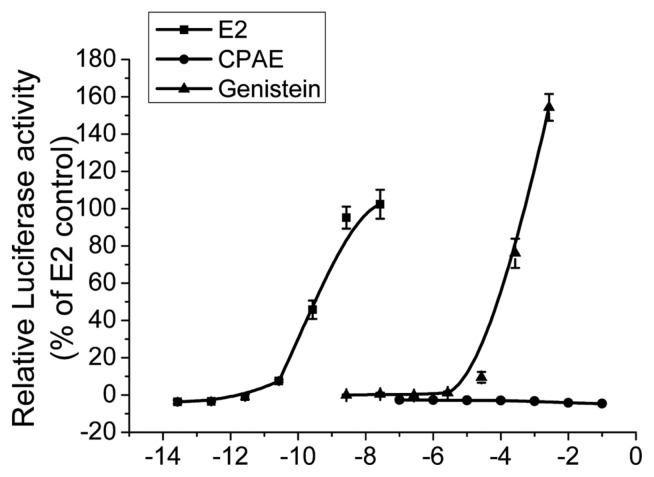
The STTA assay developed by the CERI was accepted as the official TG 455 in 2009 (11). This assay is used to evaluate the ability of chemicals to function as ERα ligands and induce agonistic responses in HeLa9903 cells stably transfected with the hERα-Luc gene. In the STTA assay performed in this study, CPAE did not exhibit any estrogenic activity at any tested concentrations while E2 and genistein, well-known phytoestrogens used as reference chemicals, showed significant estrogenic activity in a concentration-dependent manner (Fig. 1).
Fig. 1.
Concentration-response curve of transcriptional activation by Cynanchum wilfordii Hemsley, Phlomis umbrosa Turczaninow, and Angelica gigas Nakai extracts (CPAE) using HeLa9903 cells. Estradiol (E2, 10−13.6–10−7.6 g/L, 10−4–102 pM), genistein (10−8.6–10−2.6 g/L, 10−6–10 μM), and CPAE (10−7–10−1 g/L). Cells were harvested and assayed for luciferase activity. All experiments were performed in triplicate. Bars represent mean ± SD.
Estrogenic activity of CPAE in pERE-Luc transfected MCF-7 cells
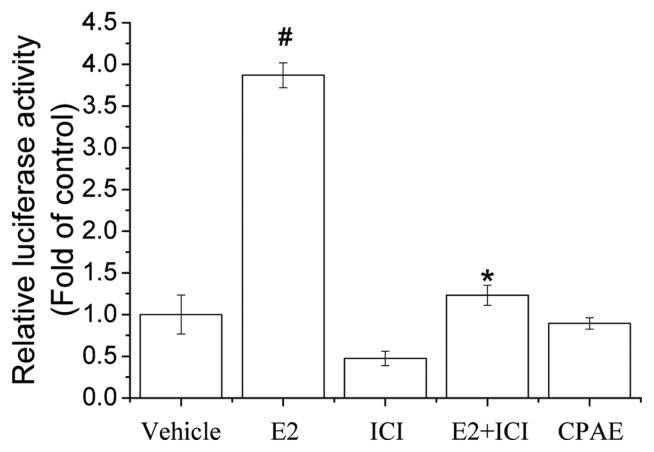
MCF-7 human breast cancer cells were also used to examine the estrogenic activity of CPAE. To determine if CPAE has classical ER agonist activity, we transiently transfected MCF-7 cells with an ERE-luciferase reporter gene construct and used this system to indirectly measure the transcription induced by ERs activated by CPAE. We found that 100 μg/mL CPAE did not induce luciferase activity while E2 caused a robust response (Fig. 2). To confirm the reliability of the transient transfection assays, the ER antagonist ICI was used and it strongly inhibited E2-induced luciferase activity. This observation suggests that CPAE did not show ERE-mediated estrogenic activity in MCF-7 cells. The extract of Larrea nitida is used to treat menstrual pain in South America and was reported to have no significant effect on ERE-Luc gene expression (15), which is similar to our present result.
Fig. 2.
Effect of estrogen response element (ERE) luciferase promoter activity induced by Cynanchum wilfordii Hemsley, Phlomis umbrosa Turczaninow, and Angelica gigas Nakai extract (CPAE) in MCF-7 cells. MCF-7 cells were transfected with pERE-Luc, treated with CPAE (100 μg/mL), estradiol (E2; 10 nM), ICI 182,780 (ICI, 10 μM) or a combination of these compounds for 24 hr. Cells were harvested and assayed for luciferase activity, which was normalized to β-galactosidase activity. All experiments were performed in triplicate. Bars represent mean ± SD. #P < 0.05 vs. control and *P < 0.05 vs. E2.
Competitive binding affinities of CPAE to ERs
The comparative ER binding assay is also generally used to detect ligand-receptor binding and measure the affinity of chemicals to ER receptor proteins (16). The binding affinities of CPAE to the ERs as an hER ligand were determined based on its ability to displace [3H]-E2 bound either the hERα or recombinant purified ERs, and the binding of the E2 standard was set to 100. As expected, E2 showed strong affinities in the ER binding assay while CPAE showed low binding affinities for hERα, hERβ, and non-selective ER (Table 1). A previous study also reported that none of the compounds contained in the extracts of Cimicifuga racemosa, one of the most promising available alternative therapies to HRT, was bound to the recombinant ERα or ERβ (17). These results suggest that CPAE does not have significant effects on ER signaling and the associated adverse effects.
Table 1.
Competitive binding affinities of CPAE to ERs
| Type | CPAE concentration (μg/mL) | Inhibition rate (%)* |
|---|---|---|
| ERα | 100 | 21 ± 5.3 |
| 10 | 3 ± 6.2 | |
| ERβ | 100 | 20 ± 2.3 |
| 10 | 8 ± 1.8 | |
| Non-selective ER | 100 | 11 ± 6.8 |
| 10 | −2 ± 6.3 |
No significant results noted.
Inhibition rate of ≥ 50% is considered to be significant.
CPAE stimulates estrogen-related genes in MCF-7 cells
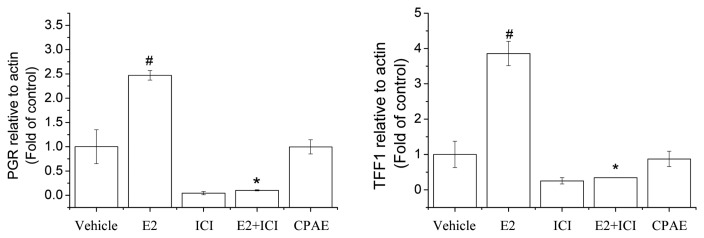
To elucidate the specificity of the estrogenic response, we assessed the effects of CPAE on the expression of estrogen-regulated genes, PGR and TFF1, which are known E2-inducible genes in MCF-7 cells. American Society of Clinical Oncology (ASCO) regards PGR as a standard marker in all invasive breast cancers (18). Activation of TFF1 gene is one of the earliest responses to E2 treatment (19), and while CPAE did not significantly change the expression of TFF1 and PGR, E2 increased the expression of these estrogen-regulated genes (Fig. 3). To confirm the reliability of the observed ER-mediated change in gene expression, we used an ER antagonist, ICI, which strongly inhibited the E2-induced gene expression of TFF1 and PGR. A diarylheptanoid isolated from Curcuma comosa Roxb. recently identified as a phytoestrogen did not significantly change the gene expression of TFF1 and PGR (20). This observation indicates that potent oncogenes induced by E2 may not be associated with the use of CPAE treatment in menopausal women.
Fig. 3.
Gene expression of progesterone receptor (PGR) and trefoil factor 1 (TFF1) in MCF-7 cells. Effect of CPAE (100 μg/mL) on PGR and TFF1 mRNA levels determined using quantitative reverse transcription-polymerase chain reaction (qRT-PCR). All experiments were performed in triplicate. Bars represent mean ± SD. #P < 0.05 vs. control and *P< 0.05 vs. E2.
Effect of CPAE on growth of MCF-7 cells
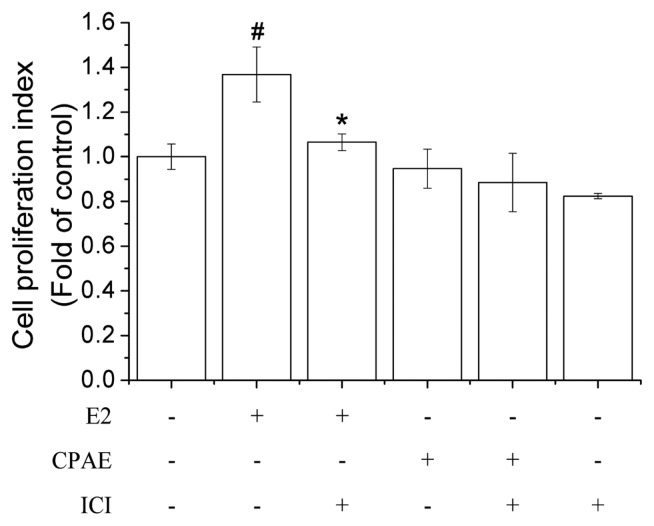
The E-screen assay is an MCF-7 cell proliferation assay that measures the number of target cells during the exponential phase of proliferation (21). The MCF-7 cell line is known to be a hormone-dependent breast cancer cell line that expresses both ERα and ERβ. We attempted to determine if CPAE exposure affects the proliferation of MCF-7 cells. The result showed that the CPAE did not affect the proliferation of MCF-7 cells compared with the effects of the positive control E2 (Fig. 4), indicating that CPAE did not affect the development of E2-mediated breast cancer. Wild yam extract, which is known as “natural progesterone” and popularly used to alleviate menopausal symptoms, was reported not to induce the growth of MCF-7 cells (22), which is similar to our present result.
Fig. 4.
Effects of Cynanchum wilfordii Hemsley, Phlomis umbrosa Turczaninow, and Angelica gigas Nakai extract (CPAE) on MCF-7 cell growth. MCF-7 cells were exposed to CPAE (100 μg/mL), estradiol (E2; 10 nM), ICI 182,780 (ICI, 10 μM) or a combination of these compounds for 24 hr. Cell proliferation was measured using water-soluble tetrazolium (WST)-1 assay. Bars represent mean ± SD. #P < 0.05 vs. control and *P < 0.05 vs. E2.
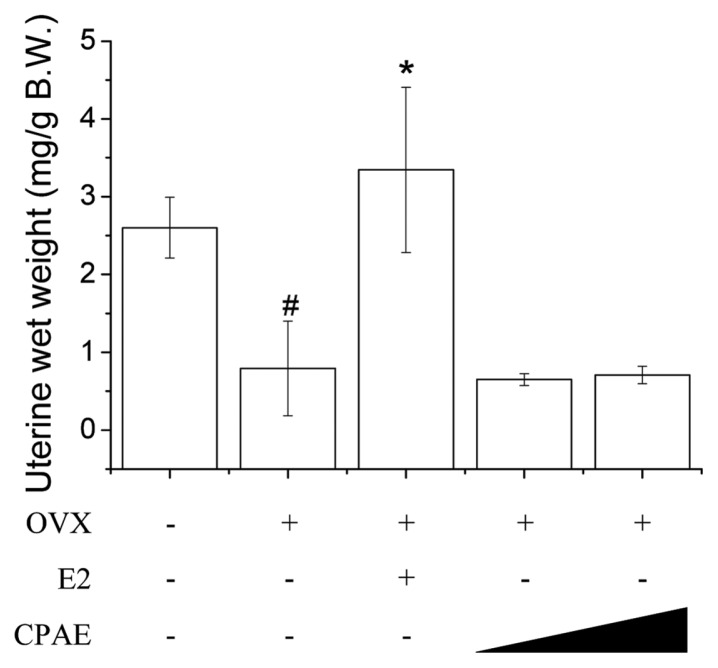
In vivo effect of CPAE in uterotrophic assay
An in vivo rodent uterotrophic assay was developed in which the estrogen-induced uterotrophic response was estimated based on an increase in the uterine tissue mass in OVX rats. Several studies have reported that some phytoestrogen-rich herbs inhibit bone loss in OVX rats without inducing any or only mild increases in uterine weight (23–25). CPAE was tested in vivo to determine if it induces uterine weight gain in rats and the results show that it did not increase uterine wet weight; however, E2 significantly increased both the absolute and relative wet weight compared to that of animals receiving saline only. The result demonstrated that CPAE was not estrogenic in adult female Sprague-Dawley rats in vivo (Fig. 5). A previous study reported that the mixture of CW and PU extracts decreased the uterus wall thickness and increased the body weight of OVX rats (26), which is consistent with our in vivo data.
Fig. 5.
Effects of Cynanchum wilfordii Hemsley, Phlomis umbrosa Turczaninow, and Angelica gigas Nakai extract (CPAE) in ovariectomized (OVX) rat uterotrophic assay. Each compound was administered subcutaneously at indicated concentration (125 and 250mg/kg per day) for 12 weeks. #P < 0.05 vs. sham group and *P < 0.05 vs. OVX group.
In conclusion, our present study provided evidence that CPAE does not show any estrogenic effects in the STTA and ERE reporter assays on HeLa9903 and MCF-7 cells, respectively. CPAE showed no significant activity against hERα, hERβ, and non-selective ER, as well as the expression of ER target genes. Furthermore, CPAE showed no significant effects in the E-screening and uterotrophic assays. These results suggest that CPAE may be a beneficial herbal medicine for the treatment of menopausal symptoms in women without adverse estrogenic activity.
ACKNOWLEDGMENTS
This work was supported by a research fund from Chungnam National University.
Abbreviations
- CPAE
Cynanchum wilfordii Hemsley, Phlomis umbrosa Turczaninow, and Angelica gigas Nakai extract
- HRT
Hormone replacement therapy
- OECD
Organization for Economic Cooperation and Development
- hER
human estrogen receptor
- CU
Cynanchum wilfordii Hemsley
- PU
Phlomis umbrosa Turczaninow
- AG
Angelica gigas Nakai
- AMA
American Medical Association
- MFDS
Ministry of Food and Administration Safety
- BMI
body mass index
- FSH
follicle-stimulating hormone
- DMSO
dimethyl sulfoxide
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- STTA
stably transfected transcriptional activation
- CERI
Chemicals Evaluation and Research Institute
- DTT
dithiothreitol
- BSA
bovine serum albumin
- OVX
ovariectomized
- ERE
estrogen response element
- PGR
progesterone receptor
- TFF1
trefoil factor 1.
Footnotes
CONFLICT OF INTEREST
None declared by all authors.
REFERENCES
- 1.American Medical Association. Guidelines for counseling postmenopausal women about preventive hormone therapy. American College of Physicians. Ann Intern Med. 1992;117:1038–1041. doi: 10.7326/0003-4819-117-12-1038. [DOI] [PubMed] [Google Scholar]
- 2.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 3.National Toxicology Program. Toxicology and carcinogenesis studies of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study) Natl Toxicol Program Techn Rep Ser. 2008;(545):1–240. [PubMed] [Google Scholar]
- 4.de Lemos ML. Effects of soy phytoestrogens genistein and daidzein on breast cancer growth. Ann Pharmacother. 2001;35:1118–1121. doi: 10.1345/aph.10257. [DOI] [PubMed] [Google Scholar]
- 5.Kim SN, Li YC, Xu HD, Yi DG, Kim MS, Lee SP, Yi KT, Lee JK, Kim JS, Kwon MS, Chang PS, Kwak BY. Phytoestrogenic effects of combined plant extracts on the change of bone metabolismof OVX rats. Korean J Food Sci Technol. 2008;40:316–320. [Google Scholar]
- 6.Lee YJ, Choi HI, Kim YC, You HK, Shin HS. Effects of dichloromethane fraction of Phlomidis radix on bone formation in human fetal osteoblasts. J Korean Acad Periodontol. 2003;33:259–269. doi: 10.5051/jkape.2003.33.2.259. [DOI] [Google Scholar]
- 7.Wong RW, Rabie AB, Hagg EU. The effect of crude extract from Radix Dipsaci on bone in mice. Phytother Res. 2007;21:596–598. doi: 10.1002/ptr.2126. [DOI] [PubMed] [Google Scholar]
- 8.Bae IY, Lee JY, Kwak BY, Lee HG. Estrogenic effects of various extracts from Chamdanggui (Angelica gigas Nakai) and Sogdan (Phlomis umbrosa Turcz) Food Sci Biotechnol. 2011;20:1113–1118. doi: 10.1007/s10068-011-0151-1. [DOI] [Google Scholar]
- 9.Lee KH, Lee DJ, Kim SM, Je SH, Kim EK, Han HS, Han IK. Evaluation of effectiveness and safety of natural plants extract (Estromon) on perimenopausal women for 1 year. J Korean Soc Menopause. 2005;11:16–26. [Google Scholar]
- 10.Chang A, Kwak BY, Yi K, Kim JS. The effect of herbal extract (EstroG-100) on pre-, peri- and post-menopausal women: a randomized double-blind, placebo-controlled study. Phytother Res. 2012;26:510–516. doi: 10.1002/ptr.3597. [DOI] [PubMed] [Google Scholar]
- 11.OECD, Organization for Economic Cooperation and Development. Ten new test guidelines and six updated test guidelineshave been adopted by council on 7 September. 2009. [Google Scholar]
- 12.Han EH, Kim HG, Hwang YP, Song GY, Jeong HG. Prostaglandin E2 induces CYP1B1 expression via ligand-independent activation of the ERalpha pathway in human breast cancer cells. Toxicol Sci. 2010;114:204–216. doi: 10.1093/toxsci/kfq013. [DOI] [PubMed] [Google Scholar]
- 13.Obourn JD, Koszewski NJ, Notides AC. Hormone- and DNA-binding mechanisms of the recombinant human estrogen receptor. Biochemistry. 1993;32:6229–6236. doi: 10.1021/bi00075a016. [DOI] [PubMed] [Google Scholar]
- 14.Kim CW, Go RE, Cho KC. Treatment of BG-1 ovarian cancer cells expressing estrogen receptors with lambda-cyhalothrin and cypermethrin caused a partial estrogenicity via an estrogen receptor-dependent pathway. Toxicol Res. 2015;31:331–337. doi: 10.5487/TR.2015.31.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn HN, Jeong SY, Bae GU, Chang M, Zhang D, Liu X, Pei Y, Chin YW, Lee J, Oh SR, Song YS. Selective estrogen receptor modulation by Larreanitida on MCF-7 cell proliferation and immature rat uterus. Biomol Ther (Seoul) 2014;22:347–354. doi: 10.4062/biomolther.2014.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akahori Y, Nakai M, Yamasaki K, Takatsuki M, Shimohigashi Y, Ohtaki M. Relationship between the results of in vitro receptor binding assay to human estrogen receptor alpha and in vivo uterotrophic assay: comparative study with 65 selected chemicals. Toxicol In Vitro. 2008;22:225–231. doi: 10.1016/j.tiv.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Wuttke W, Seidlová-Wuttke D. Black cohosh (Cimicifuga racemosa) is a non-estrogenic alternative to hormone replacement therapy. Clin Phytoscience. 2015;1:12. doi: 10.1186/s40816-015-0013-0. [DOI] [Google Scholar]
- 18.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–922. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown AM, Jeltsch JM, Roberts M, Chambon P. Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci U S A. 1984;81:6344–6348. doi: 10.1073/pnas.81.20.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winuthayanon W, Piyachaturawat P, Suksamrarn A, Ponglikitmongkol M, Arao Y, Hewitt SC, Korach KS. Diarylheptanoid phytoestrogens isolated from the medicinal plant Curcuma comosa: biologic actions in vitro and in vivo indicate estrogen receptor-dependent mechanisms. Environ Health Perspect. 2009;117:1155–1161. doi: 10.1289/ehp.0900613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995;103(Suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park MK, Kwon HY, Ahn WS, Bae S, Rhyu MR, Lee Y. Estrogen activities and the cellular effects of natural progesterone from wild yam extract in mcf-7 human breast cancer cells. Am J Chin Med. 2009;37:159–167. doi: 10.1142/S0192415X09006746. [DOI] [PubMed] [Google Scholar]
- 23.Zierau O, Zheng KY, Papke A, Dong TT, Tsim KW, Vollmer G. Functions of danggui buxue tang, a chinese herbal decoction containing astragali radix and angelicae sinensis radix, in uterus and liver are both estrogen receptor-dependent and -independent. Evid Based Complement Alternat Med. 2014;2014:438531. doi: 10.1155/2014/438531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng X, Huang H, Zheng X, Li B. Olive oil exhibits osteoprotection in ovariectomized rats without estrogenic effects. Exp Ther Med. 2016;11:1881–1888. doi: 10.3892/etm.2016.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lomnitski L, Padilla-Banks E, Jefferson WN, Nyska A, Grossman S, Newbold RR. A natural antioxidant mixture from spinach does not have estrogenic or antiestrogenic activity in immature CD-1 mice. J Nutr. 2003;133:3584–3587. doi: 10.1093/jn/133.11.3584. [DOI] [PubMed] [Google Scholar]
- 26.Han SH, Lee TH, Jang JY, Song HK, Hong SK, Kim YR, Han BS. Mixture of extracts of Cynanchum wilfordii and Phlomis umbrosa Turcz. doesnot have an estrogenic effect in ovariectomized rats. Korean J Food Sci Technol. 2015;47:667–672. doi: 10.9721/KJFST.2015.47.5.667. [DOI] [Google Scholar]