Supplemental Digital Content is available in the text.
Keywords: arteries, brain infarction, cerebral angiography, meta-analysis, stroke
Abstract
Background and Purpose—
Computed tomographic angiography and magnetic resonance angiography are used increasingly to assess arterial patency in patients with ischemic stroke. We determined which baseline angiography features predict response to intravenous thrombolytics in ischemic stroke using randomized controlled trial data.
Methods—
We analyzed angiograms from the IST-3 (Third International Stroke Trial), an international, multicenter, prospective, randomized controlled trial of intravenous alteplase. Readers, masked to clinical, treatment, and outcome data, assessed prerandomization computed tomographic angiography and magnetic resonance angiography for presence, extent, location, and completeness of obstruction and collaterals. We compared angiography findings to 6-month functional outcome (Oxford Handicap Scale) and tested for interactions with alteplase, using ordinal regression in adjusted analyses. We also meta-analyzed all available angiography data from other randomized controlled trials of intravenous thrombolytics.
Results—
In IST-3, 300 patients had prerandomization angiography (computed tomographic angiography=271 and magnetic resonance angiography=29). On multivariable analysis, more extensive angiographic obstruction and poor collaterals independently predicted poor outcome (P<0.01). We identified no significant interaction between angiography findings and alteplase effect on Oxford Handicap Scale (P≥0.075) in IST-3. In meta-analysis (5 trials of alteplase or desmoteplase, including IST-3, n=591), there was a significantly increased benefit of thrombolytics on outcome (odds ratio>1 indicates benefit) in patients with (odds ratio, 2.07; 95% confidence interval, 1.18–3.64; P=0.011) versus without (odds ratio, 0.88; 95% confidence interval, 0.58–1.35; P=0.566) arterial obstruction (P for interaction 0.017).
Conclusions—
Intravenous thrombolytics provide benefit to stroke patients with computed tomographic angiography or magnetic resonance angiography evidence of arterial obstruction, but the sample was underpowered to demonstrate significant treatment benefit or harm among patients with apparently patent arteries.
Clinical Trial Registration—
URL: http://www.isrctn.com. Unique identifier: ISRCTN25765518.
Computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) are increasingly used to assess arterial patency in patients with ischemic stroke.1,2 Angiography may also improve selection of patients with ischemic stroke for treatment with intravenous thrombolytics. However, it is unclear if arterial obstruction, or collateral supply, influences the response to intravenous thrombolytics. A retrospective stroke registry observed a trend for more favorable clinical outcome after intravenous alteplase in patients with intracranial arterial occlusion versus those with no/minimal obstruction in whom it was unclear if intravenous alteplase was beneficial or not.3
Few randomized controlled trials (RCTs) of intravenous thrombolytics included CTA or MRA to determine whether angiographic obstruction–occlusion or collateral supply modifies the treatment response. A pooled analysis of DIAS (Desmoteplase in Acute Ischemic Stroke), DIAS-2, and DEDAS (Dose Escalation of Desmoteplase for Acute Ischemic Stroke) demonstrated that patients with complete arterial occlusion or severe obstruction had better outcomes after desmoteplase than placebo, but in patients with minimal obstruction or normal arteries, there was no significant difference between desmoteplase versus placebo.4 In EPITHET (Echoplanar Imaging Thrombolytic Evaluation Trial), patients with middle cerebral artery (MCA) rather than internal carotid artery (ICA) obstruction demonstrated better outcome after intravenous alteplase, but they did not test for an alteplase–arterial patency interaction.5
The IST-3 (Third International Stroke Trial) was a large (n=3035) RCT that tested whether intravenous alteplase given within 6 hours of ischemic stroke improved functional outcome at 6 months compared with control.6 In IST-3, some centers routinely performed CTA or MRA prerandomization. As prespecified,7 we investigated whether arterial obstruction or collateral status on prerandomization CTA and MRA influenced outcome after alteplase versus control. In addition, we meta-analyzed all RCTs of intravenous thrombolytics in ischemic stroke with baseline angiography data.
Methods
Third International Stroke Trial
IST-3 was an international, multicenter, PROBE trial (prospective, randomized, open-label, blinded end point) of intravenous alteplase in acute ischemic stroke. Ethical approval was granted by the Multicentre Research Ethics Committees, Scotland (MREC/99/0/78) and by local ethics committees. Enrollment, randomization, data collection and analysis, and adherence to CONSORT (Consolidated Standards of Reporting Trials) recommendations have been published.6,8,9 Briefly, adults with acute stroke of any severity, in whom CT or MR imaging had excluded intracranial hemorrhage and structural stroke mimics, who did not meet the prevailing license criteria for alteplase but had no clear contraindication to treatment, were eligible for the trial if treatment could be started within 6 hours of known stroke onset. Informed consent was obtained for all patients. Baseline stroke severity before randomization was assessed with the National Institutes of Health Stroke Scale (NIHSS). After entry of baseline data, patients were randomly allocated to immediate intravenous alteplase (0.9 mg/Kg) or control. Functional outcome was assessed at 6 months with the Oxford Handicap Scale (OHS), which is similar to the modified Rankin Scale.
Angiographic Imaging in IST-3
IST-3 centers that routinely performed CTA or MRA in stroke submitted these images for assessment. Here, we include only IST-3 patients with angiography performed prerandomization. Images were anonymized and uploaded to a web-based rating platform, the Systematic Image Review System V2. Systematic Image Review System V2 provides secure anonymized image viewing via a browser (http://www.neuroimage.co.uk) and simultaneously records the scan interpretation using a validated proforma10,11 (http://www.sbirc.ed.ac.uk/research/imageanalysis.html).
All angiographic imaging in IST-3 was assessed centrally by a panel of 10 experts in stroke imaging (7 neuroradiologists and 3 neurologists/stroke physicians). Readers were masked to all clinical, treatment, and functional outcome data and to imaging from different time points (concurrent plain scans were viewed with angiography). IST-3 angiography was analyzed independently from the plain CT and MR imaging scans of the main IST-3 trial.
Scan readers recorded the presence, location, and severity of all arterial obstructions, largest affected artery first plus ≤2 additional segments (Table I in the online-only Data Supplement). Locations were ICA, MCA, anterior cerebral artery, posterior cerebral artery, vertebral artery, or basilar artery. We grouped arterial segments as proximal (ICA, main stem MCA, vertebral artery, and basilar artery) or distal (Sylvian branch of MCA, anterior cerebral artery, and posterior cerebral artery).
Readers coded the focus of arterial obstruction using a modified Thrombolysis in Cerebral Infarction (TICI) and the IST-3 angiography scores.7,12 Both are scalar and range from occlusion (0), through decreasing grades of obstruction, to normal (3 for TICI and 4 for IST-3). For this analysis, we categorized the IST-3 score 0 to 2b and the TICI score 0 to 2a as obstruction and IST-3 score 3 to 4 and the TICI score 2b to 3 as patent.
Readers also coded the clot burden score in patients with anterior circulation obstruction (subtracts the sum of the number of arterial segments affected from 10,13 where score 0=all segments affected and 10=no segments affected) and the collateral vessel supply in patients with ICA or MCA obstruction (categorized as good where the entire MCA distal to obstruction reconstituted with contrast, moderate where there was some MCA branch reconstitution, or poor if only distal superficial branches reconstituted).14
We tested previously interobserver reliability for the IST-3 panel’s CTA assessments (but because of the small number of cases, not MRA) on 15 representative scans,15 finding substantial agreement for arterial obstruction on TICI, IST-3 angiography, and clot burden scores (Krippendorff α=0.60, 0.66, and 0.63, respectively) and moderate agreement for collateral score (0.56).
Statistical Analysis: IST-3 Angiography Data
For univariate analysis of normally distributed data, we compared ratios and means with t tests; for nonparametric data, we used Mann–Whitney U tests to compare medians and Spearman ρ to assess for correlation. We used multifactor ANOVA to look for interactions between combinations of angiographic findings and OHS. To test for associations between angiography findings, alteplase versus control and outcome, we used multivariable ordinal regression to calculate common odds ratios (ORs) with OHS at 6 months as the dependent variable. We first tested the impact of different angiography characteristics on outcome individually, controlled for patient age, NIHSS, time from stroke onset to scan, and alteplase treatment allocation (here, OR <1 indicates worse outcome with alteplase). We then compared alteplase treatment effect on outcome (here, OR <1 favors control) for dichotomies (more normal versus less normal) of each angiography characteristic individually, controlled for patient age, NIHSS, and time from stroke onset to scan and tested for treatment interactions by comparing ORs between dichotomies. For this latter analysis only, we dichotomized clot burden and collateral scores as better (8–9 and good) versus worse (0–7 and moderate–poor, respectively).
Meta-Analysis
We identified all RCTs comparing intravenous thrombolytics with control among patients with baseline angiography after ischemic stroke from the Cochrane Collaboration systematic review on “Thrombolysis for Acute Ischaemic Stroke”16 and contacted investigators for additional data where necessary. We used the random effects method to perform 2 separate meta-analyses to obtain summary ORs for the effect of intravenous thrombolytics (any formulation or dose) on outcome (OHS or mRS at 3–6 months) in patients with (1) arterial obstruction (IST-3 angiography score 0 to 2b or Thrombolysis in Myocardial Infarction 0–1 or 0–2) versus patent vessels (IST-3 angiography score 3–4 or Thrombolysis in Myocardial Infarction 2–3 or 3) and (2) ICA versus MCA obstruction, including tests for interactions between intravenous thrombolytics and arterial status on functional outcome. We tested for between-study heterogeneity using I2 statistics. We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for meta-analysis reporting (Appendix II in the online-only Data Supplement).
We used Comprehensive Meta-Analysis software (Biostat, Englewood, NJ) to compute summary ORs and test for interactions. We performed all other analyses using IBM SPSS Statistics software, version 20.0 (IBM Corporation, Armonk, NY). We considered P<0.05 significant.
Results
Third International Stroke Trial
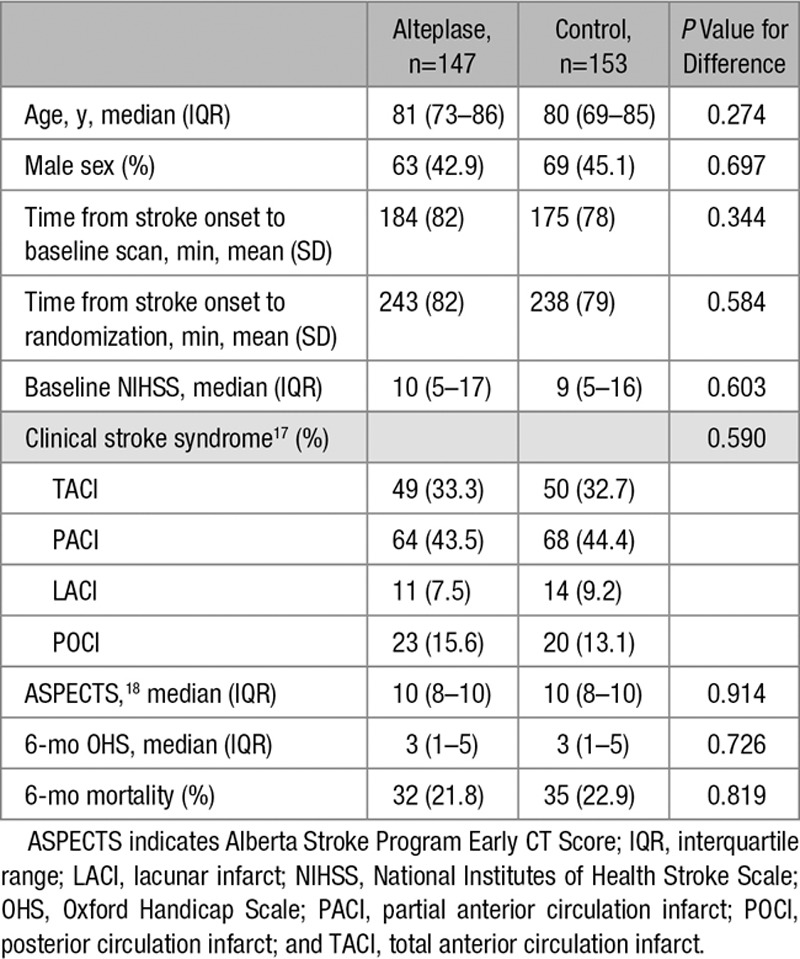
From 3035 IST-3 patients, 300 (9.9%) had prerandomization angiography, 271 with CTA, 29 with MRA. Compared with the full IST-3 trial, patients with angiography had less severe strokes at baseline (median NIHSS score of 9 versus 11; P=0.003), had baseline scanning slightly later (mean 180 versus 164 minutes; P<0.0001) and were randomized slightly later after stroke (mean 241 versus 231 minutes; P=0.019), and had better 6-month outcomes (median OHS 3 versus 4; P=0.001). There was no significant difference in age, sex, or treatment allocation between patients with angiograms versus the full IST-3 trial (Table II in the online-only Data Supplement). Among patients with angiography, there were no significant differences between alteplase and control groups (Table 1).
Table 1.
Comparison of Baseline Data and Main Outcomes in IST-3 (Third International Stroke Trial) Patients With Angiography Allocated to Alteplase and Control (n=300)
Angiography Findings: IST-3
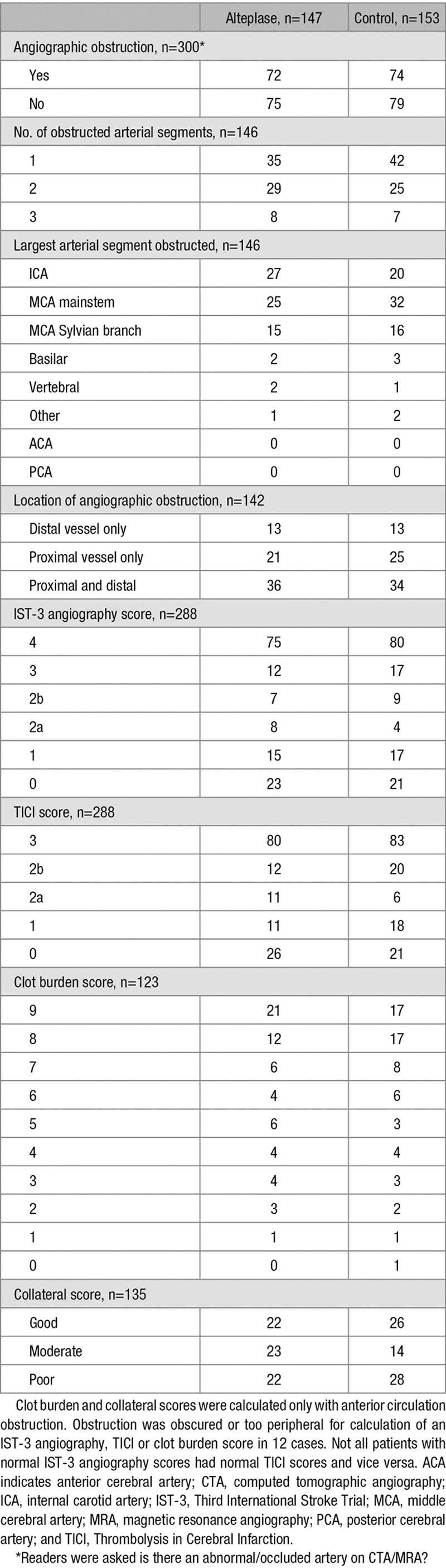
In IST-3, 146 of 300 (48.7%) patients had intracranial arterial obstruction on angiography (Table 2). ICA (47/146=32.2%) or MCA mainstem (57/146=39.0%) obstruction was most common. For those with arterial obstruction, >1 arterial segment was affected in approximately half, 2 segments in 54 of 146 (37.0%), and 3 segments in 15 of 146 (10.3%). From 288 patients with an IST-3 Angiography score, 104 (36.1%) scored 0 to 2b, whereas 184 (63.9%) scored 3 to 4 (Table 2 for concurrent TICI scores). The median clot burden score was 8 (2 segments affected). Similar proportions had good (48/135=35.6%), moderate (37/135=27.4%), or poor (50/135=37.0%) collateral scores.
Table 2.
Angiography Findings for the 300 Patients Randomized in IST-3 With CTA or MRA
Angiography, Stroke Severity, and Functional Outcome: IST-3
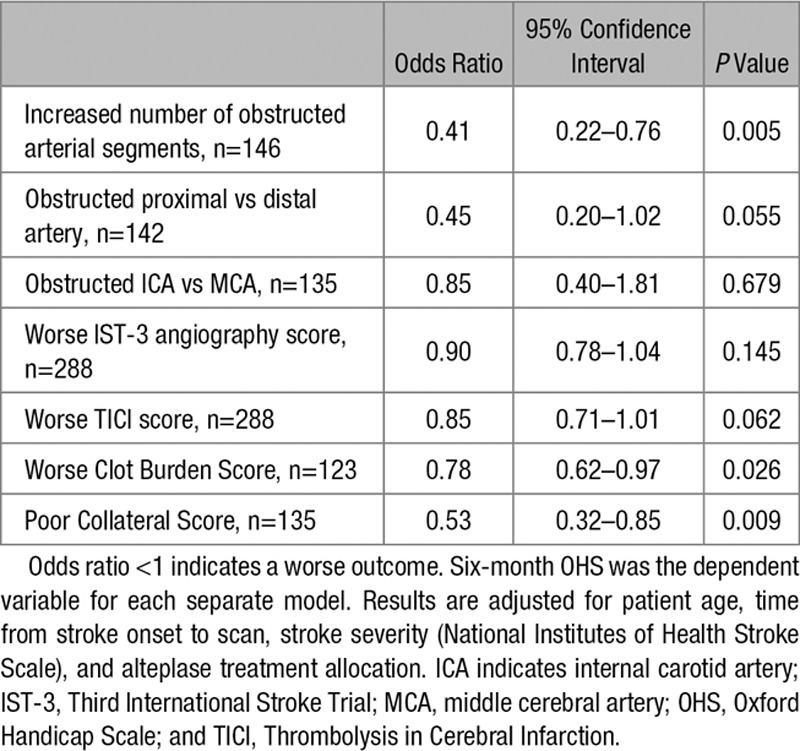
On univariate analysis, all categories of angiographic abnormality were associated with significantly worse baseline stroke severity and poorer 6-month outcome (P<0.0001 for most; Table III in the online-only Data Supplement). A 3-way ANOVA found no interaction between any combination of the effects of TICI, clot burden, and collateral scores on OHS (F=0.814; P=0.564; Table IV in the online-only Data Supplement). In multivariable ordinal regression analysis, controlled for age, NIHSS, time from stroke onset to scan and alteplase treatment allocation; having a greater number of obstructed arterial segments (OR, 0.41; 95% confidence interval [CI], 0.22–0.76; P=0.005), a worse clot burden score (OR, 0.78; 95% CI, 0.62–0.97; P=0.026), or poorer collaterals (OR, 0.53; 95% CI, 0.32–0.85; P=0.009) were all independent predictors of worse 6-month outcome, but not proximal versus distal (or ICA versus MCA) arterial obstruction, or the residual arterial caliber at the point of obstruction on IST-3 angiography or TICI scores (Table 3).
Table 3.
Multivariable Ordinal Regression Analyses Testing for Independent Associations Between Angiographic Findings in IST-3 and 6-Month Functional Outcome on the OHS
Angiography and Effect of Intravenous Alteplase: IST-3
In IST-3, in multivariable ordinal regression, we found no significant interactions between any individual angiogram feature and the effect of alteplase on 6-month OHS (Figure 1). There was a nonsignificant trend toward better outcomes after alteplase versus control among patients with obstructed (OR, 1.86; 95% CI, 0.76–4.53; P=0.171) versus patent (OR, 0.72; 95% CI, 0.42–1.25; P=0.241) arteries on the IST-3 angiography score, P=0.075 for interaction (Figures 1 and 2).
Figure 1.
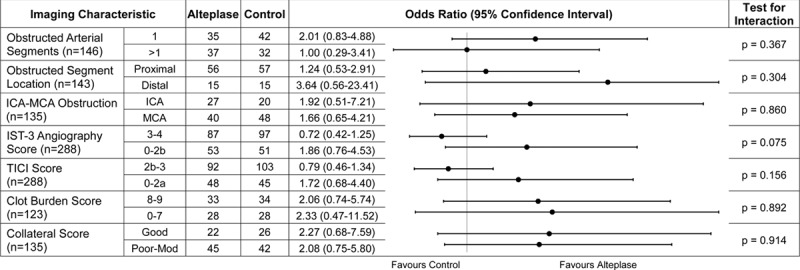
IST-3 (Third International Stroke Trial), ordinal regression analyses, of the effect of alteplase treatment on outcome (Oxford Handicap Scale [OHS] as the dependent variable) in patients with more vs less normal results by different angiography features. Results represent odds ratio of better (right of vertical line) or worse (left of line) 6-month outcome with alteplase. Adjusted for age, time from stroke onset to scan, and stroke severity (National Institutes of Health Stroke Scale). ICA indicates internal carotid artery; MCA, middle cerebral artery; and TICI, Thrombolysis in Cerebral Infarction.
Figure 2.
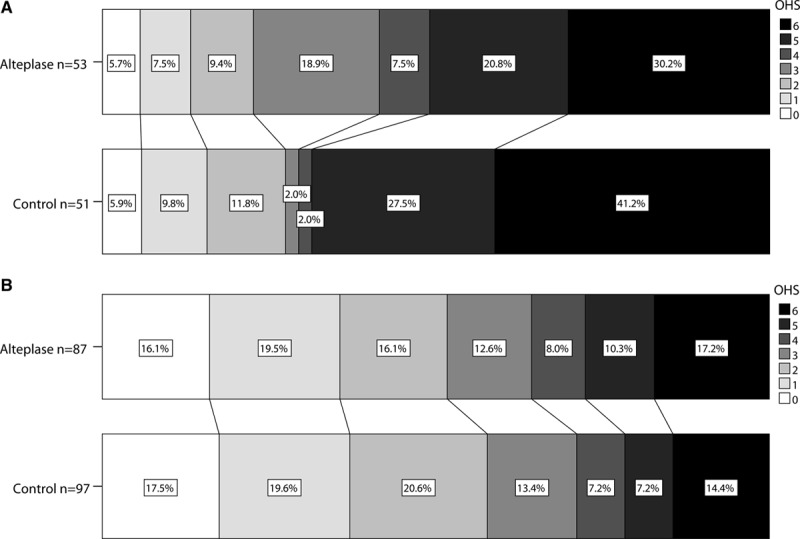
IST-3 (Third International Stroke Trial), bar charts comparing Oxford Handicap Scale (OHS) distribution for alteplase and control groups in those with (A) and without (B) arterial obstruction.
Angiography and Effect of Intravenous Thrombolytics: Meta-Analysis of All Trials
We identified 11 RCTs of intravenous thrombolytics for ischemic stroke with baseline angiography (Figure I in the online-only Data Supplement) from which data were available for meta-analysis of 4 trials in addition to IST-3 (n=591). We included 2 trials of intravenous alteplase (288 patients from IST-3 and 87 from EPITHET19) and 3 trials of intravenous desmoteplase (216 patients from DIAS, DIAS-2, and DEDAS).20–22 Meta-analysis showed that patients with arterial obstruction were significantly more likely to have better functional outcome with intravenous thrombolytics versus control (OR, 2.07; 95% CI, 1.18–3.64; P=0.011) than were patients with patent arteries (intravenous thrombolytics versus control, OR, 0.88; 95% CI, 0.58–1.35; P=0.566), for interaction P=0.017 (Figure 3).
Figure 3.
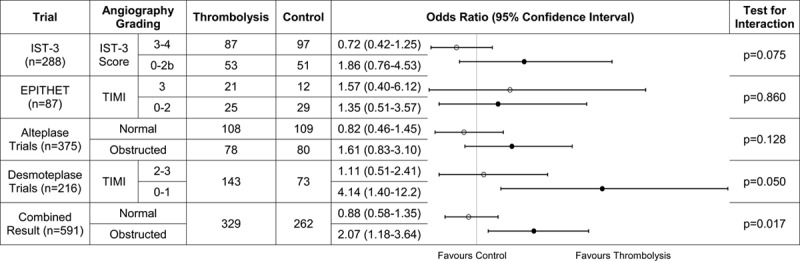
Meta-analysis of IST-3 (Third International Stroke Trial), EPITHET (Echoplanar Imaging Thrombolytic Evaluation Trial; alteplase), and the DIAS (Desmoteplase in Acute Ischemic Stroke), DIAS-2, and DEDAS (Dose Escalation of Desmoteplase for Acute Ischemic Stroke; desmoteplase) trials comparing the effect of intravenous thrombolytics on functional outcome between patients with arterial patency vs obstruction. Raw treatment data were unavailable for the Desmoteplase trials. Results represent odds ratio of better (right of vertical line) or worse (left of line) outcome after thrombolytics. Open circles represent arterial patency (test for heterogeneity, I2=0%). Closed circles represent arterial obstruction (I2=16%). TIMI indicates Thrombolysis in Myocardial Infarction.
For meta-analysis of ICA versus MCA obstruction, data were only available from IST-3 and EPITHET (n=135+54=189, respectively) and we did not find any difference between response to intravenous thrombolytics in patients with ICA versus MCA obstruction (ICA obstruction: OR, 0.65; 95% CI, 0.06–6.89; P=0.720; MCA obstruction: OR, 1.92; 95% CI, 0.90–4.09; P=0.090) for interaction P=0.391 (Figure II in the online-only Data Supplement).
Discussion
This analysis of CTA and MRA data from 5 RCTs of intravenous thrombolytics (alteplase and desmoteplase), including IST-3, comprising 591 patients treated within 6 hours of stroke, indicates that patients with angiographic obstruction/occlusion benefit significantly more from intravenous thrombolytics than patients without arterial obstruction. Therefore, where endovascular therapy is not available,23 intravenous thrombolytics remain an important treatment. The significant interaction between angiography appearance and intravenous thrombolytics does not mean that patients with normal baseline angiography gain no benefit from intravenous thrombolytics24; the meta-analysis was neutral in this group, showing neither benefit or harm, but the sample is too small to exclude modest benefit or harm. It is important to remember that patients with normal angiograms may have arterial obstruction(s) too small to see on CTA/MRA, and intravenous thrombolytics may be beneficial in these patients.
In IST-3, we also demonstrate that having a greater number of obstructed arterial segments or poor collateral supply was associated with poor 6-month functional outcome, independent of stroke severity and alteplase treatment, that is, alteplase was less likely to improve outcome in these patients. Those with a greater number of obstructed segments may, therefore, have the most to gain from endovascular therapy, which several RCTs have recently shown to be superior to intravenous alteplase alone among patients with angiography-confirmed proximal intracranial arterial obstruction.25 Consistent with our results, post hoc analysis of one of these endovascular treatment trials demonstrated that patients with poor collateral supply had poorer outcomes than those with good collaterals.26
We could not confirm in the meta-analysis that patients with MCA versus ICA obstruction responded differently to thrombolytics, but the sample available for this comparison was very small. In general, we were unable to identify available comparable trial data for most of the other angiography characteristics assessed in IST-3. To better understand thrombolysis–angiography interactions, future RCTs of acute stroke therapy with CTA or MRA should examine location, extent, completeness of arterial obstruction, and adequacy of collaterals, to refine how these findings could help treatment decisions.
Strengths and Limitations
Strengths of IST-3 include our use of robust, validated methods for scan management, of scoring angiograms,7 with blinding of readers and moderate to substantial interobserver agreement.15 Limitations of IST-3, discussed previously, include the potential introduction of bias through its open design.6 Angiographic imaging was not a requirement of IST-3, but some centers performed baseline CTA or MRA for ischemic stroke routinely; therefore, the angiography analysis was preplanned to maximize knowledge gained from an RCT. The results of angiography were not used to determine trial eligibility, and at the time of IST-3 enrollment, there were virtually no data on how angiography results should be used in this context. Therefore, clinical uncertainty as to whether treatment with alteplase would be beneficial existed even for patients with large artery obstruction. Angiography was however performed in only 10% of IST-3 centers, potentially reflecting selection bias and restricting generalizability of the findings to all current stroke centers. Nevertheless, IST-3 angiography represents real-world practice, where nearly 50% had angiographic obstruction27 and with 300 patients, is the largest angiography data set from an RCT of intravenous alteplase in ischemic stroke. Despite this, the wide CIs indicate that our sample is underpowered to estimate the effect of angiography findings on alteplase response. We used dichotomies of better versus worse angiography scoring to simplify scalar data in IST-3. We acknowledge that different dichotomies of the same scalar data could provide different results, but we felt that our technique nevertheless provided a meaningful and useable summary. For the meta-analysis, slight inconsistencies in angiogram scoring between trials mean that there is some overlap between categories (eg, Thrombolysis in Myocardial Infarction=2 is included in the better category for EPITHET but the worse category for the desmoteplase trials). Efforts to standardize angiogram rating in future trials should be encouraged because this will facilitate between-trial comparisons and meta-analysis.28
Patients recruited in IST-3 after CTA or MRA were randomized and treated, on average, 10 minutes later than were those with only plain scans. The steep time dependency of alteplase effects on outcome means that even this small delay could reduce the potential benefit of treatment,29 particularly on a population level if angiography is performed nondiscriminately in all ischemic stroke patients. Efforts should be directed at minimizing delays attributable to obtaining angiography in stroke.
Conclusions
Intravenous thrombolytic therapy is significantly more effective in improving functional outcome in patients presenting with ischemic stroke who have arterial obstruction than in those with apparently patent arteries on CTA or MRA. Intravenous thrombolytics, therefore, remain an important treatment option. The data are too sparse to determine, in this analysis, whether patients without apparent arterial obstruction benefit from intravenous thrombolytics, bearing in mind that these patients may have thrombus too small to detect on CTA or MRA.
Acknowledgments
This article acknowledges Dr Veronica Murray, IST-3 (Third International Stroke Trial) National Co-ordinator for Sweden, expert stroke neurologist, friend, and colleague, whose energy, enthusiasm, and huge contribution ensured the success of IST-3 and who died suddenly in late 2014. The IST-3 collaborative group thanks all study participants. We gratefully acknowledge the trial steering committee and national coordinators (Appendix I in the online-only Data Supplement). IST-3 centers providing baseline angiograms are listed in Appendix III in the online-only Data Supplement. We are grateful to the EPITHET trial (Echoplanar Imaging Thrombolytic Evaluation Trial) investigators, especially Stephen M. Davis, Geoffrey A. Donnan, and Mark W. Parsons, for providing raw data for meta-analysis.
Sources of Funding
IST-3 angiography substudy (Third International Stroke Trial) was funded by the National Institute for Health Research (NIHR) Efficacy and Mechanisms Evaluation Panel (EME-08-43-52). Main IST-3 trial had many funders detailed in Appendix IV in the online-only Data Supplement, primarily the UK Medical Research Council (MRC G0400069 and EME-09-800-15). Systematic Image Review System V2 (SIRS-2) development was funded by the Edinburgh MRC Hub for Trials Methodology Research funded by the UK MRC (G0800803).
Disclosures
R. von Kummer received fees from Lundbeck, Covidien, Brainsgate, Boehringer Ingelheim, and Penumbra; Dr White from Microvention Terumo, and Codman; Dr Lindley from Boehringer Ingelheim and Covidien; and P.A.G. Sandercock from Boehringer Ingelheim. Dr Wardlaw received consultancy fees from Glaxo Smith Kline paid to the institution. The other authors report no conflicts.
Supplementary Material
Footnotes
Guest Editor for this article was Louis Caplan, MD.
A list of all IST-3 Collaborative Group participants is given in the Appendix I in the online-only Data Supplement.
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.116.015164/-/DC1.
References
- 1.McDonald JS, Fan J, Kallmes DF, Cloft HJ. Pretreatment advanced imaging in patients with stroke treated with IV thrombolysis: evaluation of a multihospital data base. AJNR Am J Neuroradiol. 2014;35:478–481. doi: 10.3174/ajnr.A3797. doi: 10.3174/ajnr.A3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vagal A, Meganathan K, Kleindorfer DO, Adeoye O, Hornung R, Khatri P. Increasing use of computed tomographic perfusion and computed tomographic angiograms in acute ischemic stroke from 2006 to 2010. Stroke. 2014;45:1029–1034. doi: 10.1161/STROKEAHA.113.004332. doi: 10.1161/STROKEAHA.113.004332. [DOI] [PubMed] [Google Scholar]
- 3.Medlin F, Amiguet M, Vanacker P, Michel P. Influence of arterial occlusion on outcome after intravenous thrombolysis for acute ischemic stroke. Stroke. 2015;46:126–131. doi: 10.1161/STROKEAHA.114.006408. doi: 10.1161/STROKEAHA.114.006408. [DOI] [PubMed] [Google Scholar]
- 4.Fiebach JB, Al-Rawi Y, Wintermark M, Furlan AJ, Rowley HA, Lindstén A, et al. Vascular occlusion enables selecting acute ischemic stroke patients for treatment with desmoteplase. Stroke. 2012;43:1561–1566. doi: 10.1161/STROKEAHA.111.642322. doi: 10.1161/STROKEAHA.111.642322. [DOI] [PubMed] [Google Scholar]
- 5.de Silva DA, Brekenfeld C, Ebinger M, Christensen S, Barber PA, Butcher KS, et al. Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) Investigators. The benefits of intravenous thrombolysis relate to the site of baseline arterial occlusion in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET). Stroke. 2010;41:295–299. doi: 10.1161/STROKEAHA.109.562827. doi: 10.1161/STROKEAHA.109.562827. [DOI] [PubMed] [Google Scholar]
- 6.IST-3 Collaborative Group. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6h of acute ischaemic stroke (the Third International Stroke Trial [IST-3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wardlaw JM, von Kummer R, Carpenter T, Parsons M, Lindley RI, Cohen G, et al. Protocol for the perfusion and angiography imaging sub-study of the Third International Stroke Trial (IST-3) of alteplase treatment within six-hours of acute ischemic stroke. Int J Stroke. 2015;10:956–968. doi: 10.1111/j.1747-4949.2012.00946.x. doi: 10.1111/j.1747-4949.2012.00946.x. [DOI] [PubMed] [Google Scholar]
- 8.Sandercock P, Lindley R, Wardlaw J, Dennis M, Lewis S, Venables G, et al. IST-3 Collaborative Group. Third International Stroke Trial (IST-3) of thrombolysis for acute ischaemic stroke. Trials. 2008;9:37. doi: 10.1186/1745-6215-9-37. doi: 10.1186/1745-6215-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IST-3 Collaborative Group. Statistical analysis plan for the Third International Stroke Trial (IST-3); part of a ‘thread’ of reports of the trial. Int J Stroke. 2012;7:186–187. doi: 10.1111/j.1747-4949.2012.00782.x. doi: 10.1111/j.1747-4949.2012.00782.x. [DOI] [PubMed] [Google Scholar]
- 10.Wardlaw JM, Farrall AJ, Perry D, von Kummer R, Mielke O, Moulin T, et al. Acute Cerebral CT Evaluation of Stroke Study (ACCESS) Study Group. Factors influencing the detection of early CT signs of cerebral ischemia: an internet-based, international multiobserver study. Stroke. 2007;38:1250–1256. doi: 10.1161/01.STR.0000259715.53166.25. doi: 10.1161/01.STR.0000259715.53166.25. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw JM, von Kummer R, Farrall AJ, Chappell FM, Hill M, Perry D. A large web-based observer reliability study of early ischaemic signs on computed tomography. The Acute Cerebral CT Evaluation of Stroke Study (ACCESS). PLoS One. 2010;5:e15757. doi: 10.1371/journal.pone.0015757. doi: 10.1371/journal.pone.0015757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology; Technology Assessment Committee of the Society of Interventional Radiology. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 13.Puetz V, Dzialowski I, Hill MD, Subramaniam S, Sylaja PN, Krol A, et al. Calgary CTA Study Group. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke. 2008;3:230–236. doi: 10.1111/j.1747-4949.2008.00221.x. doi: 10.1111/j.1747-4949.2008.00221.x. [DOI] [PubMed] [Google Scholar]
- 14.Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132(pt 8):2231–2238. doi: 10.1093/brain/awp155. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 15.Mair G, von Kummer R, Adami A, White PM, Adams ME, Yan B, et al. IST-3 Collaborative Group. Observer reliability of CT angiography in the assessment of acute ischaemic stroke: data from the Third International Stroke Trial. Neuroradiology. 2015;57:1–9. doi: 10.1007/s00234-014-1441-0. doi: 10.1007/s00234-014-1441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;7:CD000213. doi: 10.1002/14651858.CD000213.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 18.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 19.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. EPITHET investigators. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 20.Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, et al. DIAS Study Group. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 21.Furlan AJ, Eyding D, Albers GW, Al-Rawi Y, Lees KR, Rowley HA, et al. DEDAS Investigators. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37:1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 22.Hacke W, Furlan AJ, Al-Rawi Y, Davalos A, Fiebach JB, Gruber F, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8:141–150. doi: 10.1016/S1474-4422(08)70267-9. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindley RI, Levi CR. The spectacular recent trials of urgent neurointervention for acute stroke: fuel for a revolution. Med J Aust. 2015;203:58–60. doi: 10.5694/mja15.00395. [DOI] [PubMed] [Google Scholar]
- 24.Lahoti S, Gokhale S, Caplan L, Michel P, Samson Y, Rosso C, et al. Thrombolysis in ischemic stroke without arterial occlusion at presentation. Stroke. 2014;45:2722–2727. doi: 10.1161/STROKEAHA.114.005757. doi: 10.1161/STROKEAHA.114.005757. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues FB, Neves JB, Caldeira D, Ferro JM, Ferreira JJ, Costa J. Endovascular treatment versus medical care alone for ischaemic stroke: systematic review and meta-analysis. BMJ. 2016;353:i1754. doi: 10.1136/bmj.i1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkhemer OA, Jansen IG, Beumer D, Fransen PS, van den Berg LA, Yoo AJ, et al. MR CLEAN Investigators. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke. 2016;47:768–776. doi: 10.1161/STROKEAHA.115.011788. doi: 10.1161/STROKEAHA.115.011788. [DOI] [PubMed] [Google Scholar]
- 27.Hansen CK, Christensen A, Ovesen C, Havsteen I, Christensen H. Stroke severity and incidence of acute large vessel occlusions in patients with hyper-acute cerebral ischemia: results from a prospective cohort study based on CT-angiography (CTA). Int J Stroke. 2015;10:336–342. doi: 10.1111/ijs.12383. doi: 10.1111/ijs.12383. [DOI] [PubMed] [Google Scholar]
- 28.Wintermark M, Albers GW, Broderick JP, Demchuk AM, Fiebach JB, Fiehler J, et al. Stroke Imaging Research (STIR) and Virtual International Stroke Trials Archive (VISTA)-Imaging Investigators. Acute stroke imaging research roadmap II. Stroke. 2013;44:2628–2639. doi: 10.1161/STROKEAHA.113.002015. doi: 10.1161/STROKEAHA.113.002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


